Nutritional Potential of Adzuki Bean Germplasm and Mining Nutri-Dense Accessions through Multivariate Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adzuki Bean Seed Material
2.2. Nutrional Composition Analysis
2.2.1. Total Moisture Content
2.2.2. Total Ash Content
2.2.3. Total Protein Content
2.2.4. Total Starch Content
2.2.5. Total Soluble Sugar (TSS) Content
2.2.6. Total Phytic Acid Content
2.2.7. Total Phenol Content
2.2.8. Antioxidant Activity
2.3. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Analysis—Descriptive Statistics of V. angularis Genotypes for Different Nutritional Components
3.1.1. Moisture Content
3.1.2. Ash Content
3.1.3. Total Protein Content
3.1.4. Total Starch Content
3.1.5. Total Soluble Sugar (TSS)
3.1.6. Phytic Acid Content
3.1.7. Phenol Content
3.1.8. Antioxidant Activity
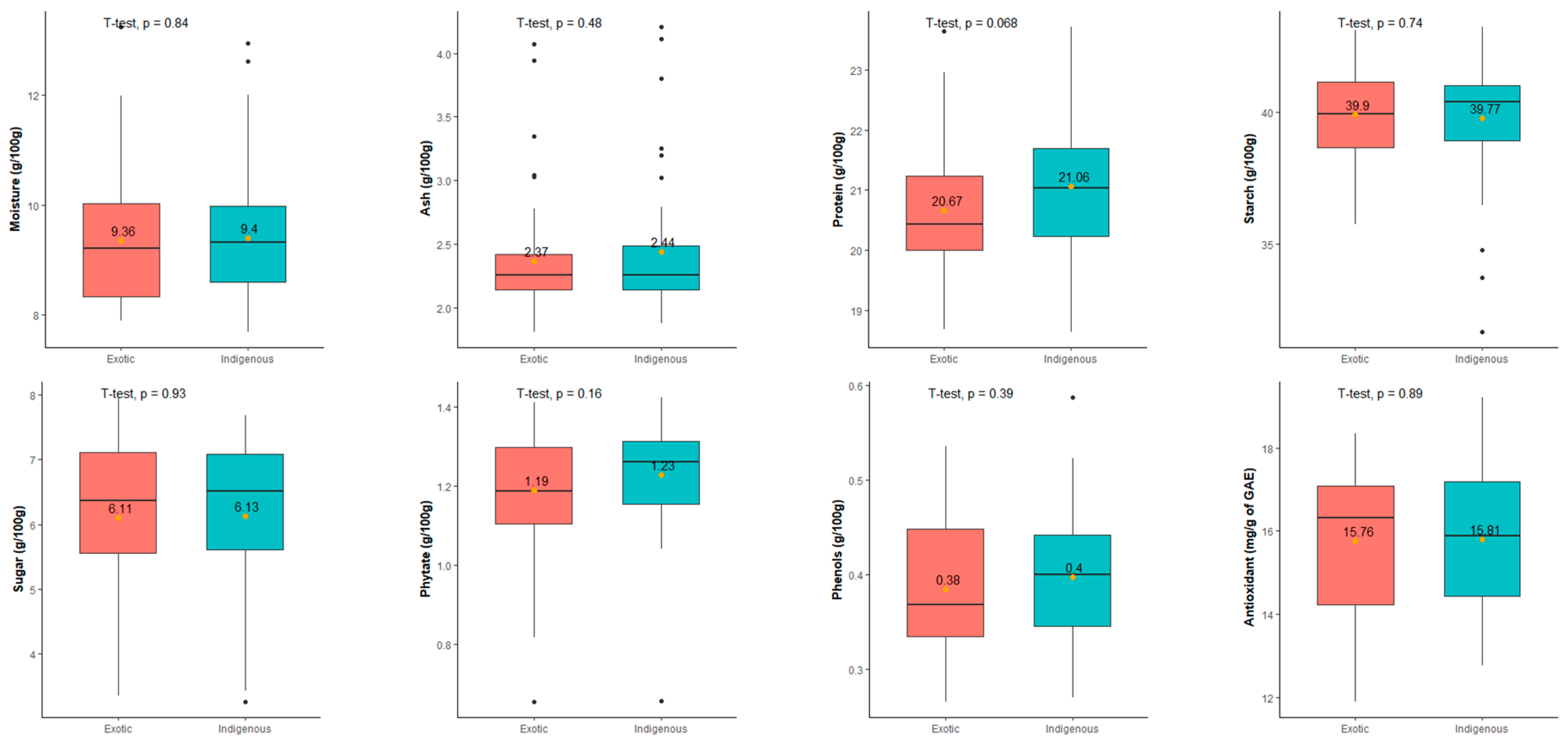
