Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Sample Preparation
2.4. Moisture Content Determination
2.5. Color of the Samples
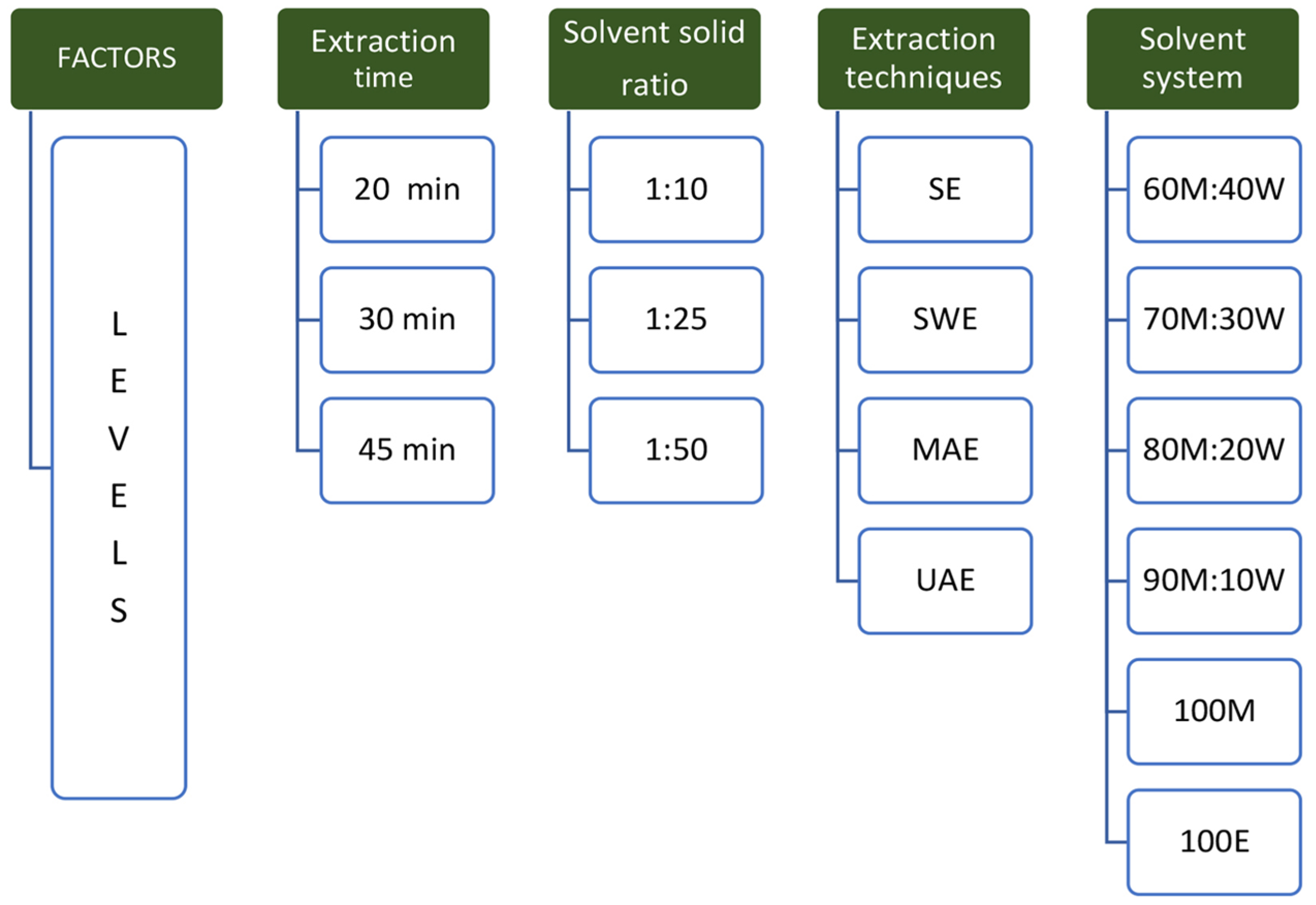
2.6. Selection of Optimum Extraction Conditions
2.6.1. Soxhlet Extraction
2.6.2. Microwave-Assisted Extraction (MAE)
2.6.3. Ultrasound-Assisted Extraction (UAE)
2.6.4. Subcritical Water Extraction (SWE)
2.7. High-Performance Liquid Chromatography (HPLC) Analysis
2.8. Particle Size Measurement
2.9. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and DPPH Radical Scavenging Activity (RSADPPH)
2.10. Statistical Analysis
3. Results
3.1. Moisture Content Determination, Color Measurements, and Pre-Sample Preparation
3.2. Selection of Optimum Extraction Conditions for Triterpenes
3.3. Investigation of Total Triterpene Content (TTC), Total Phenolics Content (TPC), Total Flavonoids Content (TFC), and DPPH Radical Scavenging Activity (RSADPPH) under Different Solvent Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernhoft, A. A brief review on bioactive compounds in plants. In Bioactive Compounds in Plants—Benefits and Risks for Man and Animals; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; Volume 50, pp. 11–17. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry and Molecular Biology of Plants; American Society of Plant Physiologists: Rockville, MD, USA, 2000; Volume 24, pp. 1250–1319. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M. Before the injection—Modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Pai, S.; Hebbar, A.; Selvaraj, S. A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector. Environ. Sci. Pollut. Res. 2022, 29, 35518–35541. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2020, 1635, 461770. [Google Scholar] [CrossRef]
- González, M.; Barrios, S.; Budelli, E.; Pérez, N.; Lema, P.; Heinzen, H. Ultrasound assisted extraction of bioactive compounds in fresh and freeze-dried Vitis vinifera cv Tannat grape pomace. Food Bioprod. Process. 2020, 124, 378–386. [Google Scholar] [CrossRef]
- Zghaibi, N.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Harun, R. Microwave-assisted brine extraction for enhancement of the quantity and quality of lipid production from Microalgae Nannochloropsis sp. Molecules 2019, 24, 3581. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Sabaragamuwa, R.; Perera, C.O.; Fedrizzi, B. Centella asiatica (Gotu kola) as a neuroprotectant and its potential role in healthy ageing. Trends Food Sci. Technol. 2018, 79, 88–97. [Google Scholar] [CrossRef]
