Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry
Abstract
1. Introduction
2. The Carotenoid Chromophore
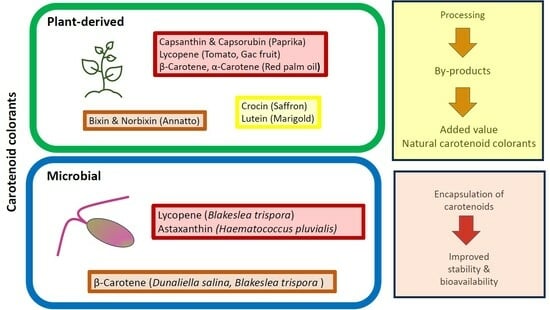
3. Carotenoid Colorants Derived from Higher Plants
3.1. Annatto Bixin and Norbixin
3.2. Paprika Capsanthin and Capsorubin
3.3. Saffron (Crocin)
3.4. Tomato and Gac Fruit Lycopene
3.5. Marigold Lutein
3.6. Red Palm Oil α- and β-Carotene
4. Encapsulation of Carotenoids
- Protection of carotenoids from undesirable environmental conditions and matrix interactions, thus avoiding degradation;
- Ease and flexibility of handling;
- Better solubility, facilitating incorporation in food products;
- Controlled release and improved bioavailability;
- Suppressing undesired aroma/flavor.
4.1. Microencapsulation
4.1.1. Recent Studies on the Microencapsulation of Carotenoids
4.1.2. Effects of Microencapsulation on Carotenoid Absorption
4.2. Nanoencapsulation
4.2.1. Recent Studies on the Nanoencapsulation of Carotenoids
4.2.2. Effects of Nanoencapsulation on Carotenoid Absorption
5. Utilization of Food Processing By-Products
5.1. Green Extraction
5.2. Biorefinery and Circular Economy Concepts
6. Microalgal Carotenoid Colorant
- Selection of species with appropriate production time and yield of biomass and pigment;
- Efficient culture system design and medium optimization (including the control of operating conditions like temperature, lighting, pH, aeration, agitation, and media components) to maximize biomass and pigment production at low cost.
- Efficient and affordable downstream processes (biomass harvesting, cell wall disruption, pigment extraction, purification, and storage).
6.1. β-Carotene
6.2. Astaxanthin
6.3. Lutein
7. Regulation and Safety Concerns
7.1. Regulation in Different Countries
- Color additives are exempt from batch certification, which includes those derived from fruits, vegetables, plants, or mineral sources. The following natural carotenoids and carotenoid-rich products belong to this category: annatto extract, carrot oil, paprika and paprika oleoresin, saffron, tomato lycopene extract, and tomato lycopene concentrate.
- Color additives are subject to batch certification, which applies to synthetic dyes, lakes, or pigments. There are no carotenoids or carotenoid-rich products in this category.
- To evaluate the safety of new food additives or propose new uses of existing food additives prior to their eventual authorization.
- To re-evaluate all food additives authorized to be used before 20 January 2009.
- To respond to ad-hoc requests from the European Commission to review food additives when relevant new scientific information is available and/or to evaluate the change in conditions of use.
7.2. Safety of Carotenoids
- The applied doses of β-carotene in the intervention studies were much higher than physiological doses: 20 mg per day in the ATBC study and 30 mg per day plus 25,000 IU of vitamin A in the CARET study. In the epidemiological studies in which β-carotene intakes were inversely associated with cancer risk, the daily intake of β-carotene was only about 4 mg [322].
- The intervention subjects in the ATBC and CARET studies were mostly smokers and workers exposed to asbestos, thus representing high risk populations. In another large trial, the Physicians’ Health Study, long-term supplementation with β-carotene (50 mg on alternate days for 12 years) produced neither benefit nor harm in terms of lung cancer incidence or overall mortality [323]. Only 11% of the participants were current smokers, thus the study population was at substantially lower risk for lung cancer.
- It was hypothesized that reactive oxygen species of cigarette smoke (or produced as a consequence of asbestosis) in the presence of the relatively high oxygen tension in the lung, induced oxidation of β-carotene, resulting in a prooxidant effect [324].
7.3. Concern about Contaminants and Safety of Nanomaterials
8. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar]
- Jurić, S.; Jurić, M.; Król-Kilińska, Z.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar]
- de Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The colors of health: Chemistry, bioactivity, and market demand for colorful foods and natural food sources of colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [PubMed]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Current knowledge on the health benefits of carotenoids: Focus on the scientific evidence. In Global Perspectives on Astaxanthin. From Industrial Production to Food, Health, and Pharmaceutical Applications; Ravishankar, G.A., Ambati, R.R., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 693–717. [Google Scholar]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and cognitive outcomes: A meta-analysis of randomized intervention trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Abdel-Aal, E.-S.-M. Dietary lutein and cognitive function in adults: A meta-analysis of randomized controlled trials. Molecules 2021, 26, 5794. [Google Scholar] [CrossRef]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and zeaxanthin influence brain function in older adults: A randomized controlled trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90. [Google Scholar] [CrossRef]
- Lin, S.; Shen, Y. Dietary carotenoids intake and depressive symptoms in US adults, NHANES 2015–2016. J. Affec. Disord. 2021, 282, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Xue, F.; Li, Z.; Li, X.; Ai, L.; Jin, M.; Xie, M.; Yu, Y. Dietary intake of carotenoids and risk of depressive symptoms: A systematic review and meta-analysis. Antioxidants 2022, 11, 2205. [Google Scholar] [CrossRef]
- Kim, S.J.; Anh, N.H.; Diem, N.C.; Park, S.; Cho, Y.H.; Long, N.P.; Hwang, I.G.; Lim, J.; Kwon, S.W. Effects of β-cryptoxanthin on improvement in osteoporosis risk: A systematic review and meta-analysis of observational studies. Foods 2021, 10, 296. [Google Scholar] [CrossRef]
- Regu, G.M.; Kim, H.; Kim, Y.J.; Paek, J.E.; Lee, G.; Chang, N.; Kwon, O. Association between dietary carotenoid intake and bone mineral density in Korean adults aged 30–75 years using data from the Fourth and Fifth Korean National Health and Nutrition Examination Surveys (2008–2011). Nutrients 2017, 9, 1025. [Google Scholar] [CrossRef]
- Xu, J.; Song, C.; Song, X.; Zhang, X.; Li, X. Carotenoids and risk of fracture: A meta-analysis of observational studies. Oncotarget 2017, 8, 2391–2399. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology, and Technology; IFT Press-Wiley: Oxford, UK, 2016. [Google Scholar]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigment. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Esquivel, P.; Rodriguez-Amaya, D.B. Comprehensive review on carotenoid composition: Transformations during processing and storage of foods. Food Res. Int. 2023, 169, 112773. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Carle, R. Alterations of natural pigments. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 265–327. [Google Scholar]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene-properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Raddatz-Mota, D.; Pérez-Flores, L.J.; Carrari, F.; Mendoza-Espinoza, J.A.; de León-Sánchez, F.D.; Pinzón-López, L.L.; Godóy-Hernández, G.; Rivera-Cabrera, F. Achiote (Bixa orellana L.): A natural source of pigment and vitamin E. J. Food Sci. Technol. 2017, 54, 1729–1741. [Google Scholar] [CrossRef]