3.1.9. Comparisons among the Indigenous and Exotic Collections of Adzuki Bean Accessions for Nutritional Traits
3.2. Statistical Analysis
3.2.1. Analysis of Variations (ANOVA) of Nutritional Traits of V. angularis Accessions
3.2.2. Genetic Parameters Analysis for Eight Nutritional Traits for 100 Accessions of V. angularis
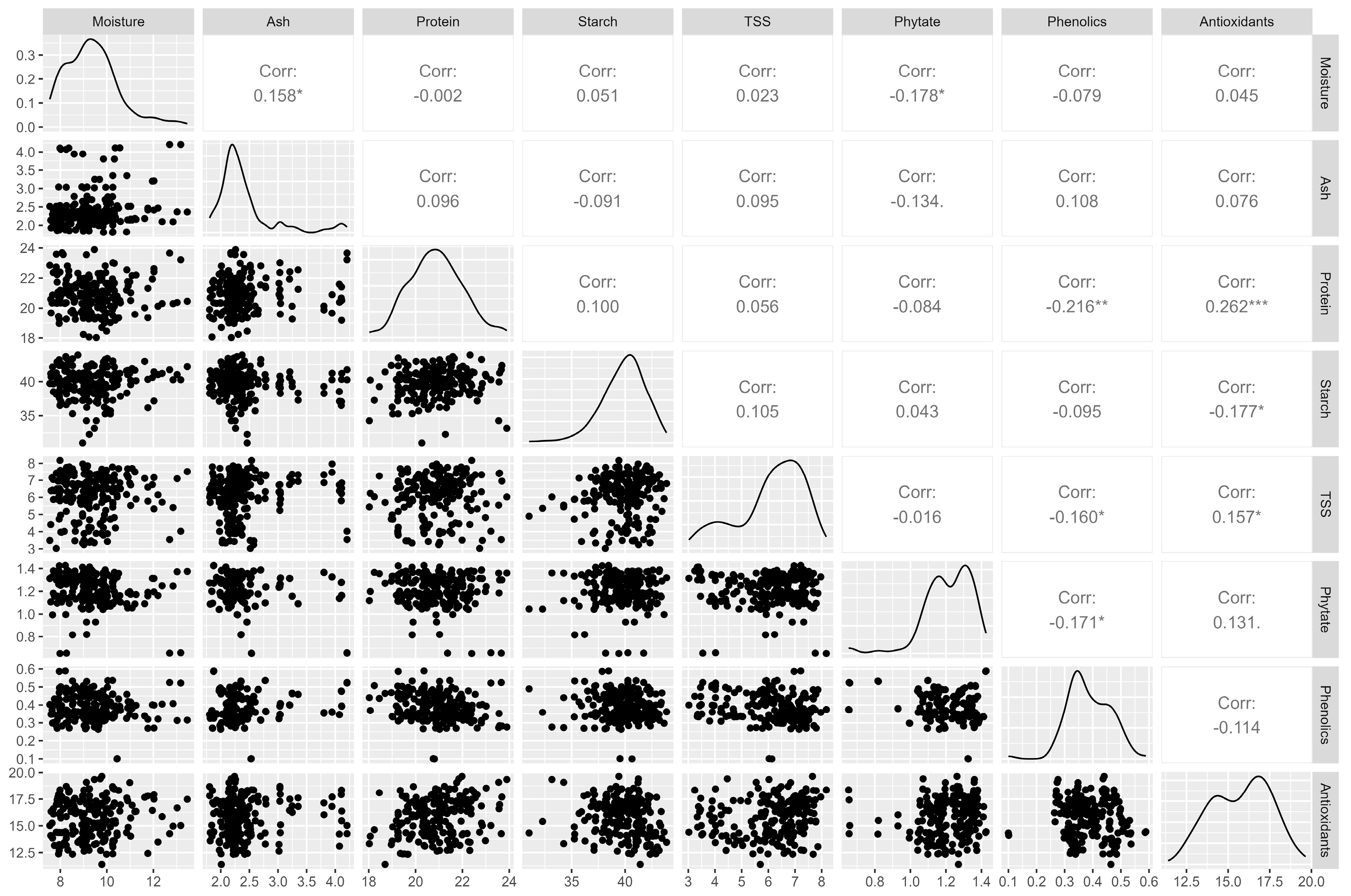
3.2.3. Correlation Analysis of the Nutritional Parameters
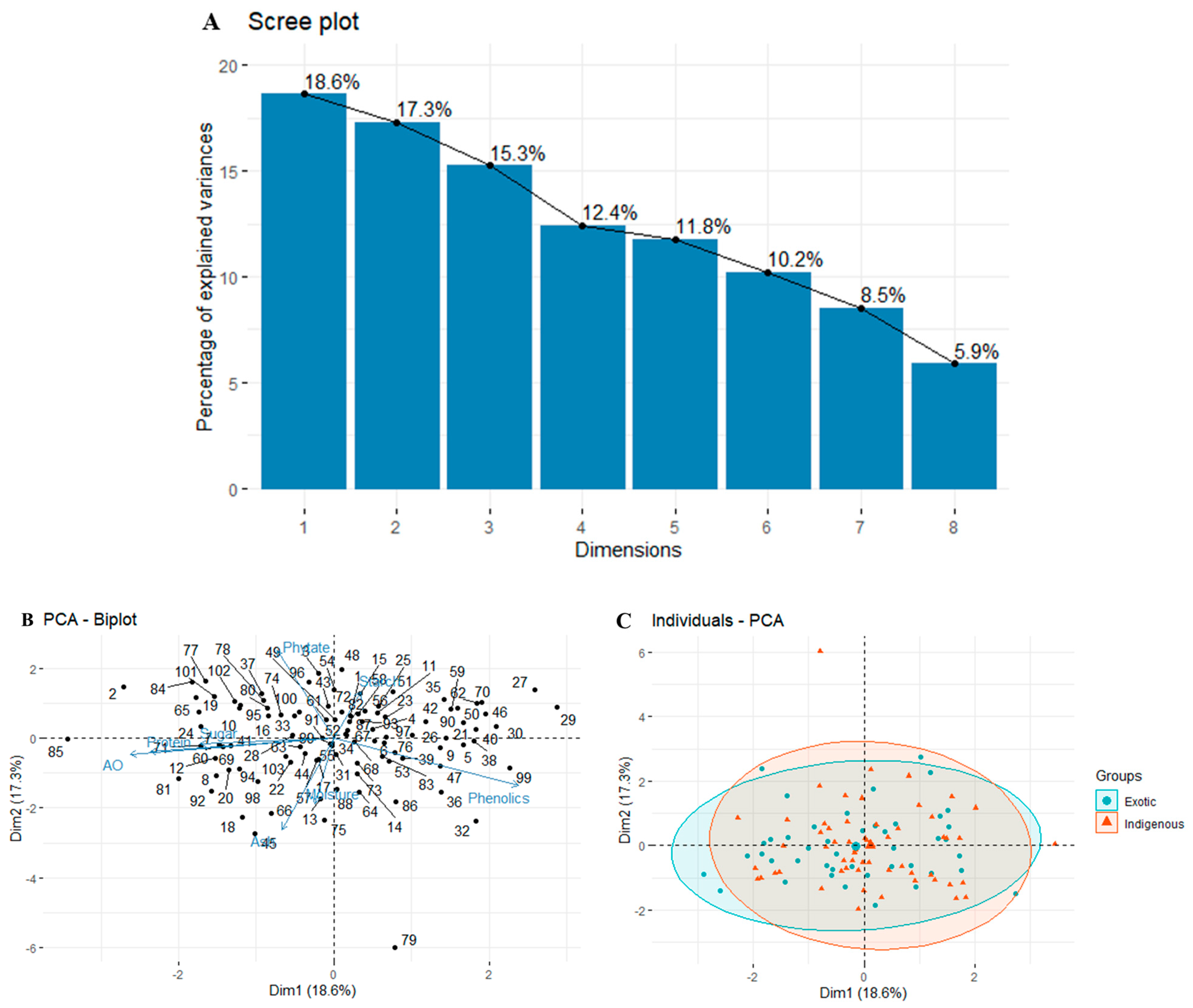
3.2.4. Principal Component Analysis (PCA)
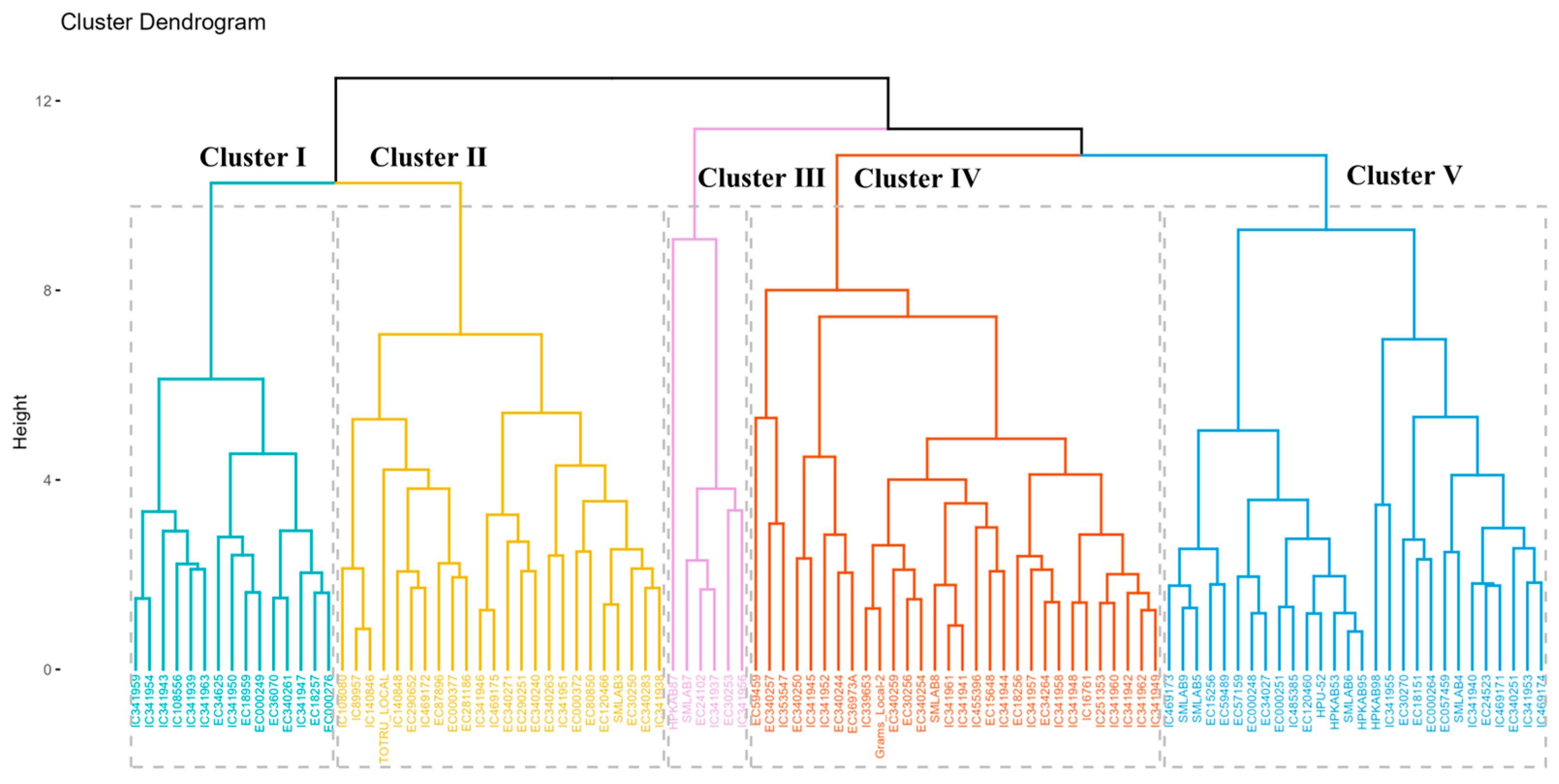
3.2.5. Hierarchical Clustering Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omomowo, O.I.; Babalola, O.O. Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Front. Plant Sci. 2021, 12, 751731. [Google Scholar] [CrossRef] [PubMed]
- Kaga, A.; Isemura, T.; Tomooka, N.; Vaughan, D.A. The genetics of domestication of the azuki bean (Vigna angularis). Genetics 2008, 178, 1013–1036. [Google Scholar] [CrossRef]
