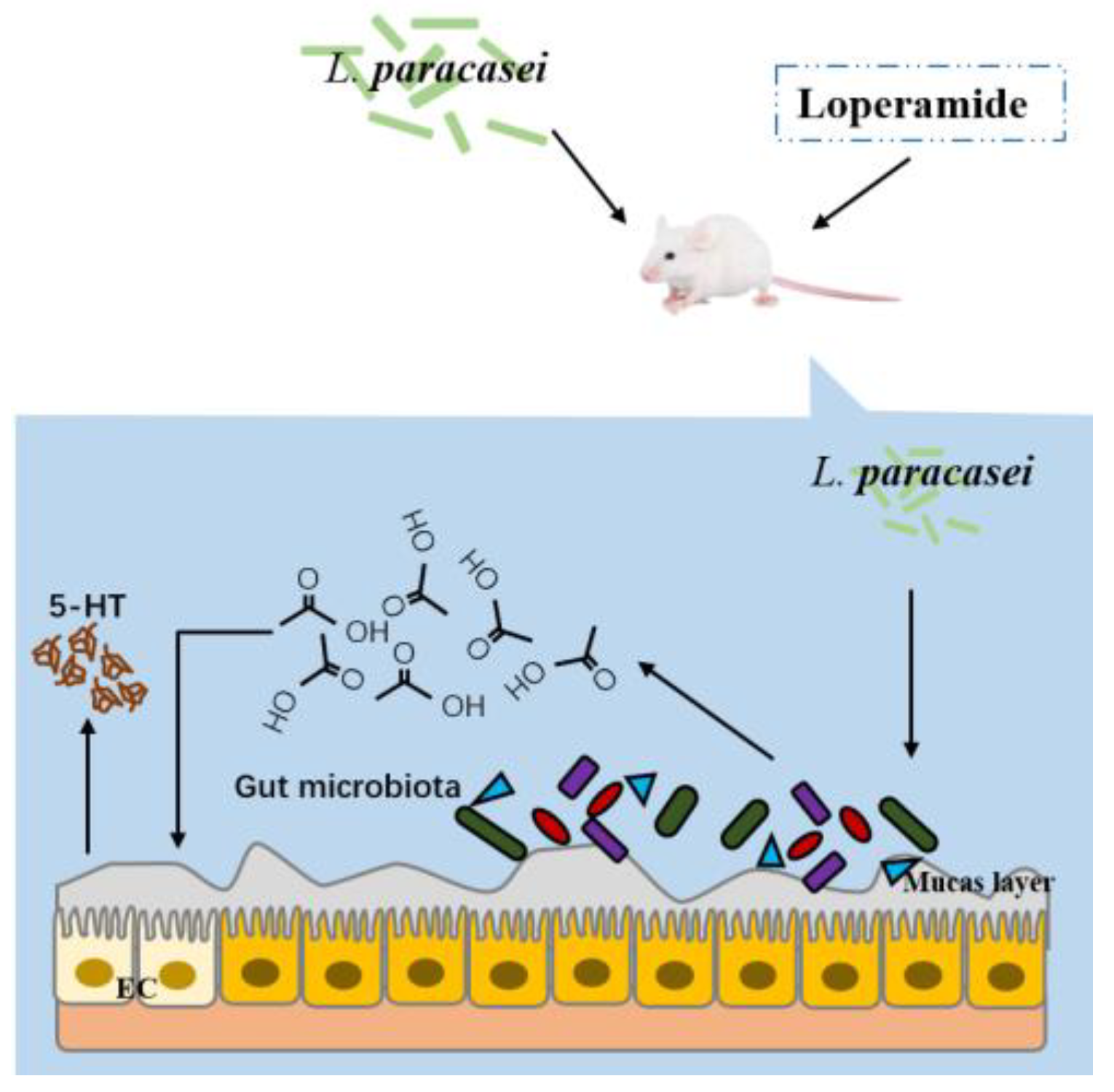
Lactobacillus paracasei Relieves Constipation by Acting on the Acetic Acid-5-HT-Intestinal Motility Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Bacteria Preparation
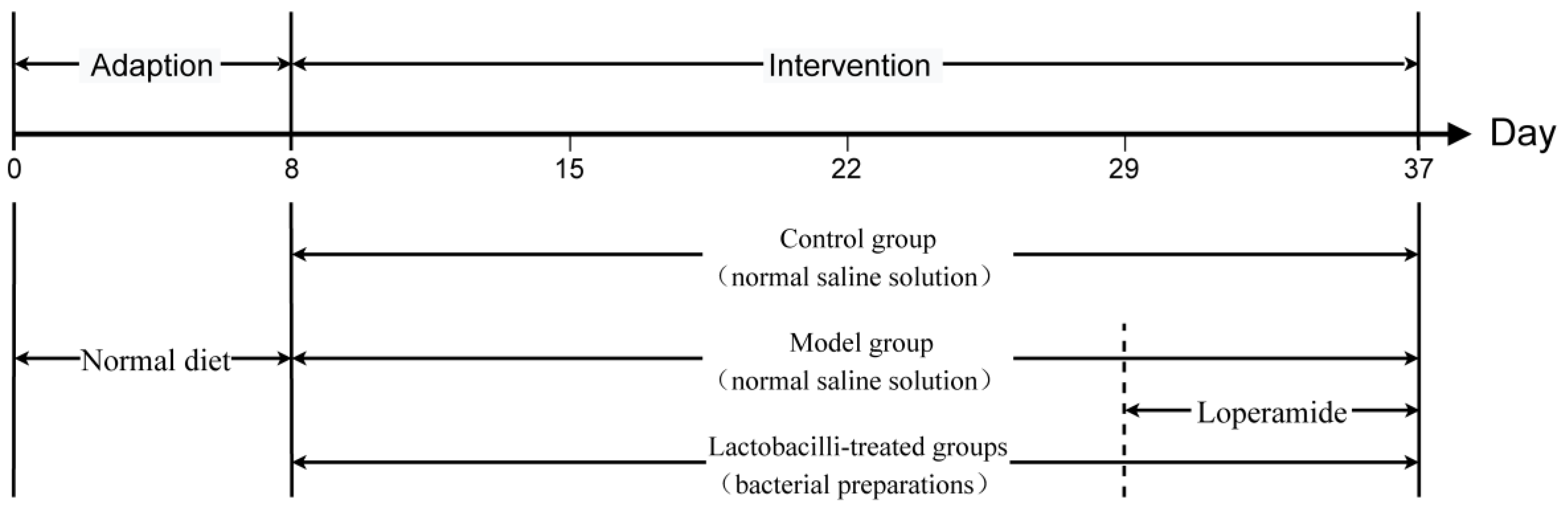
2.3. Animals and Experimental Design
2.4. Faecal Water Content
2.5. Time to First Black Defecation
