Total Lipid Extracts of Honeybee Drone Larvae Are Modulated by Extraction Temperature and Display Consistent Anti-Inflammatory Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Sample Preparation
2.4. Instrument Conditions and Methods
2.4.1. LC-MS Analysis
2.4.2. GC-MS Analysis
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Determination of Nitric Oxide (NO) Concentration in Cell Culture (Greiss Reaction)
2.8. Total RNA Isolation and Quantitative Real-Time PCR
2.9. Capture of Intracellular ROS Generation
2.10. Statistical Analysis
3. Results and Discussion
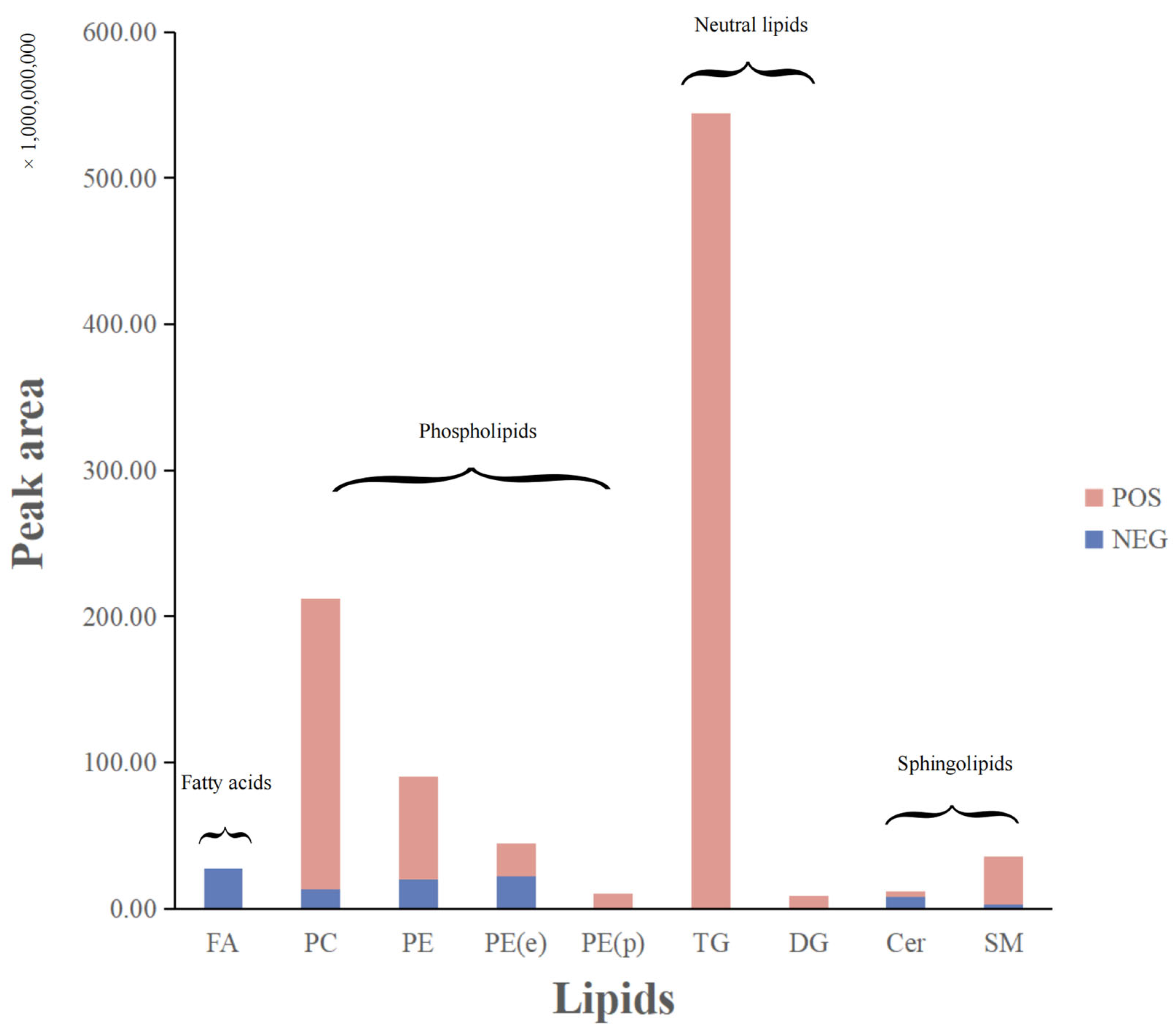
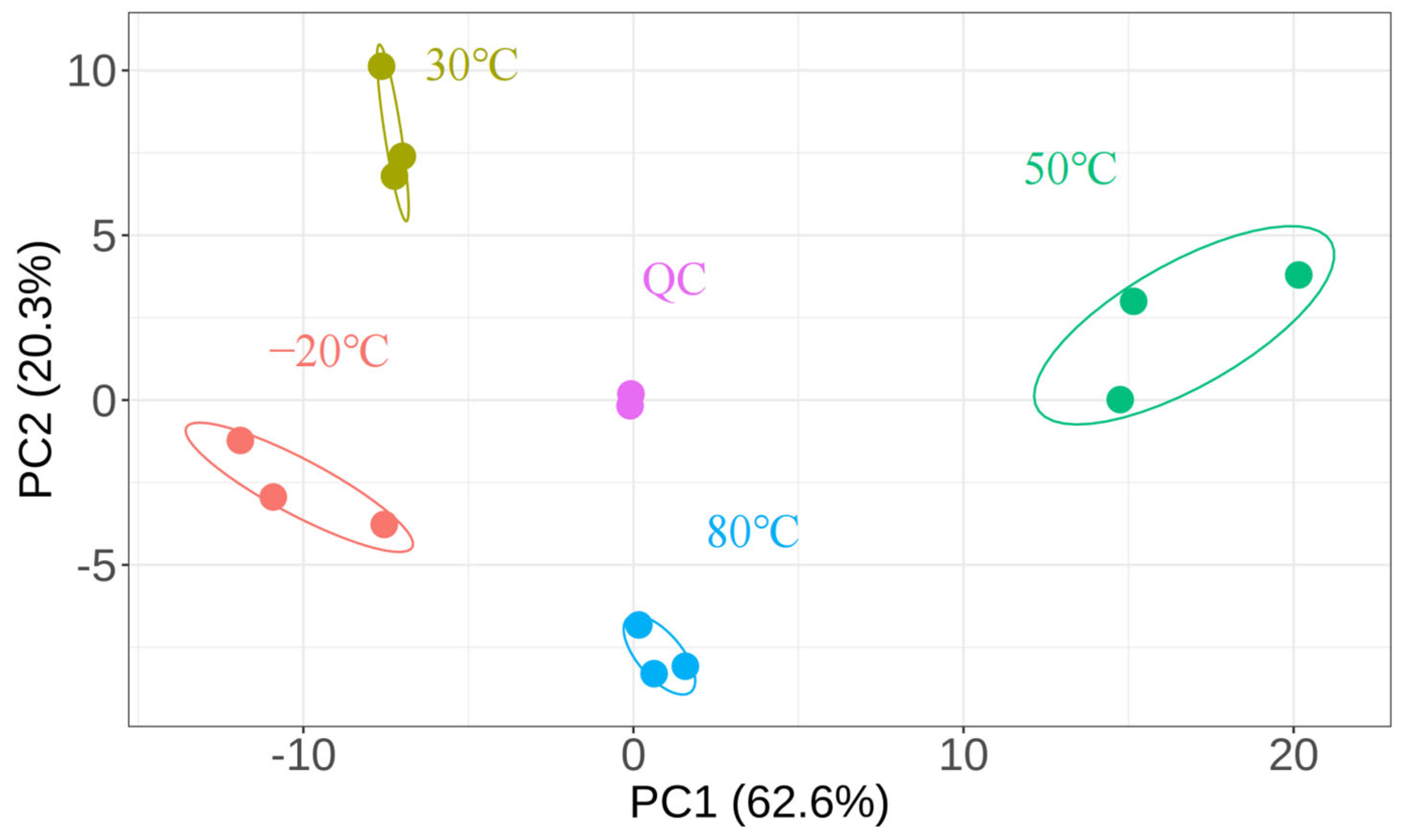
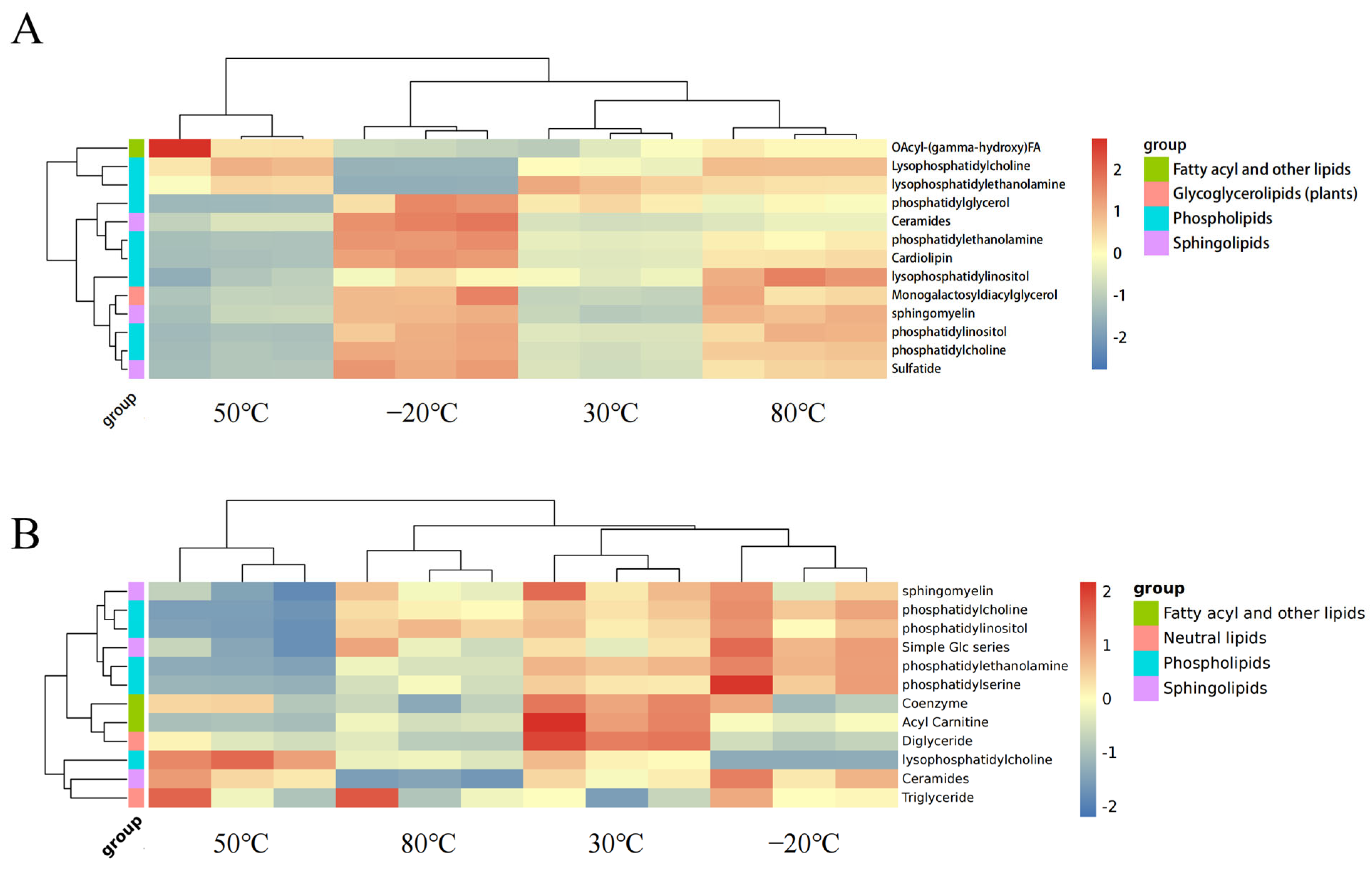
3.1. Lipid Composition of DLL
3.1.1. Untargeted Lipidomics Analysis
3.1.2. Free Fatty Acid (FFA) Analysis
3.2. In Vitro Anti-Inflammatory Activity of DLL
3.2.1. Effect of DLL on Cell Viability
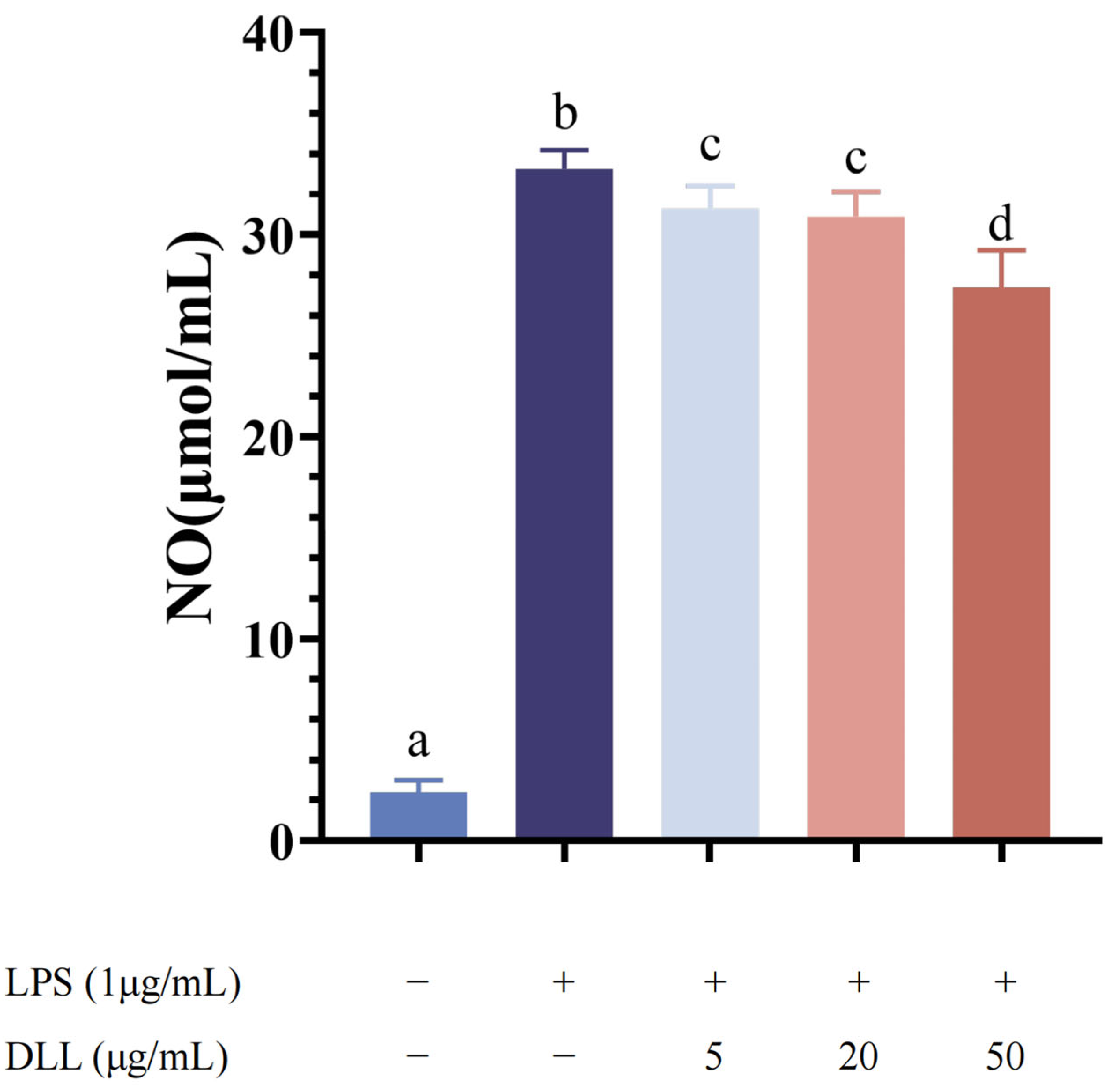
3.2.2. DLL Inhibited the Production of NO in LPS-Activated RAW264.7 Macrophages
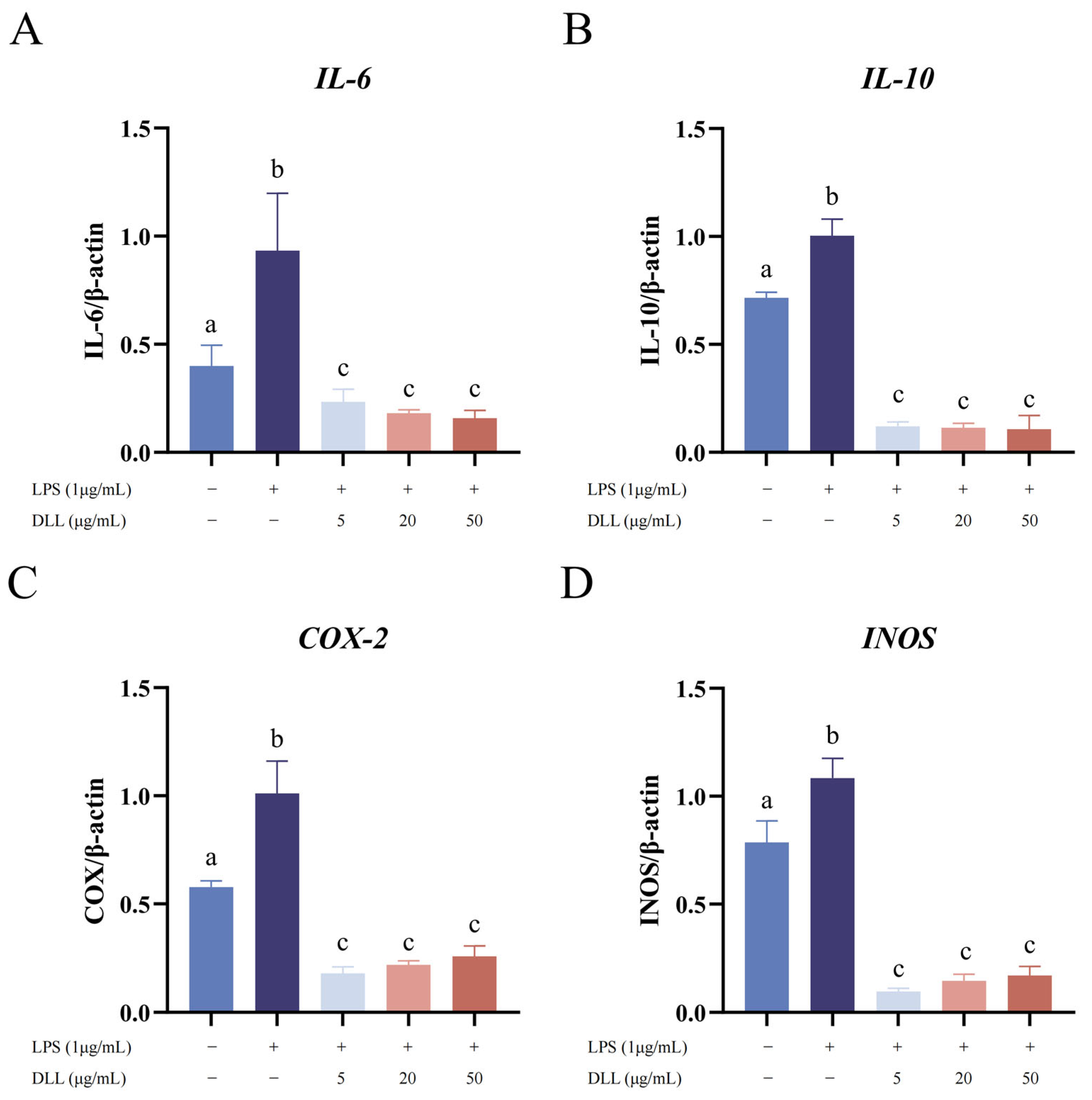
3.2.3. DLLs Inhibited LPS-Induced IL-6, IL-10, COX-2, and iNOS mRNA Expression in RAW264.7 Macrophages
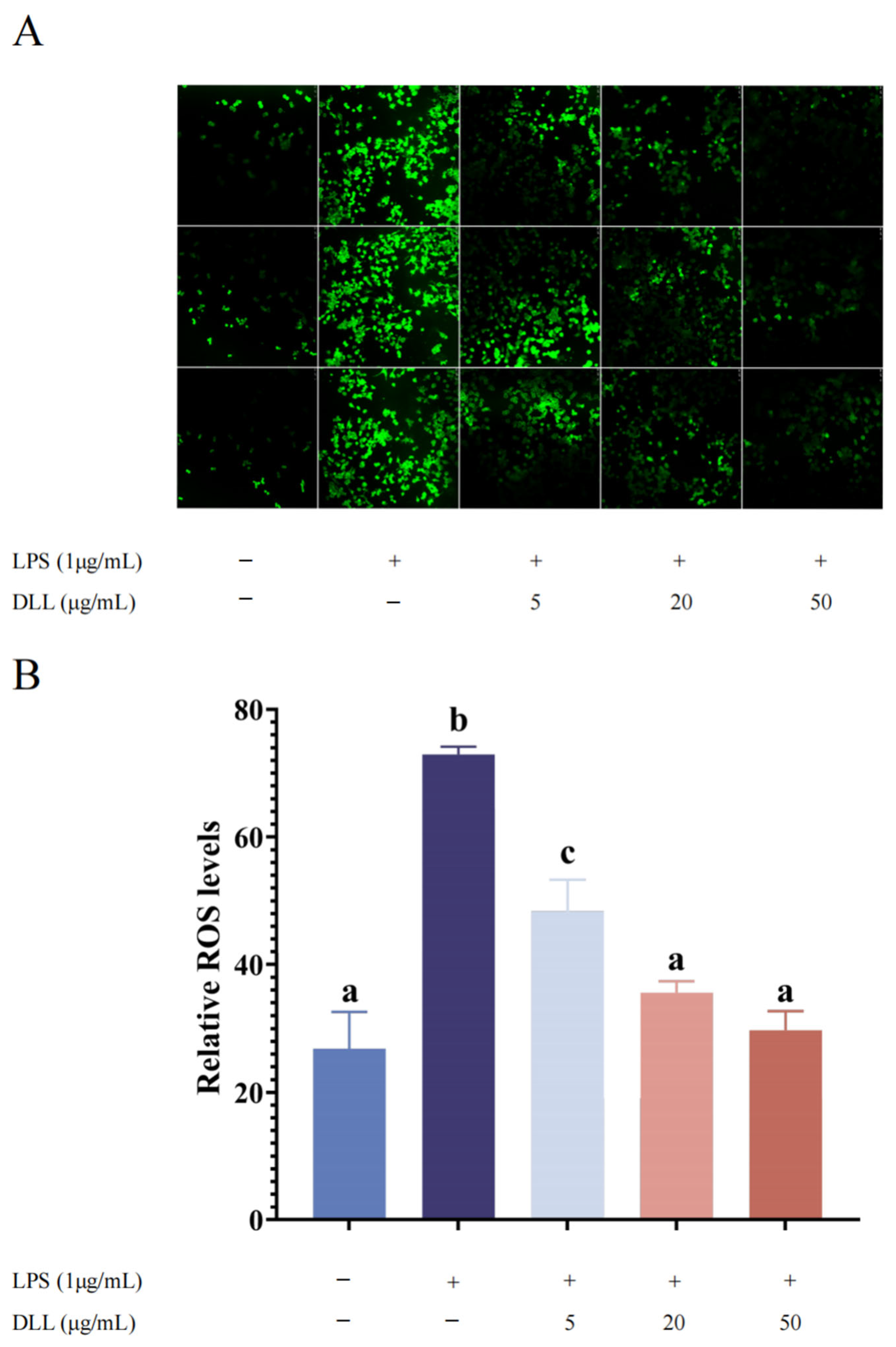
3.2.4. DLLs Inhibited LPS-Induced ROS Level in RAW264.7 Macrophages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, P.J. Clinical nutrition: 7. Functional foods—More than just nutrition. CMAJ 2002, 166, 1555–1563. [Google Scholar]