- Jain, K.K. The Handbook of Neuroprotection; Humana Press: New York, NY, USA, 2011; p. 547. [Google Scholar]
- Cooper, E.L.; Ma, M.J. Alzheimer Disease: Clues from traditional and complementary medicine. J. Tradit. Complement. Med. 2017, 7, 380–385. [Google Scholar] [CrossRef]
- Al-Nahain, A.; Jahan, R.; Rahman, T.; Nurunnabi, M.; Rahmatullah, M. Centella asiatica: From Ethnomedicinal Uses to the Possibility of New Drug Discovery. In Therapeutic Medicinal Plants: From Lab to the Market; CRC Press: Boca Raton, FL, USA, 2015; Volume 380, p. 702. [Google Scholar]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomedicine 2000, 7, 427–448. [Google Scholar] [CrossRef] [PubMed]
- James, J.T.; Dubery, I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants–Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Allan, H.H. Flora of New Zealand. Vol. I. Indigenous Tracheophyta: Psilopsida, Lycopsida, Filicopsida, Gymnospermae, Dicotyledones; Government Printer: Wellington, New Zealand, 1961. [Google Scholar]
- She, M.; Watson, M.F. Centella. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Vol. 14 Apiaceae through Ericaceae; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MI, USA, 2005. [Google Scholar]
- Poyraz, I. Comparison of ITS, RAPD and ISSR from DNA-based genetic diversity techniques. Comptes Rendus Biol. 2016, 339, 171–178. [Google Scholar] [CrossRef]
- Council of Europe, European Pharmacopoeia Commission. European Pharmacopoeia; European Council: Strasbourg, France, 2008. [Google Scholar]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and analysis of terpenes/terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Rafamantanana, M.; Rozet, E.; Raoelison, G.; Cheuk, K.; Ratsimamanga, S.; Hubert, P.; Quetin-Leclercq, J. An improved HPLC-UV method for the simultaneous quantification of triterpenic glycosides and aglycones in leaves of Centella asiatica (L.) Urb (APIACEAE). J. Chromatogr. B 2009, 877, 2396–2402. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- WHO Guidelines on Good Agricultural and Collection Practices [GACP] for Medicinal Plants (9241546271). 2003. Available online: https://apps.who.int/medicinedocs/documents/s23449en/s23449en.pdf (accessed on 15 July 2023).