- Mercer, D.G.; Rodriguez-Amaya, D.B. Reactions and interactions of some food additives. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 579–635. [Google Scholar]
- Møller, A.H.; Jahangiri, A.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Dalsgaard, T.K. Effect of light, pH, metal ions and antioxidants on the colour stability of norbixin in aqueous solution. Int. J. Food Sci. Technol. 2018, 54, 1625–1632. [Google Scholar] [CrossRef]
- Møller, A.H.; Jahangiri, A.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Dalsgaard, T.K. Mechanism behind the degradation of aqueous norbixin upon storage in light and dark environment. Food Chem. 2020, 310, 125967. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Eun, J.B. Effects of different extraction methods on the extraction yield, degradation of bixin and formation of harmful volatile compounds in the extracts from annatto seeds. Food Res. 2021, 5, 42–48. [Google Scholar] [CrossRef]
- Jayakumar, J.; Sudha, P.; Rajkumar, P.; Pandiselvam, R.; Gurusamy, K.; Kumaran, K.; Subramanian, P. Comparative study on the effect of solvents on extraction of bixin from annatto seed (Bixa orellana L.) and optimization of process parameters using Box–Behnken design. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Bachtler, S.; Bart, H.-J. Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave assisted extraction technologies. Food Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Yolmeh, M.; Habibi Najafi, M.B.; Farhoosh, R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM). Food Chem. 2014, 155, 319–324. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Osorio-Tobón, J.F.; Forster-Carneiro, T.; Meireles, M.A.A. Obtaining bixin from semi-defatted annatto seeds by a mechanical method and solvent extraction: Process integration and economic evaluation. Food Res. Int. 2017, 99 Pt 1, 393–402. [Google Scholar] [CrossRef]
- Møller, A.H.; Wijaya, W.; Jahangiri, A.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Van der Meeren, P.; Dalsgaard, T.K. Norbixin binding to whey protein isolate - alginate electrostatic complexes increases its solubility and stability. Food Hydrocoll. 2020, 101, 105559. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Xiong, Y.; Peng, S.; McClements, D.J.; Zou, L.; Liang, R.; Liu, W. Improving norbixin dispersibility and stability by liposomal encapsulation using the pH-driven method. J. Sci. Food Agric. 2022, 102, 2070–2079. [Google Scholar] [CrossRef]
- Yolmeh, M.; Najafi, M.B.H.; Farhoosh, R.; Salehi, F. Modeling of antibacterial activity of annatto dye on Escherichia coli in mayonnaise. Food Biosci. 2014, 8, 8–13. [Google Scholar] [CrossRef]
- Handayani, I.; Haryanti, P.; Sulistyo, S.B. Color and antibacterial activity of annatto extracts at various pH of distilled water solvent and extraction temperature. Food Res. 2021, 5, 247–253. [Google Scholar] [CrossRef]
- Shakeri, A.; Soheili, V.; Karimi, M.; Hosseininia, S.A.; Bazzaz, B.S.F. Biological activities of three natural plant pigments and their health benefits. J Food Meas Charact. 2018, 12, 356–361. [Google Scholar] [CrossRef]
- Habibi Najafi, M.B.; Fatemizadeh, S.S.; Boroojerdi, S.R.; Hosseini, F.; Karazhyan, R. In vitro evaluation of antimold activity of annatto natural dye and its effects on microbial, physicochemical, and sensory properties of bread. J. Food Prot. 2018, 81, 1598–1604. [Google Scholar] [CrossRef]
- Beni, A.A.; Rodrigues, R.F.; Conte, L.; Costa, I.F.; Delalibera, E.A.; Roehrs, M.; Rampelotto, C.; Emanuelli, T.; Somacal, S. Dietary supplementation with annatto food-coloring extracts increases the resistance of human erythrocytes to hemolysis. Nutr. Res. 2020, 76, 71–81. [Google Scholar] [CrossRef]
- Kusmita, L.; Franyoto, Y.D.; Mutmainah, M.; Puspitaningrum, I.; Nurcahyanti, A.D.R. Bixa orellana L. carotenoids: Antiproliferative activity on human lung cancer, breast cancer, and cervical cancer cells in vitro. Nat. Prod. Res. 2022, 36, 6421–6427. [Google Scholar] [CrossRef]
- Roehrs, M.; Conte, L.; da Silva, D.T.; Duarte, T.; Maurer, L.H.; de Carvalho, J.A.M.; Moresco, R.N.; Somacal, S.; Emanuelli, T. Annatto carotenoids attenuate oxidative stress and inflammatory response after high-calorie meal in healthy subjects. Food Res. Int. 2017, 100 Pt 1, 771–779. [Google Scholar] [CrossRef]
- Kläui, H.; Bauernfeind, J.C. Carotenoids as food colors. In Carotenoids as Colorants and Vitamin A Precursors; Bauernfeind, J.C., Ed.; Academic Press: New York, NY, USA, 1981; pp. 47–317. [Google Scholar]
- Molnár, H.; Kónya, E.; Zalán, Z.; Bata-Vidács, I.; Tömösközi-Farkas, R.; Székács, A.; Adányi, N. Chemical characteristics of spice paprika of different origins. Food Control 2018, 83, 54–60. [Google Scholar] [CrossRef]
- Pang, M.; Lium, Q.; Yu, Y.L.; Cai, S.L. Ultrasonic-microwave synergistic extraction of paprika pigment. E3S Web Conf. 2019, 78, 02009. [Google Scholar] [CrossRef]
- Huang, P.; Yu, Q.; Feng, X.; Ma, C.; Kan, J. Optimization of accelerated solvent extraction of paprika oleoresin and its effect on capsaicinoid and carotenoid composition. J. Food Compost. Anal. 2022, 110, 104589. [Google Scholar] [CrossRef]
- Procopio, F.R.; Ferraz, M.C.; do Prado-Silva, L.; Paulino, B.N.; Sant’Ana, A.S.; Pastore, G.M.; Sobral, P.J.A.; Hubinger, M.D. Antifungal synergistic effect of paprika and cinnamon oleoresins and their coencapsulation by spray chilling technique to produce a carotenoid-cinnamaldehyde-rich food powder. Food Bioprocess Technol. 2022, 15, 2826–2838. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Vigano, J.; Anthero, A.G.S.; Dias, A.L.B.; Hubinger, M.D.; Martínez, J. Supercritical fluids and fluid mixtures to obtain high-value compounds from Capsicum peppers. Food Chem. X 2022, 13, 100228. [Google Scholar] [CrossRef]
- Kim, G.H.; Chin, K.B. Characteristics of low-nitrite pork emulsified-sausages with paprika oleoresin solution during refrigerated storage. J. Anim. Sci. Technol. 2021, 63, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Thakur, R.; Kumar, R. Safron (Crocus sativus L.): Gold of the spices—A comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; safron. Trends Food Sci. Technol. 2016, 58, 69–78. [Google Scholar] [CrossRef]
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. Int. J. Biol. Macromol. 2017, 99, 401–408. [Google Scholar] [CrossRef]
- Chranioti, C.; Nikoloudaki, A.; Tzia, C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: Incorporation in a chewing gum system. Carbohydr. Polym. 2015, 127, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Esfanjani, A.F.; Jafari, S.M.; Assadpour, E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem. 2017, 221, 1962–1969. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M.; et al. Nutritional and health beneficial properties of saffron (Crocus sativus L): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683–2706. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef]