- Kang, Y.J.; Satyawan, D.; Shim, S.; Lee, T.; Lee, J.; Hwang, W.J.; Lee, S.H. Draft genome sequence of adzuki bean, Vigna angularis. Sci. Rep. 2015, 5, 8069. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A. Archaeological perspectives on the origins of azuki (Vigna angularis). Holocene 2013, 23, 453–459. [Google Scholar] [CrossRef]
- Yang, K.; Tian, Z.; Chen, C.; Luo, L.; Zhao, B.; Wang, Z.; Wan, P. Genome sequencing of adzuki bean (Vigna angularis) provides insight into high starch and low-fat accumulation and domestication. Proc. Natl. Acad. Sci. USA 2015, 112, 13213–13218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Verma, R.; Singh, N.; Dhaliwal, Y.S. Assessment of anti-nutritional factors and antioxidants in three genotypes of adzuki beans. J. Pharmacogn. Phytochem. 2019, 8, 1376–1378. [Google Scholar]
- Yadav, U.; Singh, N.; Arora, S.; Arora, B. Physicochemical, pasting, and thermal properties of starches isolated from different adzuki bean (Vigna angularis) cultivars. J. Food Process. Preserv. 2019, 43, e14163. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Jia, B.; Zhang, H.; Zuo, F. Evaluation of Protein Nutritional Value of Main Cultivated Adzuki Bean in Heilongjiang Province. J. Chin. Cereals Oils Assoc. 2021, 36, 29–33. [Google Scholar]
- Takahashi, Y.; Nemoto, K.; Sharma, S.; Dongol, D.M.S.; Shrestha, D.S.; Joshi, G.D.; Tomooka, N. Collection and conservation of leguminous crops and their wild relatives in Western Nepal from October 29 to November 10, 2016. AREIPGR 2017, 33, 295–329. [Google Scholar]
- Anonymous. Food and Agricultural Organization of the United Nations. 2019. Available online: https://www.fao.org/faostat/en/ (accessed on 16 May 2023).