- Muhammad Sajid, A.; Waseem, K.; Rabia Shabir, A.; Muhammad Kamran, K.; Muhammad Haseeb, A.; Saira, S.; Safura, K.; Haroon, M.; Umair, S.; Muhammad, Z. Functional Foods and Human Health: An Overview. In Functional Foods; IntechOpen: London, UK, 2021; Chapter 1. [Google Scholar] [CrossRef]
- Asma, S.T.; Bobiş, O.; Bonta, V.; Acaroz, U.; Shah, S.R.A.; Istanbullugil, F.R.; Arslan-Acaroz, D. General Nutritional Profile of Bee Products and Their Potential Antiviral Properties against Mammalian Viruses. Nutrients 2022, 14, 3579. [Google Scholar] [CrossRef]
- Jiang, Q.; Charoensiddhi, S.; Xue, X.; Sun, B.; Liu, Y.; El-Seedi, H.R.; Wang, K. A review on the gastrointestinal protective effects of tropical fruit polyphenols. Crit. Rev. Food Sci. Nutr. 2023, 63, 7197–7223. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Mazur, A.; Maier, J. Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Haydak, M.H. The Food of the Drone Larvae. Ann. Entomol. Soc. Am. 1957, 50, 73–75. [Google Scholar] [CrossRef]
- Woyke, J. Drone larvae from fertilized eggs of the honeybee. J. Apic. Res. 1963, 2, 19–24. [Google Scholar] [CrossRef]
- Ghosh, S.; Herren, P.; Meyer-Rochow, V.B.; Jung, C. Nutritional Composition of Honey Bee Drones of Two Subspecies Relative to Their Pupal Developmental Stages. Insects 2021, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Hoover, S.E.; Higo, H.A.; Winston, M.L. Worker honey bee ovary development: Seasonal variation and the influence of larval and adult nutrition. J. Comp. Physiol. B 2006, 176, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tozetto, S.d.O.; Engels, W. Classification of substages in preimaginal development of honey bee drones (Hymenoptera: Apidae). Entomol. Gen. 2013, 34, 287–293. [Google Scholar] [CrossRef]