- Ali, A.; Yu, L.; Kousar, S.; Khalid, W.; Maqbool, Z.; Aziz, A.; Arshad, M.S.; Aadil, R.M.; Trif, M.; Riaz, S.; et al. Crocin: Functional characteristics, extraction, food applications and efficacy against brain related disorders. Front Nutr. 2022, 9, 1009807. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Manzoor, M.; Dhar, M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother. 2019, 109, 21–27. [Google Scholar] [CrossRef]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules 2020, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Rahiman, N.; Akaberi, M.; Sahebkar, A.; Emami, S.A.; Tayarani-Najaran, Z. Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc. Res. 2018, 18, 82–89. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases (Review). Exp. Ther. Med. 2023, 25, 235. [Google Scholar] [CrossRef]
- Zhou, W.E.; Zhang, Y.; Li, Y.; Ling, Y.; Li, H.N.; Li, S.H.; Jiang, S.J.; Ren, Z.Q.; Huang, Z.Q.; Zhang, F. Determination of gardenia yellow colorants in soft drink, pastry, instant noodles with ultrasound-assisted extraction by high performance liquid chromatography-electrospray ionization tandem mass spectrum. J Chromatogr. A 2016, 1446, 59–69. [Google Scholar] [CrossRef]
- Giovannucci, E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp. Biol. Med. 2002, 227, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Lycopene and prostate cancer risk. Methodological considerations in the epidemiologic literature. Pure Appl. Chem. 2002, 74, 1427–1434. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and risk of prostate cancer: A systematic review and meta-analysis. Medicine 2015, 94, e1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of carotene and lycopene on the risk of prostate cancer: A systematic review and dose-response meta-analysis of observational studies. PLoS ONE 2015, 10, e0137427. [Google Scholar]
- Rowles, J.L., 3rd; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W., Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Liao, X.; Rosner, B.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am. J. Clin. Nutr. 2015, 101, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-X.; Xing, M.-Y.; Yu, L.-F.; Shen, P. Carotenoid intake and esophageal cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 1911–1918. [Google Scholar] [CrossRef]
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid intake from natural sources and head and neck cancer: A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 1003–1011. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Li, X.; Xu, J. Dietary and circulating lycopene and stroke risk: A meta-analysis of prospective studies. Sci. Rep. 2014, 4, 5031. [Google Scholar] [CrossRef]
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61, 9. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.T.; Franke, A.A.; Custer, L.J.; Murphy, S.P. Momordica cochinchinensis Spreng. (gac) fruit carotenoids reevaluated. J. Food Compos. Anal. 2006, 19, 664–668. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensis Spreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2014, 50, 567–577. [Google Scholar] [CrossRef]
- Khalil, M.; Raila, J.; Ali, M.; Islan, R.N.S.; Schenk, R. Stability and bioavailability of lutein ester supplements from Tagetes flower prepared under processing conditions. J. Funct. Foods 2012, 4, 602–610. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dou, H.L.; Wu, Y.Q.; Huang, Y.M.; Huang, Y.B.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef]
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 56, 252–258. [Google Scholar] [CrossRef]
- Cui, Y.H.; Jing, C.X.; Pan, H.W. Association of blood antioxidants and vitamins with risk of age-related cataract: A meta-analysis of observational studies. Am. J. Clin. Nutr. 2013, 98, 778–786. [Google Scholar] [CrossRef]
- Liu, X.H.; Yu, R.B.; Liu, R.; Hao, Z.X.; Han, C.C.; Zhu, Z.H.; Ma, L. Association between lutein and zeaxanthin status and the risk of cataract: A meta-analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef]
- Ma, L.; Hao, Z.X.; Liu, R.R.; Yu, R.B.; Shi, Q.; Pan, J.P. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 63–70. [Google Scholar] [CrossRef]
- Chen, F.; Hu, J.; Liu, P.; Li, J.; Wei, Z.; Liu, P. Carotenoid intake and risk of non-Hodgkin lymphoma: A systematic review and dose-response meta-analysis of observational studies. Ann. Hematol. 2017, 96, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.M.; Darweesh, S.K.L.; Baena, C.P.; Moreira, E.M.; van Lent, D.M.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 481–494. [Google Scholar] [CrossRef]
- Feeney, J.; O’Leary, N.; Moran, R.; O’Halloran, A.M.; Nolan, J.M.; Beatty, S.; Young, I.S.; Kenny, R.A. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: Findings from the Irish Longitudinal Study on Aging. J. Gerontol. 2017, 72, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xia, H.; Wang, F.; Sun, G. The effect of red palm oil on vitamin A deficiency: A meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red palm oil: A review on processing, health benefits and its application in food. J. Oleo Sci. 2021, 70, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, R.; Subramaniam, K.M.; Radhakrishnan, A.K.; Choo, Y.-M.; Teng, K.-T. Health-promoting effects of red palm oil: Evidence from animal and human studies. Nutr. Rev. 2017, 75, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L. Red palm oil. Inform 2017, 28, 6–10. [Google Scholar] [CrossRef]
- Purnama, K.O.; Setyaningsih, D.; Hambali, E.; Taniwiryono, D. Processing, characteristics, and potential application of red palm oil—A review. Int. J. Oil Palm 2020, 3, 40–55. [Google Scholar] [CrossRef]
- Hoe, B.C.; Chan, E.S.; Nagasundara Ramanan, R.; Ooi, C.W. Direct recovery of palm carotene by liquid-liquid extraction. J. Food Eng. 2022, 313, 110755. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- De Queiroz, J.L.C.; Medeiros, I.; Piuvezam, G.; de França Nunes, A.C.; Gomes, C.C.; Maciel, B.L.L.; de Araújo Morais, A.H.; Passos, T.S. Effect of carotenoid encapsulation on antioxidant activities: A protocol for systematic review. Medicine 2020, 99, e19772. [Google Scholar] [CrossRef]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A review of encapsulation of carotenoids using spray drying and freeze drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, J.L.C.; Medeiros, I.; Trajano, A.C.; Piuvezam, G.; Nunes, A.C.F.; Passos, T.S.; Morais, A.H.A. Encapsulation techniques perfect the antioxidant action of carotenoids: A systematic review of how this efffect is promoted. Food Chem. 2022, 385, 132593. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Bohn, T.A. Comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the food industry: A brief historical overview to recent developments. Food Sci. Nutr. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Vimala Bharathi, S.K.; Moses, J.A.; Anandharamakrishnan, C. Nano and microencapsulation using food grade polymers. In Polymers for Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 357–400. [Google Scholar]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits—A review of recent advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Dos Santos, P.P.; Andrade, L.A.; Flôres, S.H.; Rios, A.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef]