- Kitano-Okada, T.; Ito, A.; Koide, A.; Nakamura, Y.; Han, K.H.; Shimada, K.; Fukushima, M. Anti-obesity role of adzuki bean extract containing polyphenols: In vivo and in vitro effects. J. Sci. Food Agric. 2012, 92, 2644–2651. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, X.; Ren, G. α-Glucosidase inhibitory activity of protein-rich extracts from extruded adzuki bean in diabetic KK-Ay mice. Food Funct. 2014, 5, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, B.; Zheng, X. Bioactive ingredients in adzuki bean sprouts. J. Med. Plants Res. 2011, 5, 5894–5898. [Google Scholar]
- Liu, R.; Zheng, Y.; Cai, Z.; Xu, B. Saponins and flavonoids from adzuki bean (Vigna angularis L.) ameliorate high-fat diet-induced obesity in ICR mice. Front. Pharmacol. 2017, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Chauhan, E.S. Adzuki beans-physical and nutritional characteristics of beans and its health benefits. Int. J. Health Sci. Res. 2019, 9, 304–310. [Google Scholar]
- Lin, P.Y.; Lai, H.M. Bioactive compounds in legumes and their germinated products. J. Agric. Food Chem. 2006, 54, 3807–3814. [Google Scholar] [CrossRef]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) seed coats attenuate vascular oxidative stress and inflammation in spontaneously hypertensive rats. J. Nutr. Biochem. 2011, 22, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Maruyama, N.; Salleh, M.R.M.; Mikami, B.; Utsumi, S. Characterization and crystallography of recombinant 7S globulins of Adzuki bean and structure− function relationships with 7S globulins of various crops. J. Agric. Food Chem. 2008, 56, 4145–4153. [Google Scholar] [CrossRef] [PubMed]
- Bednar, G.E.; Patil, A.R.; Murray, S.M.; Grieshop, C.M.; Merchen, N.R.; Fahey, G.C., Jr. Starch and fiber fractions in selected food and feed ingredients affect their small intestinal digestibility and fermentability and their large bowel fermentability in vitro in a canine mode. J. Nutr. 2001, 131, 276–286. [Google Scholar] [CrossRef]
- Oliva, M.E.; Ferreira, M.R.; Chicco, A.; Lombardo, Y.B. Dietary Salba (Salvia hispanica L) seed rich in α-linolenic acid improves adipose tissue dysfunction and the altered skeletal muscle glucose and lipid metabolism in dyslipidemic insulin-resistant rats. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 279–289. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Iroaganachi, M.A.; Kalu, W. Starch digestibility and predicted glycemic indices of raw and processed forms of hausa potato (S olenostemon rotundifolius poir). J. Food Biochem. 2017, 41, e12355. [Google Scholar] [CrossRef]
- Lestari, P.; Kang, Y.J.; Han, K.S.; Gwag, J.G.; Moon, J.K.; Kim, Y.H.; Lee, S.H. Genome-wide single nucleotide polymorphism discovery and validation in adzuki bean. Mol. Breed. 2014, 33, 497–501. [Google Scholar] [CrossRef]
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International; Agricultural Chemicals, Contaminants, Drugs; AOAC International: Gaithersburg, ML, USA, 1997; Volume I.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Nitesh, S.D.; Patroti, P.; Parashuram, S. TraitStasts: Statistical Data Analysis for Randomized Block Design Experiments, Version 1.0.2; R Package; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R 4.2.3 Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to ‘ggplot2’. 2022. Available online: https://github.com/ggobi/ggally (accessed on 28 October 2023).
- Kassambara, A. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Padhi, S.R.; Bartwal, A.; John, R.; Tripathi, K.; Gupta, K.; Wankhede, D.P.; Mishra, G.P.; Kumar, S.; Archak, S.; Bhardwaj, R. Evaluation and Multivariate Analysis of Cowpea [Vigna unguiculata (L.) Walp] Germplasm for Selected Nutrients—Mining for Nutri-Dense Accessions. Front. Sustain. Food Syst. 2022, 6, 888041. [Google Scholar] [CrossRef]
- Bhatt, K.C.; Malav, P.K.; Gore, P.G.; Tripathi, K.; Rathi, R.S.; Tiwari, U.L.; Ahlawat, S.P. A note on distribution and potential of Japanese wild adzuki bean [Vigna angularis var. nipponensis (Ohwi) Ohwi and H. Ohashi] in India. Genet. Resour. Crop Evol. 2021, 68, 2157–2166. [Google Scholar] [CrossRef]
- Gohara, A.K.; Souza, A.H.P.D.; Gomes, S.T.M.; Souza, N.E.D.; Visentainer, J.V.; Matsushita, M. Nutritional and bioactive compounds of adzuki beans cultivars using chemometric approach. Ciência E Agrotecnol. 2016, 40, 104–113. [Google Scholar] [CrossRef]