- Czerkasowa, A.; Prochoda, I. A New Addition of Drone Larvae–An Alternative to Royal Jelly; NfaU: Kharkiv, Ukraine, 2006; pp. 65–69. [Google Scholar]
- Silici, S. Chemical content and bioactive properties of drone larvae (Apilarnil). Mellifera 2019, 19, 14–22. [Google Scholar]
- Ahmad, S.C.; Graça, M. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef]
- Pisani, D.F.; Ailhaud, G. Involvement of polyunsaturated fatty acids in the control of energy storage and expenditure. Lipids Health 2019, 26, 37. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Ghasemi Fard, S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Mitchell, T.W. Advances in mass spectrometry for lipidomics. Annu. Rev. Anal. Chem. 2010, 3, 433–465. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal. Chem. 2014, 61, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C.; Han, X. Selection of internal standards for accurate quantification of complex lipid species in biological extracts by electrospray ionization mass spectrometry—What, how and why? Mass Spectrom. Rev. 2017, 36, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, W.; Zhang, X.; Chen, X. Neocryptotanshinone inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppression of NF-κB and iNOS signaling pathways. Acta Pharm. Sin. B 2015, 5, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, M.; Zhou, L.; Zhang, M.; Liu, J.; Marchioni, E. Identification and differentiation of wide edible mushrooms based on lipidomics profiling combined with principal component analysis. J. Agric. Food Chem. 2021, 69, 9991–10001. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Salimon, J.; Omar, T.A.; Salih, N. Comparison of two derivatization methods for the analysis of fatty acids and trans fatty acids in bakery products using gas chromatography. Sci. World J. 2014, 2014, 906407. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Jiao, Y.; Liu, X. Assessment of potential false positives via orbitrap-based untargeted lipidomics from rat tissues. Talanta 2018, 178, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.; Scherer, M.; Schmitz, G.; Liebisch, G. A rapid GC–MS method for quantification of positional and geometric isomers of fatty acid methyl esters. J. Chromatogr. B 2012, 897, 98–104. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhang, C.; Hu, F. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus × canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Koşum, N.; Yücel, B.; Kandemir, Ç.; Taşkın, T.; Duru, M.E.; Küçükaydın, S.; Margaoan, R.; Cornea-Cipcigan, M. Chemical Composition and Androgenic Effect of Bee Drone Larvae (Apilarnil) for Goat Male Kids. Chem. Biodivers. 2022, 19, e202200548. [Google Scholar] [CrossRef]
- Bărnuţiu, L.-I.; Mărghitaş, L.A.; Dezmirean, D.; Bobiş, O.; Mihai, C.; Pavel, C. Physicochemical composition of Apilarnil (bee drone larvae). Lucr. Ştiinţifice Ser. Zooteh. 2013, 59, 199–202. [Google Scholar]