- Santos, P.D.F.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Ashraf, W.; Latif, A.; Lianfu, Z.; Jian, Z.; Chenqiang, W.; Rehman, A.; Hussain, A.; Siddiquy, M.; Karim, A. Technological advancement in the processing of lycopene: A review. Food Rev. Int. 2022, 38, 857–883. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids microencapsulation by spray drying method and supercritical micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Jafari, S.M.; Arpagaus, C.; Cerqueira, M.A.; Samborska, K. Nano spray drying of food ingredients; Materials, processing and applications. Trends Food Sci. Technol. 2021, 109, 632–646. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Molina, C.V.; Netto, F.M.; Pinho, S.C. Encapsulation of beta-carotene in lipid microparticles stabilized with hydrolyzed soy protein isolate: Production parameters, alpha-tocopherol coencapsulation and stability under stress conditions. J. Food Sci. 2017, 82, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-X.; Chen, Z.; Huang, Q.; Fu, X.; Tang, C.-H. Spray-drying microencapsulation of β-Carotene by soy protein isolate and/or OSA-modified starch. J. Appl. Polym. Sci. 2014, 131, 40399. [Google Scholar] [CrossRef]
- Deng, X.-X.; Zhang, N.; Tang, C.-H. Soy protein isolate as a nanocarrier for enhanced water dispersibility, stability and bioaccessibility of β-carotene. J. Sci. Food Agric. 2017, 97, 2230–2237. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharjee, P. Spray dried powder of lutein-rich supercritical carbon dioxide extract of gamma-irradiated marigold flowers: Process optimization, characterization and food application. Powder Technol. 2018, 327, 512–523. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, X.; Guo, M. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLoS ONE 2018, 13, e0192511. [Google Scholar] [CrossRef]
- Do Nascimento Filho, E.; Silva, N.N.B.; Converti, A.; Grosso, C.R.F.; Santos, A.M.P.; Ribeiro, D.S.; Maciel, M.I.S. Microencapsulation of acerola (Malpighia emarginata DC) and ciriguela (Spondias purpurea L.) mixed juice with different wall materials. Food Chem. Adv. 2022, 1, 100046. [Google Scholar] [CrossRef]
- Carmona, P.A.O.; Garcia, L.C.; Ribeiro, J.A.A.; Valadares, L.F.; Marçal, A.F.; França, L.F.; Mendonça, S. Effect of solids content and spray-drying operating conditions on the carotenoids microencapsulation from pressed palm fiber oil extracted with supercritical CO2. Food Bioproc. Technol. 2018, 11, 1703–1718. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Perez-Masiá, R.; González-Barrio, R.; Periago, M.J.; López-Rubio, A. Potential of microencapsulation through emulsion-electrospraying to improve the bioaccesibility of β-Carotene. Food Hydrocoll. 2017, 73, 1–12. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Encapsulation of carotenoid-rich oil from gac peel: Optimisation of the encapsulating process using a spray drier and the storage stability of encapsulated powder. Powder Technol. 2019, 344, 373–379. [Google Scholar] [CrossRef]
- García, J.M.; Giuffrida, D.; Dugo, P.; Mondello, L.; Osorio, C. Development and characterisation of carotenoid-rich microencapsulates from tropical fruit by-products and yellow tamarillo (Solanum betaceum Cav.). Powder Technol. 2018, 339, 702–709. [Google Scholar] [CrossRef]
- Klettenhammer, S.; Ferrentino, G.; Zendehbad, H.S.; Morozova, K.; Scampicchio, M. Microencapsulation of linseed oil enriched with carrot pomace extracts using particles from gas saturated solutions (PGSS) process. J. Food Eng. 2022, 312, 110746. [Google Scholar] [CrossRef]
- Tupuna-Yerovi, D.S.; Paese, K.; Flôres, S.H.; Guterres, S.S.; Rios, A. Addition of norbixin microcapsules obtained by spray drying in an isotonic tangerine soft drink as a natural dye. J. Food Sci. Technol. 2020, 57, 1021–1031. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, O.; Luna-Guevara, J.J.; Ramírez-Rodrigues, M.M.; Luna-Vital, D.; Luna-Guevara, M.L. Microencapsulation of Renealmia alpinia (Rottb.) Maas pulp pigment and antioxidant compounds by spray-drying and its incorporation in yogurt. J. Food Sci. Technol. 2022, 59, 1162–1172. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of palm oil by complex coacervation for application in food systems. Food Chem. 2017, 220, 59–66. [Google Scholar] [CrossRef]
- Wen, C.; Xu, X.; Zhou, D.; Yu, Q.; Wang, T.; Zou, Y. The effects of canthaxanthin microencapsulation on yolk color and canthaxanthin deposition in cgg yolk of laying hens. Poult. Sci. 2022, 101, 101889. [Google Scholar] [CrossRef] [PubMed]
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crop. Prod. 2018, 111, 846–855. [Google Scholar] [CrossRef]
- Ursache, F.M.; Andronoiu, D.G.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleţ, M.; Dumitraşcu, L.; Botez, E.; Râpeanu, G.; Stănciuc, N. Valorizations of carotenoids from sea buckthorn extract by microencapsulation and formulation of value-added food products. J. Food Eng. 2018, 219, 16–24. [Google Scholar] [CrossRef]
- Curi-Borda, C.K.; Tannira, V.; Gentile, N.; Alvarado, J.A.; Bergenståhl, B. Model for measuring light stability of photolabile substances in powder beds using spray dried bixin microcapsules. Coll. Surf. A Physicochem. Eng. Asp. 2021, 627, 127131. [Google Scholar] [CrossRef]
- Quintero Quiroz, J.; Velazquez, V.; Corrales-Garcia, L.L.; Torres, J.D.; Delgado, E.; Ciro, G.; Rojas, J. Use of plant proteins as microencapsulating agents of bioactive compounds extracted from annatto seeds (Bixa orellana L.). Antioxidants 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Gayathiri, S.; Preetha, P.; Pandiselvam, R.; Jeevarathinam, G.; Delfiya, D.A.; Kothakota, A. Microencapsulation of bixin pigment by spray drying: Evaluation of characteristics. LWT-Food Sci. Technol. 2021, 145, 111343. [Google Scholar] [CrossRef]
- Neto, A.Á.M.; Tomazini, L.F.; Mizuta, A.G.; Corrêa, R.C.G.; Madrona, G.S.; de Moraes, F.F.; Peralta, R.M. Direct microencapsulation of an annatto extract by precipitation of psyllium husk mucilage polysaccharides. Food Hydrocoll. 2021, 112, 106333. [Google Scholar] [CrossRef]
- Lobato, K.B.S.; Paese, K.; Forgearini, J.C.; Guterres, S.S.; Jablonski, A.; Rios, A.O. Evaluation of stability of bixin in nanocapsules in model systems of photosensitization and heating. LWT-Food Sci. Technol. 2015, 60, 8–14. [Google Scholar] [CrossRef][Green Version]
- Bitencourt, A.P.; Duarte, J.L.; Oliveira, A.E.; Cruz, R.A.; Carvalho, J.C.; Gomes, A.T.; Ferreira, I.M.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.; Fernandes, C.P. Preparation of aqueous nanodispersions with annatto (Bixa orellana L.) extract using an organic solvent-free and low energy method. Food Chem. 2018, 257, 196–205. [Google Scholar] [CrossRef]
- Anthero, A.G.d.S.; Bezerra, E.O.; Comunian, T.A.; Procopio, F.R.; Hubinger, M.D. Effect of modified starches and gum arabic on the stability of carotenoids in paprika oleoresin microparticles. Dry. Technol. 2021, 39, 1927–1940. [Google Scholar] [CrossRef]
- No, J.; Shin, M. Preparation and characteristics of octenyl succinic anhydride-modified partial waxy rice starches and encapsulated paprika pigment powder. Food Chem. 2019, 295, 466–474. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpoor, E.; Mohammadi, A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng. 2015, 165, 149–155. [Google Scholar] [CrossRef]