- Hayakawa, I.; Breene, W.M. A study on the relationship between cooking properties of adzuki bean and storage conditions. J. Fac. Agr.-Kyushu Univ. 1982, 27, 83–88. [Google Scholar] [CrossRef]
- Yousif, A.M.; Kato, J.; Deeth, H.C. Effect of storage on the biochemical structure and processing quality of adzuki bean (Vigna angularis). Food Rev. Int. 2007, 23, 1–33. [Google Scholar] [CrossRef]
- Michikawa, K.; Kobayashi, R.; Watanabe, T. Comparison of physical and chemical properties of “An” (Bean Jam) prepared from Otebo (Phaseolus vulgaris) and Oshirahand (Phaseolus coccineus). J. Jap. Soc. Food Sci. Technol. 1992, 39, 663–670. [Google Scholar] [CrossRef]
- International Seed Testing Association. International Rules for Seed Testing. Rules 1999; CABI: Wallingford, UK, 1999. [Google Scholar]
- Yadav, U.; Singh, N.; Kaur, A.; Thakur, S. Physico-chemical, hydration, cooking, textural and pasting properties of different adzuki bean (Vigna angularis) accessions. J. Food Sci. Technol. 2018, 55, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Moongngarm, A. Chemical compositions and resistant starch content in starchy foods. Am. J. Agric. Biol. Sci. 2013, 8, 107–113. [Google Scholar] [CrossRef]
- Jide, A.O.; Alademeyin, J.O.; Ajongbolo, K.F. Adzuki (Vigna angularis) beans as food: Chemical composition, nutrition and quality identities. J. Adv. Educ. Sci. 2023, 3, 05–08. [Google Scholar]
- Ukom, A.N.; Egejuru, F.C.; Ojimelukwe, P.C.; Fabian, U.U. Effect of Plant Ash on Nutrient Quality and Antioxidant Activity of African Yam Bean Seed Porridge in Relation to Cooking Time. Food Nutr. Sci. 2020, 11, 591–602. [Google Scholar] [CrossRef]
- Wu, X.; Xue, W.; Zhang, H. Study on Quality Evaluation of Different Adzuke Beans. J. Chin. Cereals Oils Assoc. 2011, 26, 20–24. [Google Scholar]
- Shi, Z.; Yao, Y.; Zhu, Y.; Ren, G. Nutritional composition and biological activities of 17 Chinese adzuki bean (Vigna angularis) varieties. Food Agric. Immunol. 2017, 28, 78–89. [Google Scholar] [CrossRef]
- Johnson, J.B.; Neupane, P.; Bhattarai, S.P.; Trotter, T.; Naiker, M. Partitioning of nutritional and phytochemical constituents in nine Adzuki bean genotypes from Australia. J. Agric. Food Res. 2022, 10, 100398. [Google Scholar] [CrossRef]
- Longvah, T.; Anantan, I.; Bhaskarachary, K.; Venkaiah, K.; Longvah, T. Indian Food Composition Tables; National Institute of Nutrition, Indian Council of Medical Research: Hyderabad, India, 2017; pp. 2–58.
- Durak, A.; Baraniak, B.; Jakubczyk, A.; Świeca, M. Biologically active peptides obtained by enzymatic hydrolysis of Adzuki bean seeds. Food Chem. 2013, 141, 2177–2183. [Google Scholar] [CrossRef]
- Philadelpho, B.; Souza, V.; Souza, F.; Santos, J.; Batista, F.; Silva, M.; Ferreira, E. Chromatography-independent fractionation and newly identified molecular features of the adzuki bean (Vigna angularis Willd.) β-vignin protein. Int. J. Mol. Sci. 2021, 22, 3018. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.K.; Go, E.; Song, K.B. Application of adzuki bean starch in antioxidant films containing cocoa nibs extract. Polymers 2018, 10, 1210. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Khan, S. Baking and Nutritional Characteristics of Adzuki Beans and Its Health impacts. Interdiscip. J. Appl. Basic Subj. 2021, 1, 50–57. [Google Scholar]
- Yang, X.; Wang, L.; Ding, L.; Zhou, X.; Li, C.; Zhou, S. Effects of Different Processing Methods on Physicochemical Properties and Estimate Glycemic Index of Adzuki Bean (Vigna angularis) Powder. J. Chin. Cereals Oils Assoc. 2021, 36, 33–38. [Google Scholar]
- Han, K.H.; Iijuka, M.; Shimada, K.I.; Sekikawa, M.; Kuramochi, K.; Ohba, K.; Fukushima, M. Adzuki resistant starch lowered serum cholesterol and hepatic 3-hydroxy-3-methylglutaryl-CoA mRNA levels and increased hepatic LDL-receptor and cholesterol 7α-hydroxylase mRNA levels in rats fed a cholesterol diet. Br. J. Nutr. 2005, 94, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q.; Li, S.; Yue, Y.; Ma, Y.; Ren, G. Convenient food made of extruded adzuki bean attenuates inflammation and improves glycemic control in patients with type 2 diabetes: A randomized controlled trial. Ther. Clin. Risk Manag. 2018, 14, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hou, D.; Fu, Y.; Xue, Y.; Guan, X.; Shen, Q. Adzuki bean alleviates obesity and insulin resistance induced by a high-fat diet and modulates gut microbiota in mice. Nutrients 2021, 13, 3240. [Google Scholar] [CrossRef]