- Seki, H.; Tani, Y.; Arita, M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 2009, 89, 126–130. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-inflammatory Effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- Whitehouse, M.; Macrides, T.; Kalafatis, N.; Betts, W.; Haynes, D.; Broadbent, J. Anti-inflammatory activity of a lipid fraction (Lyprinol) from the NZ green-lipped mussel. Inflammopharmacology 1997, 5, 237–246. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2011, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Iwata, M.; Inoue, T.; Asai, Y.; Hori, K.; Fujiwara, M.; Matsuo, S.; Tsuchida, W.; Suzuki, S. The protective role of localized nitric oxide production during inflammation may be mediated by the heme oxygenase-1/carbon monoxide pathway. Biochem. Biophys. Rep. 2020, 23, 100790. [Google Scholar] [CrossRef]
- Jia, J.; Sun, Y.; Hu, Z.; Li, Y.; Ruan, X. Propofol inhibits the release of interleukin-6, 8 and tumor necrosis factor-α correlating with high-mobility group box 1 expression in lipopolysaccharides-stimulated RAW 264.7 cells. BMC Anesthesiol. 2017, 17, 148. [Google Scholar] [CrossRef]
- Ernst, O.; Glucksam-Galnoy, Y.; Bhatta, B.; Athamna, M.; Ben-Dror, I.; Glick, Y.; Gerber, D.; Zor, T. Exclusive Temporal Stimulation of IL-10 Expression in LPS-Stimulated Mouse Macrophages by cAMP Inducers and Type I Interferons. Front. Immunol. 2019, 10, 1788. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Arai, T.; Endo, N.; Yamashita, K.; Fukuda, K.; Sasada, M.; Uchiyama, T. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life Sci. 2006, 78, 926–933. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Treede, I.; Braun, A.; Jeliaskova, P.; Giese, T.; Füllekrug, J.; Griffiths, G.; Stremmel, W.; Ehehalt, R. TNF-α-induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidylcholine in intestinal epithelial cells. BMC Gastroenterol. 2009, 9, 53. [Google Scholar] [CrossRef]
- Ehehalt, R.; Braun, A.; Karner, M.; Füllekrug, J.; Stremmel, W. Phosphatidylcholine as a constituent in the colonic mucosal barrier—Physiological and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 983–993. [Google Scholar] [CrossRef]
- Eros, G.; Varga, G.; Varadi, R.; Czobel, M.; Kaszaki, J.; Ghyczy, M.; Boros, M. Anti-inflammatory action of a phosphatidylcholine, phosphatidylethanolamine and N-acylphosphatidylethanolamine-enriched diet in carrageenan-induced pleurisy. Eur. Surg. Res. 2009, 42, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sampalis, F.; Bunea, R.; Pelland, M.F.; Kowalski, O.; Duguet, N.; Dupuis, S. Evaluation of the effects of Neptune Krill Oil™ on the management of premenstrual syndrome and dysmenorrhea. Altern. Med. Rev. 2003, 8, 171–179. [Google Scholar] [PubMed]
- Almeida, J.L.; Dakic, A.; Kindig, K.; Kone, M.; Letham, D.L.; Langdon, S.; Peat, R.; Holding-Pillai, J.; Hall, E.M.; Ladd, M.; et al. Interlaboratory study to validate a STR profiling method for intraspecies identification of mouse cell lines. PLoS ONE 2019, 14, e0218412. [Google Scholar] [CrossRef]