- Rahaiee, S.; Shojaosadati, S.A.; Hashemi, M.; Moini, S.; Razavi, S.H. Improvement of crocin stability by biodegradeble nanoparticles of chitosan-alginate. Int. J.Biol. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Fu, G.; Feng, S.; He, X.; Cai, W.; Wan, Y. Fabrication of casein-crocin nanocomplexes: Interaction mechanism, impact on stability and bioavailability of crocin. Food Hydrocoll. 2023, 136, 108279. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. Microencapsulation of gac oil by spray drying: Optimization of wall material concentration and oil load using response surface methodology. Dry. Technol. 2014, 32, 385–397. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. A storage study of encapsulated gac (Momordica cochinchinensis) oil powder and its fortification into foods. Food Bioprod. Process. 2015, 96, 113–125. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Kong, F. Beta-carotene: Digestion, microencapsulation, and in vitro bioavailability. Food Bioprocess Technol. 2014, 7, 338–354. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT Food Sci. Technol. 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-grade pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The Stability and Bioaccessibility of Fucoxanthin in Spray-Dried Microcapsules Based on Various Biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Šeregelj, V.; Kalušević, A.; Lević, S.; Nedović, V.; Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G. Bioavailability and bioactivity of encapsulated phenolics and carotenoids isolated from red pepper waste. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of carotenoids as food colorants via formation of cyclodextrin inclusion complexes: A review. Polysaccharides 2021, 2, 454–476. [Google Scholar] [CrossRef]
- Katouzian, I.; Faridi Esfanjani, A.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Ahmed, A.; Ahmad, A.; Ahmad, M.S.; Sandhu, M.A. Optimization of mixed surfactants-based β-carotene nanoemulsions using response surface methodology: An ultrasonic homogenization approach. Food Chem. 2018, 253, 179–184. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Deng, Y.; McClements, D.J. Fabrication of β-carotene nanoemulsion-based delivery systems using dual-channel microfluidization: Physical and chemical stability. J. Colloid Interface Sci. 2017, 490, 328–335. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Shamoon, M.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical properties of β-carotene and eugenol co-encapsulated flax seed oil powders using OSA starches as wall material. Food Hydrocoll. 2017, 73, 274–283. [Google Scholar] [CrossRef]
- Pezeshki, A.; Hamishehkar, H.; Ghanbarzadeh, B.; Fathollahy, I.; Keivani Nahr, F.; Khakbaz Heshmati, M.; Mohammadi, M. Nanostructured Lipid Carriers as a Favorable Delivery System for β-Carotene. Food Biosci. 2019, 27, 11–17. [Google Scholar] [CrossRef]
- Hejri, A.; Khosravi, A.; Gharanjig, K.; Hejazi, M. Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013, 141, 117–123. [Google Scholar] [CrossRef]
- İnanç Horuz, T.; Belibağlı, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chem. 2018, 268, 86–93. [Google Scholar] [CrossRef]
- Afonso, B.S.; Azevedo, A.G.; Gonçalves, C.; Amado, I.R.; Ferreira, E.C.; Pastrana, L.M.; Cerqueira, M.A. Bio-based nanoparticles as a carrier of β-carotene: Production, characterisation and in vitro gastrointestinal digestion. Molecules 2020, 25, 4497. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Lee, J.-S.; Lee, H.G. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int. J. Biol. Macromol. 2016, 85, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Encapsulation of lutein in liposomes using supercritical carbon dioxide. Food Res. Int. 2017, 100, 168–179. [Google Scholar] [PubMed]
- Lacatusu, I.; Mitrea, E.; Badea, N.; Stan, R.; Oprea, O.; Meghea, A. Lipid nanoparticles based on omega-3 fatty acids as effective carriers for lutein delivery. preparation and in vitro characterization studies. J. Funct. Foods 2013, 5, 1260–1269. [Google Scholar] [CrossRef]
- Chen, L.; Yokoyama, W.; Alves, P.; Tan, Y.; Pan, J.; Zhong, F. Effect of encapsulation on β-carotene absorption and metabolism in mice. Food Hydrocoll. 2021, 121, 107009. [Google Scholar] [CrossRef]
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and bioaccessibility of β-carotene in nanoemulsions stabilized by modified starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Wen, Z.; Zhao, L. Physicochemical stability and in vitro bioaccessibility of β-carotene nanoemulsions stabilized with whey protein-dextran conjugates. Food Hydrocoll. 2017, 63, 256–264. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, C.; Dai, L.; Zhan, X.; Gao, Y. Structure, physicochemical stability and in vitro simulated gastrointestinal digestion properties of β-carotene loaded zein-propylene glycol alginate composite nanoparticles fabricated by emulsification-evaporation method. Food Hydrocoll. 2018, 81, 149–158. [Google Scholar] [CrossRef]
- Salem, A.; Ramadan, A.R.; Shoeib, T. Entrapment of β-carotene and zinc in whey protein nanoparticles using the pH cycle method: Evidence of sustained release delivery in intestinal and gastric fluids. Food Biosci. 2018, 26, 161–168. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Y.; Chen, G.; Shi, Y.; Li, X.; Zhang, H.; Shen, Y. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Controlled release of β-carotene in β-lactoglobulin–dextran-conjugated nanoparticles’ in vitro digestion and transport with caco-2 monolayers. J. Agric. Food Chem. 2014, 62, 8900–8907. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Verkempinck, S.; Rijal, S.K.; van Loey, A.; Grauwet, T.; Hendrickx, M. Lipid nanoparticles with fats or oils containing β-carotene: Storage stability and in vitro digestibility kinetics. Food Chem. 2019, 278, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.-S.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release 2017, 248, 117–124. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Ghoshal, G.; Kesharwani, P.; Singh, B.; Shivhare, U.S.; Katare, O.P. Lycopene loaded whey protein isolate nanoparticles: An innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int. J. Pharm. 2018, 546, 97–105. [Google Scholar] [CrossRef]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.-S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef]
- Sotomayor-Gerding, D.; Oomah, B.D.; Acevedo, F.; Morales, E.; Bustamante, M.; Shene, C.; Rubilar, M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016, 199, 463–470. [Google Scholar] [CrossRef]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato protein-based carriers for enhancing bioavailability of astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Hu, Z.; Li, G.; Hu, L.; Chen, X.; Hu, Y. Chitosan-based films with antioxidant of bamboo leaves and ZnO nanoparticles for application in active food packaging. Int. J. Biol. Macromol. 2021, 189, 363–369. [Google Scholar] [CrossRef]
- Teo, A.; Lee, S.J.; Goh, K.K.T.; Wolber, F.M. Kinetic stability and cellular uptake of lutein in WPI-stabilised nanoemulsions and emulsions prepared by emulsification and solvent evaporation method. Food Chem. 2017, 221, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Harish Prashanth, K.V.; Baskaran, V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: Characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013, 141, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Tandon, R.; Tandom, N. Utilizing fruits and vegetable wastes as functional food: A review. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 41. [Google Scholar]