- Zhao, Q.; Hou, D.; Laraib, Y.; Xue, Y.; Shen, Q. Comparison of the effects of raw and cooked adzuki bean on glucose/lipid metabolism and liver function in diabetic mice. Cereal Chem. 2021, 98, 1081–1090. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Shi, Y.; Li, X.; Zhang, Y. Effects of drought stress on the physiological characteristics of different adzuki bean varieties at the seedling stage. Plant Sci. J. 2014, 32, 493–501. [Google Scholar]
- Tamang, J.P.; Thapa, N.; Bhalla, T.C.; Savitri. Ethnic fermented foods and beverages of India. In Ethnic Fermented Foods and Alcoholic Beverages of Asia; Tamang, J., Ed.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Gore, P.G.; Gupta, V.; Singh, R.; Tripathi, K.; Kumar, R.; Kumari, G.; Pratap, A. Insights into the genetic diversity of an underutilized Indian legume, Vigna stipulacea (Lam.) Kuntz., using morphological traits and microsatellite markers. PLoS ONE 2022, 17, e0262634. [Google Scholar] [CrossRef]
- Han, N.; Woo, K.S.; Lee, J.Y.; Song, S.B.; Lee, Y.Y.; Kim, M.; Kim, H.J. Comparison of physicochemical characteristics, functional compounds, and physiological activities in adzuki bean cultivars. J. Korean Soc. Food Sci. Nutr. 2022, 51, 428–438. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.; Raigond, P.; Sahu, C.; Mishra, U.N.; Sharma, S.; Lal, M.K. Phytic acid: Blessing in disguise, a prime compound required for both plant and human nutrition. Food Res. Int. 2021, 142, 110193. [Google Scholar] [CrossRef] [PubMed]
- Pramitha, J.L.; Rana, S.; Aggarwal, P.R.; Ravikesavan, R.; Joel, A.J.; Muthamilarasan, M. Diverse role of phytic acid in plants and approaches to develop low-phytate grains to enhance bioavailability of micronutrients. Adv. Genet. 2021, 107, 89–120. [Google Scholar] [PubMed]
- Banni, K.; Moe, K.T.; Park, Y.-J. Assessing genetic diversity, population structure and gene flow in the Korean red bean [Vigna angularis (Willd.) Ohwi & Ohashi] using SSR markers. Plant Genet. Resour. 2012, 10, 74–82. [Google Scholar]
- Yao, Y.; Cheng, X.; Wang, S.; Wang, L.; Ren, G. Influence of altitudinal variation on the antioxidant and antidiabetic potential of azuki bean (Vigna angularis). Int. J. Food Sci. Nutr. 2011, 63, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.G.; Deng, Z.; Yan, A.; Shah, N.P.; Lui, W.; Chan, C.; Corke, H. Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects. LWT-Food Sci. Technol. 2016, 73, 168–177. [Google Scholar] [CrossRef]
- Jeong, E.-W.; Park, S.-Y.; Yang, Y.-S.; Baek, Y.-J.; Yun, D.-M.; Kim, H.-J.; Go, G.-W.; Lee, H.-G. Black Soybean and Adzuki Bean Extracts Lower Blood Pressure by Modulating the Renin-Angiotensin System in Spontaneously Hypertensive Rats. Foods 2021, 10, 1571. [Google Scholar] [CrossRef]
- Cheng, A.; Wu, J.; Qin, H.; Yang, Q.; Liu, C.; Guo, X.; Sun, J. Content and Antioxidant Activity of Polyphenols and Flavonoids from 4 Food Beans. J. Chin. Oils Assoc. 2017, 32, 28–32. [Google Scholar]
- Hori, Y.; Sato, S.; Hatai, A. Antibacterial activity of plant extracts from azuki beans (Vigna angularis) in vitro. Phytother. Res. 2006, 20, 162–164. [Google Scholar] [CrossRef]
- Kim, E.-H.; Song, H.-K.; Park, Y.-J.; Lee, J.-R.; Kim, M.-Y.; Chung, I.-M. Determination of Phenolic Compounds in Adzuki bean (Vigna angularis) Germplasm. Korean J. Crop Sci. 2011, 56, 375–384. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Matemu, A.; Nakamura, S.; Katayama, S. Health benefits of antioxidative peptides derived from legume proteins with a high amino acid score. Antioxidants 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Seczyk, L.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a Cardioprotective Diet. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef] [PubMed]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between phenolic acids, proteins, and carbohydrates—Influence on dough and bread properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.M.; Amodio, M.L.; Babellahi, F.; de Chiara, M.L.; Rubio, J.M.A.; Colelli, G. Hyperspectral imaging and multivariate accelerated shelf-life testing (MASLT) approach for determining shelf life of rocket leaves. J. Food Eng. 2018, 238, 122–133. [Google Scholar] [CrossRef]