| Fatty Acid | RT (min) | Content (μg/g) | |||
|---|---|---|---|---|---|
| −20 °C | 30 °C | 50 °C | 80 °C | ||
| Caprylic | 5.153 | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.14 ± 0.02 | 0.16 ± 0.01 |
| Capric | 6.111 | 1.52 ± 0.12 a,b | 1.40 ± 0.15 a,b | 1.30 ± 0.13 a | 1.67 ± 0.03 b |
| Lauric | 6.832 | 2.67 ± 1.89 a,c | 2.56 ± 1.17 a,b,c | 2.27 ± 1.96 b,c | 2.85 ± 0.29 a,c |
| Tridecanoic | 7.179 | \ | 0.07 ± 0.1 | \ | \ |
| Myristic | 7.552 | 246.99 ± 13.19 a | 250.21 ± 5.51 a | 216.32 ± 14.37 b | 249.66 ± 4.58 a |
| Myristoleic | 7.722 | 2.10 ± 0.04 a,b | 2.25 ± 0.29 a,b | 1.76 ± 0.19 a | 2.33 ± 0.25 b |
| Pentadecanoic | 7.98 | 0.76 ± 0.04 | 0.69 ± 0.04 | 0.68 ± 0.10 | 0.74 ± 0.02 |
| Palmitic | 8.494 | 1707.87 ± 60.53 a,b | 1721.35 ± 12.41 a,b | 1553.33 ± 69.13 c | 1668.02 ± 25.92 a,b,c |
| Palmitoleic | 8.684 | 52.51 ± 4.56 a | 53.52 ± 3.43 a | 42.51 ± 2.07 b | 51.78 ± 2.07 a |
| Margaric | 9.133 | 2.89 ± 0.14 a,b | 2.83 ± 0.10 a,b | 2.59 ± 0.19 a | 3.17 ± 0.10 b |
| Stearic | 9.946 | 852.32 ± 24.17 | 844.87 ± 44.72 | 772.05 ± 56.99 | 839.63 ± 46.73 |
| Oleic | 10.192 | 2463.03 ± 149.61 | 2363.70 ± 198.71 | 2189.96 ± 134.92 | 2437.91 ± 83.85 |
| Linoleic | 10.71 | 41.84 ± 0.39 | 41.88 ± 5.68 | 36.03 ± 3.22 | 43.43 ± 3.07 |
| Linolenic | 11.452 | 44.35 ± 1.73 | 47.24 ± 2.37 | 34.66 ± 1.79 | 45.08 ± 3.87 |