- Puligundla, P.; Obulam, V.S.R.; Oh, S.E.; Mok, C. Biotechnological potentialities and valorization of mango peel waste: A review. Sains Malaysiana 2014, 43, 1901–1906. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- García, S.L.R.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Kaur, B.; Panesar, P.S.; Anal, A.K.; Chu-Ky, S. Recent trends in the management of mango by-products. Food Rev. Int. 2022, 39, 4159–4179. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Cassani, L.; Marcovich, N.E.; Gomez-Zavaglia, A. Valorization of fruit and vegetables agro-wastes for the sustainable production of carotenoid-based colorants with enhanced bioavailability. Food Res. Int. 2022, 152, 110924. [Google Scholar] [CrossRef]
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Characterization of carotenoids (lyco-red) extracted from tomato peels and its uses as natural colorants and antioxidants of ice cream. Ann. Agric. Sci. 2014, 59, 53–61. [Google Scholar] [CrossRef]
- Da Silva, L.M.R.; de Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; de Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral valorisation of tomato by-products towards bioactive compounds recovery: Human health benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef]
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G.A. Comprehensive overview of tomato processing by-product valorization by conventional methods versus emerging technologies. Foods 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Kumar, S.; Sunil, C.K.; Radhakrishnan, M.; Rawson, A. Recent advances in the utilization of industrial byproducts and wastes generated at different stages of tomato processing: Status report. J. Food Process. Preserv. 2022, 46, e17063. [Google Scholar] [CrossRef]
- Méndez-Carmona, J.Y.; Ascacio-Valdes, J.A.; Alvarez-Perez, O.B.; Hernández-Almanza, A.Y.; Ramírez-Guzman, N.; Sepúlveda, L.; Aguilar-González, M.A.; Ventura-Sobrevilla, J.M.; Aguilar, C.N. Tomato waste as a bioresource for lycopene extraction using emerging technologies. Food Biosci. 2022, 49, 101966. [Google Scholar] [CrossRef]
- Strati, F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent advances in recovery of lycopene from tomato waste: A potent antioxidant with endless benefits. Molecules 2021, 26, 4495. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive compounds extracted from tomato processing by-products as a source of valuable nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Food. 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Multari, S.; Carlin, S.; Sicari, V. Differences in the composition of phenolic compounds, carotenoids, and volatiles between juice and pomace of four citrus fruits from Southern Italy. Eur. Food Res. Technol. 2020, 246, 1991–2005. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable waste and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Citrus processing by-products: An overlooked repository of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 67–86. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of vegetable and fruit by-products as functional ingredient and food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Deeksha, K.; Sunita, M. Utilization of mango and its by-products by different processing methods. Asian J. Sci. Technol. 2018, 9, 8896–8901. [Google Scholar]
- Majid, I.; Khan, S.; Aladel, A.; Dar, A.H.; Adnan, M.; Khan, M.I.; Awadelkareem, A.M.; Ashraf, S.A. Recent insights into green extraction techniques as efficient methods for the extraction of bioactive components and essential oils from foods. CyTA–J. Food 2023, 21, 101–114. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.; Singh, J.; Gani, A.; Noor, N. Valorization of natural colors as health-promoting bioactive compounds: Phytochemical profile, extraction techniques, and pharmacological perspectives. Food Chem. 2021, 362, 130141. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Rixier, A.-S.; Albert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Machmudah, S.; Zakaria; Winardi, S.; Sasaki, M.; Goto, M.; Kusumoto, N.; Hayakawa, K. Lycopene extraction from tomato peel by-product containing tomato seed using supercritical carbon dioxide. J. Food Eng. 2012, 108, 290–296. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- De Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT-Food Sci. Technol. 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Perretti, G.; Troilo, A.; Bravi, E.; Marconi, O.; Galgano, F.; Fantozzi, P. Production of a lycopene-enriched fraction from tomato pomace using supercritical carbon dioxide. J. Supercrit. Fluids 2013, 82, 177–182. [Google Scholar] [CrossRef]
- Linares, G.; Rojas, M.L. Ultrasound-assisted extraction of natural pigments from food processing by-products: A review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trends Anal Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Pinzón-Zarate, L.X.; González-Salcedo, L.O. Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrason. Sonochem. 2015, 27, 560–566. [Google Scholar] [CrossRef]
- Ordoñez-Santos, L.E.; Martínez-Girón, J.; Rodríguez, D.X. Extraction of total carotenoids from peach palm fruit (Bactris gasipaes) peel by means of ultrasound application and vegetable oil. DYNA 2019, 86, 91–96. [Google Scholar]
- Portillo-López, R.; Morales-Contreras, B.E.; Lozano-Guzmán, E.; Basilio-Heredia, J.; Muy-Rangel, M.D.; Ochoa-Martínez, L.A.; Rosas-Flores, W.; Morales-Castro, J. Vegetable oils as green solvents for carotenoid extraction from pumpkin (Cucurbita argyrosperma Huber) byproducts: Optimization of extraction parameters. J. Food Sci. 2021, 86, 3122–3136. [Google Scholar] [CrossRef]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-assisted extraction of carotenoids from orange peel using olive oil and its encapsulation in Ca-alginate beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and ultrasound-assisted extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Purohit, A.J.; Gogate, P.R. Ultrasound-assisted extraction of β-carotene from waste carrot residue: Effect of operating parameters and type of ultrasonic irradiation. Sep. Sci. Technol. 2015, 50, 1507–1517. [Google Scholar] [CrossRef]
- Lara-Abia, S.; Welti-Chanes, J.; Cano, M.P. Effect of ultrasound-assisted extraction of carotenoids from papaya (Carica papaya L. cv. Sweet Mary) using vegetable oils. Molecules 2022, 27, 638. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Esparza-Estrada, J.; Vanegas-Mahecha, P. Ultrasound-assisted extraction of total carotenoids from mandarin epicarp and application as natural colorant in bakery products. LWT-Food Sci. Technol. 2021, 139, 110598. [Google Scholar] [CrossRef]
- Rahimi, S.; Mikani, M. Lycopene green ultrasound-assisted extraction using edible oil accompany with response surface methodology (RSM) optimization performance: Application in tomato processing wastes. Microchem. J. 2019, 146, 1033–1042. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving carotenoid extraction from tomato waste by pulsed electric fields. Front. Nutr. 2014, 1, 12. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef]