| Sources | Moisture (%) | Ash (%) | Protein (g/100 g) | Starch (g/100 g) | Sugar (g/100 g) | Phytate (g/100 g) | Phenols (g/100 g) | Antioxidant (mg/g GAE) |
|---|---|---|---|---|---|---|---|---|
| N | 206 | 206 | 206 | 206 | 206 | 206 | 206 | 206 |
| Mean | 9.39 | 2.40 | 20.9 | 39.8 | 6.13 | 1.21 | 0.39 | 15.9 |
| Minimum | 7.55 | 1.80 | 18.0 | 31.0 | 3.02 | 0.65 | 0.10 | 11.4 |
| Maximum | 13.5 | 4.21 | 23.9 | 43.9 | 8.17 | 1.43 | 0.59 | 19.6 |
| 1 SE at 5% | 0.23 | 0.01 | 0.47 | 0.53 | 0.19 | 0.00 | 0.01 | 0.39 |
| 2 CV (%) | 3.39 | 0.11 | 2.95 | 1.87 | 4.36 | 0.14 | 2.63 | 3.63 |
| 3 CD at 5% | 0.63 | 0.01 | 1.22 | 1.48 | 0.53 | 0.00 | 0.02 | 1.18 |
| Source of Variations | df | Moisture (g/100 g) | Ash (g/100 g) | Protein (g/100 g) | Starch (g/100 g) | Sugar (g/100 g) | Phytate (g/100 g) | Phenols (g/100 g) | Antioxidant (mg/g of GAE) |
|---|---|---|---|---|---|---|---|---|---|
| Replications | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Genotypes | 103 | 103 | 103 | 103 | 103 | 103 | 103 | 103 | 103 |
| MSS of replication | 1 | 0.03 | 0.00 * | 0.53 | 0.22 | 0.15 | 0.00 ** | 0.00 | 0.44 |
| MSS of genotypes | 102 | 2.64 *** | 0.52 *** | 2.26 *** | 8.51 *** | 2.88 *** | 0.04 *** | 0.01 *** | 5.94 *** |
| Error | 102 | 0.10 | 0.00 | 0.38 | 0.56 | 0.07 | 0.00 | 1 × 10−4 | 0.33 |
| CD | 0.63 | 0.005 | 1.22 | 1.48 | 0.53 | 0.003 | 0.0 | 1.18 |
| Parameters | GCV (%) | State | PCV (%) | State | H2 (%) | State | GAM (%) | State |
|---|---|---|---|---|---|---|---|---|
| Moisture | 11.99 | Moderate | 12.46 | Moderate | 92.61 | High | 23.78 | High |
| Ash | 21.07 | High | 21.07 | High | 99.99 | High | 43.41 | High |
| Protein | 4.63 | Low | 5.49 | Low | 71.15 | High | 8.05 | Low |
| Starch | 5.01 | Low | 5.34 | Low | 87.72 | High | 9.66 | Low |
| Sugar | 19.29 | Moderate | 19.78 | Moderate | 95.13 | High | 38.77 | High |
| Phytate | 11.37 | Moderate | 11.37 | Moderate | 99.98 | High | 23.43 | High |
| Phenols | 19.42 | Moderate | 19.60 | Moderate | 98.20 | High | 39.65 | High |
| Antioxidant | 10.60 | Moderate | 11.21 | Moderate | 89.50 | High | 20.67 | High |
| Parameters | PC 1 | PC 2 | PC 3 | PC 4 |
|---|---|---|---|---|
| Moisture | 0.27 | 17.21 | 14.23 | 6.44 |
| Ash | 2.04 | 33.11 | 0.02 | 1.62 |
| Protein | 25.12 | 0.75 | 0.68 | 47.5 |
| Starch | 0.54 | 8.44 | 45.33 | 0.58 |
| Sugar | 13.32 | 0.1 | 7.86 | 31.27 |
| Phytate | 2.43 | 30.46 | 6.68 | 10.88 |
| Phenols | 25.38 | 8.91 | 8.76 | 0.11 |
| Antioxidant | 30.86 | 0.98 | 16.4 | 1.57 |
| Subcluster | Count | Moisture (g/100 g) | Ash (g/100 g) | Protein (g/100 g) | Starch (g/100 g) | TSS (g/100 g) | Phenolics (g/100 g) | Phytate (g/100 g) | Antioxidant (mg/g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 8.9 a | 2.2 b | 21.1 ab | 40.0 b | 4.0 b | 0.396 ab | 1.24 a | 15.8 a |
| 2 | 24 | 9.3 a | 2.3 b | 20.6 b | 41.2 a | 6.3 a | 0.373 b | 1.18 a | 13.7 b |
| 3 | 6 | 9.8 a | 4.0 a | 20.9 ab | 39.3 bc | 6.3 a | 0.408 ab | 1.15 a | 16.0 a |
| 4 | 30 | 9.2 a | 2.4 b | 20.4 b | 38.1 c | 6.5 a | 0.432 a | 1.21 a | 16.4 a |
| 5 | 28 | 9.8 a | 2.3 b | 21.5 a | 40.3 ab | 6.7 a | 0.341 b | 1.25 a | 16.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D., D.D.; Padhi, S.R.; Gore, P.G.; Tripathi, K.; Katral, A.; Chandora, R.; Abhishek, G.J.; Kondal, V.; Singh, R.; Bharadwaj, R.; et al. Nutritional Potential of Adzuki Bean Germplasm and Mining Nutri-Dense Accessions through Multivariate Analysis. Foods 2023, 12, 4159. https://doi.org/10.3390/foods12224159
D. DD, Padhi SR, Gore PG, Tripathi K, Katral A, Chandora R, Abhishek GJ, Kondal V, Singh R, Bharadwaj R, et al. Nutritional Potential of Adzuki Bean Germplasm and Mining Nutri-Dense Accessions through Multivariate Analysis. Foods. 2023; 12(22):4159. https://doi.org/10.3390/foods12224159
Chicago/Turabian StyleD., Deepika D., Siddhant Ranjan Padhi, Padmavati G. Gore, Kuldeep Tripathi, Ashvinkumar Katral, Rahul Chandora, G. J. Abhishek, Vishal Kondal, Rakesh Singh, Rakesh Bharadwaj, and et al. 2023. "Nutritional Potential of Adzuki Bean Germplasm and Mining Nutri-Dense Accessions through Multivariate Analysis" Foods 12, no. 22: 4159. https://doi.org/10.3390/foods12224159