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Petitcolas, E.; Maache-Rezzoug, Z.; Chemat, F.; Rezzoug, S. Impact of ultrasound on solid–liquid extraction of phenolic compounds from maritime pine sawdust waste. Kinetics, optimization and large scale experiments. Ultrason. Sonochem. 2016, 28, 230–239. [Google Scholar] [CrossRef]
- Ko, M.-J.; Cheigh, C.-I.; Chung, M.-S. Optimization of Subcritical Water Extraction of Flavanols from Green Tea Leaves. J. Agric. Food Chem. 2014, 62, 6828–6833. [Google Scholar] [CrossRef]
- Liang, X.; Fan, Q. Application of sub-critical water extraction in pharmaceutical industry. J. Mater. Sci. Chem. Eng. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2015, 8, 23–34. [Google Scholar] [CrossRef]
- Kim, W.-J.; Kim, J.; Veriansyah, B.; Kim, J.-D.; Lee, Y.-W.; Oh, S.-G.; Tjandrawinata, R.R. Extraction of bioactive components from Centella asiatica using subcritical water. J. Supercrit. Fluids 2009, 48, 211–216. [Google Scholar] [CrossRef]
- Luong, D.; Sephton, M.A.; Watson, J.S. Subcritical water extraction of organic matter from sedimentary rocks. Anal. Chim. Acta 2015, 879, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yingngam, B.; Chiangsom, A.; Brantner, A. Modeling and optimization of microwave-assisted extraction of pentacyclic triterpenes from Centella asiatica leaves using response surface methodology. Ind. Crop. Prod. 2020, 147, 112231. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, A.; Ye, M.; Wang, L.; Chen, J.; Wang, X.-R.; Han, C. Analysis of biologically active constituents in Centella asiatica by microwave-assisted extraction combined with LC–MS. Chromatographia 2009, 70, 431–438. [Google Scholar] [CrossRef]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Alqahtani, A.; Tongkao-On, W.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K.; Li, G.Q. Seasonal variation of triterpenes and phenolic compounds in Australian Centella asiatica(L.) Urb. Phytochem. Anal. 2015, 26, 436–443. [Google Scholar] [CrossRef]
- Arora, R.; Kumar, R.; Agarwal, A.; Reeta, K.; Gupta, Y. Comparison of three different extracts of Centella asiatica for anti-amnesic, antioxidant and anticholinergic activities: In vitro and in vivo study. Biomed. Pharmacother. 2018, 105, 1344–1352. [Google Scholar] [CrossRef]
- Azerad, R. Chemical structures, production and enzymatic transformations of sapogenins and saponins from Centella asiatica (L.) Urban. Fitoterapia 2016, 114, 168–187. [Google Scholar] [CrossRef]
- Barbosa, N.; Pittella, F.; Gattaz, W. Centella asiatica water extract inhibits iPLA2 and cPLA2 activities in rat cerebellum. Phytomedicine 2008, 15, 896–900. [Google Scholar] [CrossRef]
- Gray, N.E.; Magana, A.A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2017, 17, 161–194. [Google Scholar] [CrossRef]
- Long, H.; Stander, M.; Van Wyk, B.-E. Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. S. Afr. J. Bot. 2012, 82, 53–59. [Google Scholar] [CrossRef]
- Albright, P.S.; Gosting, L.J. Dielectric Constants of the Methanol-Water System from 5 to 55°. J. Am. Chem. Soc. 1946, 68, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Nair, G.R.; Liplap, P.; Gariepy, Y.; Orsat, V.; Raghavan, V. Effect of dielectric properties of a solvent-water mixture used in microwave-assisted extraction of antioxidants from potato peels. Antioxidants 2014, 3, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Scalbert, A.; Andres-Lacueva, C.; Arita, M.; Kroon, P.; Manach, C.; Urpi-Sarda, M.; Wishart, D. Databases on Food Phytochemicals and Their Health-Promoting Effects. J. Agric. Food Chem. 2011, 59, 4331–4348. [Google Scholar] [CrossRef]



| Step | Time (min) | Power (W) | Temperature (°C) | |
|---|---|---|---|---|
| 01 | Heating | 05 | 0–600 | 20–70 |
| 02 | Holding | 20 | 0–180 | 70 |
| 03 | Cooling | 10 | - | 70–0 |
| Bioactive Compound | Extraction Yield (mg/g) | ||
|---|---|---|---|
| Soxhlet | Microwave-Assisted | Ultrasound-Assisted | |
| Madicassoside | ND | 25.05 ± 0.19 a | 23.95 ± 0.63 a |
| Asiaticoside | 19.93 ± 0.46 a | 48.49 ± 0.64 b | 51.58 ± 0.44 b |
| Madicassic acid | 11.68 ± 1.18 a | 5.91 ± 0.97 b | 6.82 ± 0.16 b |
| Asiatic acid | ND | 1.85 ± 0.47 a | 2.31 ± 0.47 a |
| Total triterpenes | 30.94 ± 1.92 a | 81.30 ± 1.08 b | 84.66 ± 1.32 b |
| TTC | TPC | TFC | RSA | |
|---|---|---|---|---|
| TTC | 1 | 0.86 | 0.83 | 0.98 ** |
| TPC | 0.86 | 1 | 0.98 ** | 0.94 * |
| TFC | 0.83 | 0.98 ** | 1 | 0.89 * |
| RSADPPH | 0.98 ** | 0.94 * | 0.89 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabaragamuwa, R.; Perera, C.O. Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques. Foods 2023, 12, 3972. https://doi.org/10.3390/foods12213972
Sabaragamuwa R, Perera CO. Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques. Foods. 2023; 12(21):3972. https://doi.org/10.3390/foods12213972
Chicago/Turabian StyleSabaragamuwa, Rasangani, and Conrad O. Perera. 2023. "Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques" Foods 12, no. 21: 3972. https://doi.org/10.3390/foods12213972
APA StyleSabaragamuwa, R., & Perera, C. O. (2023). Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques. Foods, 12(21), 3972. https://doi.org/10.3390/foods12213972