- Shahram, H.; Dinani, S.T. Optimization of ultrasonic-assisted enzymatic extraction of β-carotene from orange processing waste. J. Food Process Eng. 2019, 42, 13042. [Google Scholar] [CrossRef]
- Strati, F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural colorants from vegetable food waste: Recovery, regulatory aspects, and stability- A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of vegetable fresh-processing residues as functional powdered ingredients. a review on the potential impact of pretreatments and drying methods on bioactive compounds and their bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- Villacís-Chiriboga, J.; Ver, E.; Van Camp, J.; Ruales, J.; Elst, K. Valorization of byproducts from tropical fruits: A review, Part 2: Applications, economic, and environmental aspects of biorefinery via supercritical fluid extraction. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2305–2331. [Google Scholar] [CrossRef]
- Taghian Dinani, S.; van der Goot, A.J. Challenges and solutions of extracting value-added ingredients from fruit and vegetable by-products: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7749–7771. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Yoon, S.H.; Lee, S.H.; Kim, J.Y.; Oh, D.K.; Kim, S.W. An update on microbial carotenoid production: Application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007, 77, 505–512. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2022, 62, 6932–6946. [Google Scholar] [CrossRef]
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Nakada, T.; Ota, S. What is the correct name for the type of Haematococcus Flot. (Volvocales, Chlorophyceae)? Taxon 2016, 65, 343–348. [Google Scholar] [CrossRef]
- Chekanov, K. Diversity and distribution of carotenogenic algae in Europe: A review. Mar. Drugs 2023, 21, 108. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: Photobioreactors for large scale algal cultivation. World J. Microbiol. Biotechnol. 2015, 31, 1409–1417. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.J.; Murray, P.M.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014. 64, 332–344. [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Maldonade, I.R. Potentials and challenges in the production of microalgal pigments with reference to carotenoids, chlorophylls, and phycobiliproteins. In Handbook of Algal Technology and Phytochemicals Volume 1 Food, Health and Neutraceutical Applications; Ravishankar, G.A., Ambati, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 109–118. [Google Scholar]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein production from microalgae: A review. Bioresour. Technol. 2023, 376, 128875. [Google Scholar] [CrossRef] [PubMed]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. 2016, 14, 214. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef]
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent advances in supercritical CO2 extraction of pigments, lipids and bioactive compounds from microalgae. Molecules 2023, 28, 1410. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, J.; Hou, D.; Fan, J.; Li, Y.; Huang, J.; Wang, J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol. 2014, 167, 276–283. [Google Scholar] [CrossRef]
- Wichuk, K.; Brynjólfsson, S.; Fu, W. Biotechnological production of value-added carotenoids from microalgae: Emerging technology and prospects. Bioengineered 2014, 5, 204–208. [Google Scholar] [CrossRef]
- Poonkum, W.; Powtongsook, S.; Pavasant, P. Astaxanthin induction in microalga H. pluvialis with flat panel airlift photobioreactors under indoor and outdoor conditions. Prep. Biochem. Biotechnol. 2015, 45, 1–17. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, J.; Li, K.; Zhou, J.; Cen, K. Optimizing gas transfer to improve growth rate of Haematococcus pluvialis in a raceway pond with chute and oscillating baffles. Bioresour. Technol. 2016, 214, 276–283. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.J.; Sun, D.; Lee, Y.; Chen, F. Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour. Technol. 2017, 224, 515–522. [Google Scholar] [CrossRef]
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef]
- Kim, B.; Youn Lee, S.; Lakshmi Narasimhan, A.; Kim, S.; Oh, Y.K. Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances. Bioresour. Technol. 2022, 343, 126124. [Google Scholar] [CrossRef]
- Koopmann, I.K.; Möller, S.; Elle, C.; Hindersin, S.; Kramer, A.; Labes, A. Optimization of astaxanthin recovery in the downstream process of Haematococcus pluvialis. Foods 2022, 11, 1352. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Haque, F.; Dutta, A.; Thimmanagarib, M.; Chiang, Y.W. Intensified green production of astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 1–11. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, G.; Dash, S.K.; Sen, R. Development of an optimal light-feeding strategy coupled with semi-continuous reactor operation for simultaneous improvement of microalgal photosynthetic efficiency, lutein production and CO2 sequestration. Biochem. Eng. J. 2016, 113, 47–56. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lu, I.C.; Nagarajan, D.; Chang, C.H.; Ng, I.S.; Lee, D.J.; Chang, J.S. A highly efficient two-stage cultivation strategy for lutein production using heterotrophic culture of Chlorella sorokiniana MB-1-M12. Bioresour. Technol. 2018, 253, 141–147. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Kwon, J.S.; Kang, S.T.; Kim, B.R.; Jung, Y.; Han, J.G.; Park, J.H.; Hwang, J.K. Optimization of culture media for large-scale lutein production by heterotrophic Chlorella vulgaris. Biotechnol. Prog. 2014, 30, 736–743. [Google Scholar] [CrossRef]
- Flórez-Miranda, L.; Cañizares-Villanueva, R.O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Flores-Ortíz, C.M. Two stage heterotrophy/photoinduction culture of Scenedesmus incrassatulus: Potential for lutein production. J. Biotechnol. 2017, 262, 67–74. [Google Scholar] [CrossRef]
- Mulders, K.J.; Lamers, P.P.; Martens, D.E. Wijffels. R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Zheng, H.S.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Přibyl, P.; Pilný, J.; Cepák, V.; Petr Kaštánek, P. The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal Res. 2016, 16, 69–75. [Google Scholar] [CrossRef]
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol. Rep. (Amst.) 2016, 10, 117–125. [Google Scholar] [CrossRef]
- Chiu, P.H.; Soong, K.; Chen, C.-N. Cultivation of two thermotolerant microalgae under tropical conditions: Influences of carbon sources and light duration on biomass and lutein productivity in four seasons. Bioresour. Technol. 2016, 212, 190–198. [Google Scholar] [CrossRef]
- Hong, S.J.; Yim, K.J.; Ryu, Y.J.; Lee, C.G.; Jang, H.J.; Jung, J.Y.; Kim, Z.H. Improvement of lutein and zeaxanthin production in Mychonastes sp. 247 by optimizing light intensity and culture salinity conditions. J. Microbiol. Biotechnol. 2023, 33, 260–267. [Google Scholar] [CrossRef]
- Heo, J.; Shin, D.S.; Cho, K.; Cho, D.H.; Lee, Y.J.; Kim, H.S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2023. [Google Scholar]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food Additives; EU: Brussels, Belgium, 2008. [Google Scholar]
- EFSA. Food Additives; EFSA: Parma, Italy, 2023. [Google Scholar]