| Arachidic | 12.39 | 28.76 ± 0.72 | 28.56 ± 1.49 | 29.32 ± 2.87 | 32.42 ± 1.28 |
| Eicosenic cis 5 | 12.806 | 7.00 ± 0.56 | 6.41 ± 0.34 | 6.20 ± 0.54 | 5.81 ± 0.36 |
| Behenic | 16.631 | 3.89 ± 0.10 | 3.89 ± 0.32 | 7.72 ± 0.85 | 5.07 ± 0.81 |
| Lignoceric | 19.989 | 3.19 ± 0.25 | 2.95 ± 0.61 | 37.21 ± 16.11 | 5.71 ± 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Guo, Y.; Zhao, W.; Khalifa, S.A.M.; El-Seedi, H.R.; Su, X.; Wu, L. Total Lipid Extracts of Honeybee Drone Larvae Are Modulated by Extraction Temperature and Display Consistent Anti-Inflammatory Potential. Foods 2023, 12, 4058. https://doi.org/10.3390/foods12224058
Luo Y, Guo Y, Zhao W, Khalifa SAM, El-Seedi HR, Su X, Wu L. Total Lipid Extracts of Honeybee Drone Larvae Are Modulated by Extraction Temperature and Display Consistent Anti-Inflammatory Potential. Foods. 2023; 12(22):4058. https://doi.org/10.3390/foods12224058
Chicago/Turabian StyleLuo, Yiming, Yuyang Guo, Wen Zhao, Shaden A. M. Khalifa, Hesham R. El-Seedi, Xiaoling Su, and Liming Wu. 2023. "Total Lipid Extracts of Honeybee Drone Larvae Are Modulated by Extraction Temperature and Display Consistent Anti-Inflammatory Potential" Foods 12, no. 22: 4058. https://doi.org/10.3390/foods12224058
APA StyleLuo, Y., Guo, Y., Zhao, W., Khalifa, S. A. M., El-Seedi, H. R., Su, X., & Wu, L. (2023). Total Lipid Extracts of Honeybee Drone Larvae Are Modulated by Extraction Temperature and Display Consistent Anti-Inflammatory Potential. Foods, 12(22), 4058. https://doi.org/10.3390/foods12224058