- European Commission. Food Additives Database; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- FAO. Combined Compendium of Food Additive Specifications; FAO: Rome, Italy, 2023. [Google Scholar]
- EFSA. Scientific Opinion on the re-evaluation of mixed carotenes (E 160a (i)) and beta-carotene (E 160a (ii)) as a food additive—EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2012, 10, 1–67. [Google Scholar]
- EFSA. Statement on the safety of β-carotene use in heavy smokers. EFSA J. 2012, 10, 1–7. [Google Scholar]
- EFSA. Scientific opinion on the re-evaluation of paprika extract (E 160c) as a food additive. EFSA J. 2015, 13, 4320–4371. [Google Scholar]
- Evaluation of Certain Food Additives; JECFA WHO Technical Report Series 990; WHO: Geneva, Switzerland, 2014.
- EFSA. Scientific opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010, 8, 1678–1735. [Google Scholar] [CrossRef]
- EFSA. The safety of annatto extracts (E 160b) as a food additive. EFSA J. 2016, 14, 4544–4631. [Google Scholar]
- Evaluation of Certain Food Additives and Contaminants; JECFA WHO Technical Report Series 940; WHO: Geneva, Switzerland, 2007.
- EFSA. Use of lycopene as a food colour. EFSA J. 2008, 674, 1–66. [Google Scholar]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Dias, M.G.; Borge, G.I.A.; Kljak, K.; Mandić, A.I.; Mapelli-Brahm, P.; Olmedilla-Alonso, B.; Pintea, A.M.; Ravasco, F.; Tumbas Šaponjac, V.; Sereikaitė, J.; et al. European database of carotenoid levels in foods. Factors affecting carotenoid content. Foods 2021, 10, 912. [Google Scholar] [CrossRef]
- Gavahian, M.; Mousavi Khaneghah, A.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–325. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.A.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.I.; Brahm, P.M.; et al. Carotenoids: Considerations for their use in functional foods, nutraceuticals, nutricosmetics, supplements, botanicals, and novel foods in the context of sustainability, circular economy, and climate change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef]
- Biehler, E.; Alkerwi, A.; Hoffmann, L.; Krause, E.; Guillaume, M.; Lair, M.-L.; Bohn, T. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J. Food Compos. Anal. 2012, 25, 56–65. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Benítez-González, A.M.; Estévez-Santiago, R.; Mapelli-Brahm, P.; Stinco, C.M.; Meléndez-Martínez, A.J. Assessment of food sources and the intake of the colourless carotenoids phytoene and phytofluene in Spain. Nutrients 2021, 13, 4436. [Google Scholar] [CrossRef]
- ATBC (Alpha-Tocopherol, Beta-carotene Cancer Prevention Group). The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- CARIG (Carotenoid Research Interactive Group). Beta-carotene and the carotenoids: Beyond the intervention trials. Nutr. Rev. 1996, 54, 185–188. [Google Scholar]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Mele, M.C. Interplay of carotenoids with cigarette smoking: Implications in lung cancer. Curr. Med. Chem. 2008, 15, 844–854. [Google Scholar] [CrossRef]
- Yoshida, W.S.; Murata, H.; Imaida, M. Distribution of pesticide residues in vegetables and fruits and removal by washing. Nippon Nogeikagaku Kaishi 1992, 66, 1007–1011. [Google Scholar] [CrossRef][Green Version]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Part A, Chemistry, analysis, control, exposure and risk assessment. Food Addit. Contam. 2017, 34, 335–355. [Google Scholar] [CrossRef]
- Simon, J.E.; Decker, E.A.; Ferruzzi, M.G.; Giusti, M.M.; Mejia, C.D.; Goldschmidt, M.; Talcott, S.T. Establishing standards on colors from natural sources. J. Food Sci. 2017, 82, 2539–2553. [Google Scholar] [CrossRef]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in food and agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nutr. 2018, 58, 297–317. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The safety of nanomaterials infood production and packaging. Curr. Res. Nutr. Food Sci. 2022, 5, 763–774. [Google Scholar]
- Singh, N.; Nelson, B.C.; Scanlan, L.D.; Coskun, E.; Jaruga, P.; Doak, S.H. Exposure to engineered nanomaterials: Impact on DNA repair pathways. Int. J. Mol. Sci. 2017, 18, 1515. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. npj Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
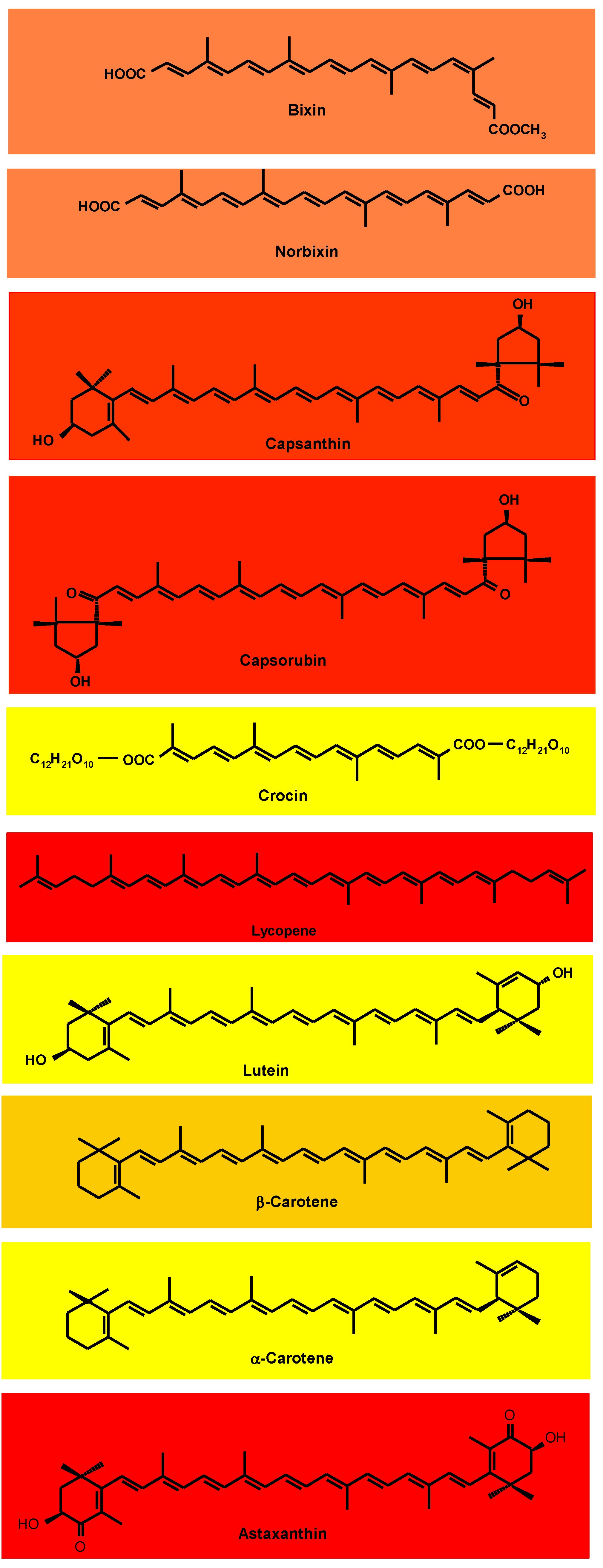
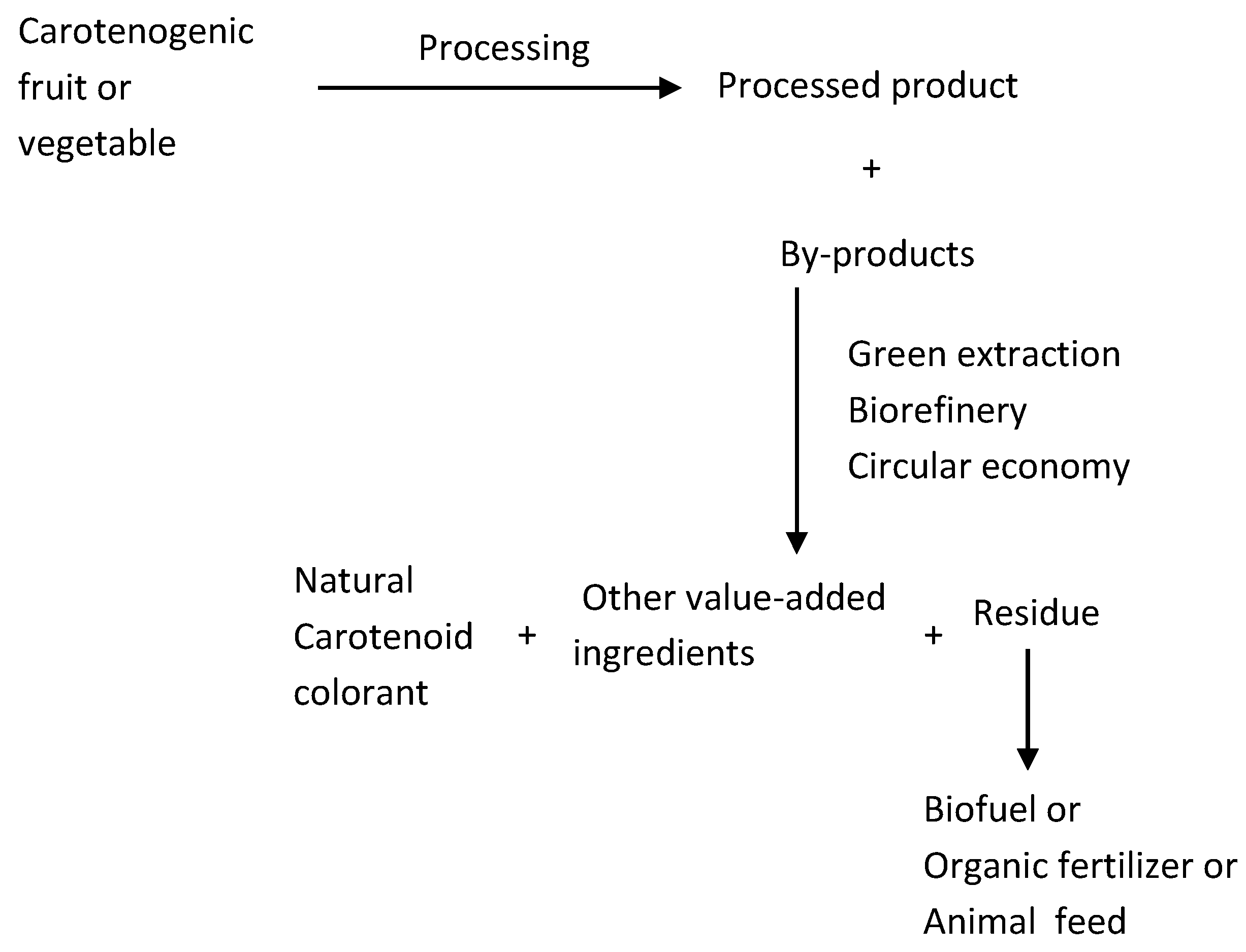
| Technique | Advantages | Disadvantages |
|---|---|---|
| Microwave-assisted extraction | Simple, fast, and economical | Can cause thermal degradation and cis-trans isomerization of carotenoids |
| Ultrasound-assisted extraction | Rapid, non-thermal and efficient extraction Low energy consumption and equipment cost | Free radical generation at high sonication power Low selectivity Low ultrasonic power may result in low yield |
| Pressurized liquid extraction | Fast, requires minimum amount of organic solvent Highly applicable to a laboratory-scale context | Difficult to apply to large volumes due to clogging caused by sugars and pectins of plant matrices |
| Pulsed electric field extraction | High extraction yield Non-thermal process Low energy use | High cost of instrumentation Bubbles in the samples may cause technical problems |
| Supercritical fluid extraction | Uses non-flammable, non-toxic and recyclable solvent (CO2 and ethanol) Continuous extraction process instead of batch processing Avoids thermal degradation High extraction yield and selectivity | High power consumption High cost of instrumentation |
| Enzyme-assisted extraction | Rapid and efficient extraction with use of solvents | High cost of the enzymes |
| FDA | EFSA (ADI) | CODEX (ADI) |
|---|---|---|
| Annatto extract | Annatto bixin (6 mg/kg bw/day) Annatto norbixin 0.3 mg/kg bw/day) | Annatto extract, bixin based (0–12 mg/kg bw) Annatto extract, norbixin based 0–0.6 mg/kg bw) |
| Paprika and paprika oleoresin | Paprika extract (24 mg/kg bw/day) | Paprika extract (0–1.5 mg/kg bw expressed as total carotenoids) |
| Tomato lycopene extract and tomato lycopene concentrate | Lycopene from tomato Blakeslea trispora lycopene (0.5 mg/kg bw/day) | Lycopene from tomato Blakeslea trispora lycopene |
| Carrot oil Saffron | ||
| Lutein from different plant sources (1 mg/kg bw/day) | Tagetes erecta lutein | |
| Dunaliella salina β-carotene | Dunaliella salina (β-Carotene) Blakeslea trispora (β-Carotene) |
| Carotenoid | Range (mg/Day) |
|---|---|
| α-Carotene | 0.14–2.43 |
| β-Carotene | 1.46–8.80 |
| β-Cryptoxanthin | 0.03–1.36 |
| Lutein + zeaxanthin | 1.00–4.84 |
| Lycopene | 0.28–10.70 |
| Phytoene | 1.89–2.0 |
| Phytofluene | 0.47–0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J. Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods 2023, 12, 4080. https://doi.org/10.3390/foods12224080
Rodriguez-Amaya DB, Esquivel P, Meléndez-Martínez AJ. Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods. 2023; 12(22):4080. https://doi.org/10.3390/foods12224080
Chicago/Turabian StyleRodriguez-Amaya, Delia B., Patricia Esquivel, and Antonio J. Meléndez-Martínez. 2023. "Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry" Foods 12, no. 22: 4080. https://doi.org/10.3390/foods12224080
APA StyleRodriguez-Amaya, D. B., Esquivel, P., & Meléndez-Martínez, A. J. (2023). Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods, 12(22), 4080. https://doi.org/10.3390/foods12224080