2.6. Small Intestinal Transit Rate
2.7. Measurement of Gastrointestinal Regulatory Peptides
2.8. Histopathological Examination
2.9. Quantified Short-Chain Fatty Acids (SCFAs) in Feces
2.10. 16S rDNA Sequencing and Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
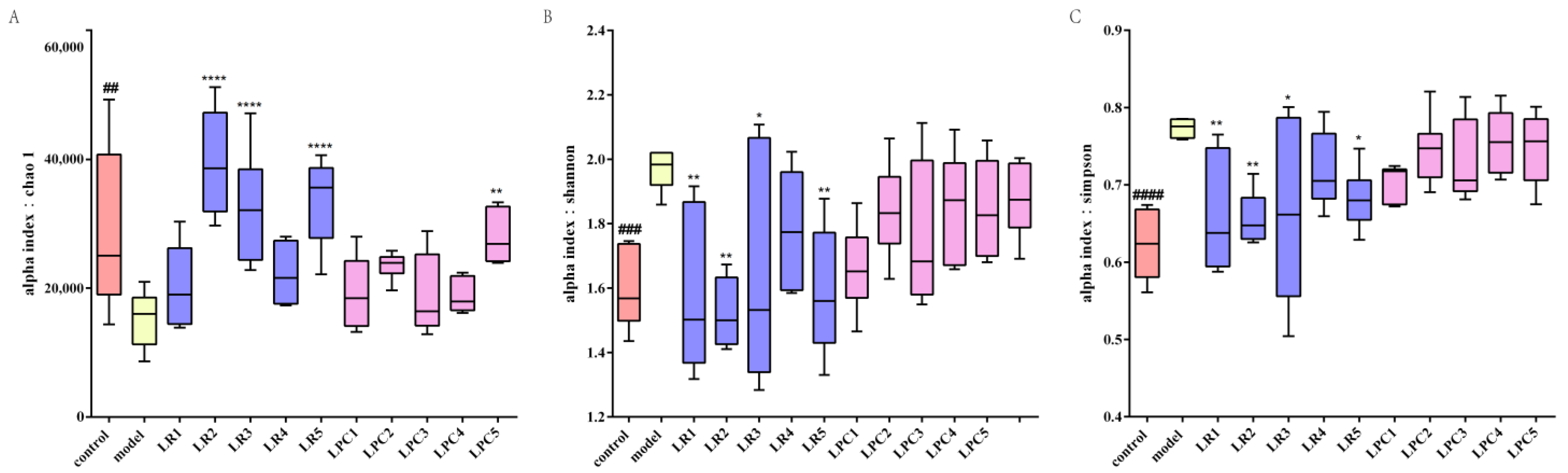
3.1. L. paracasei Relieved Constipation
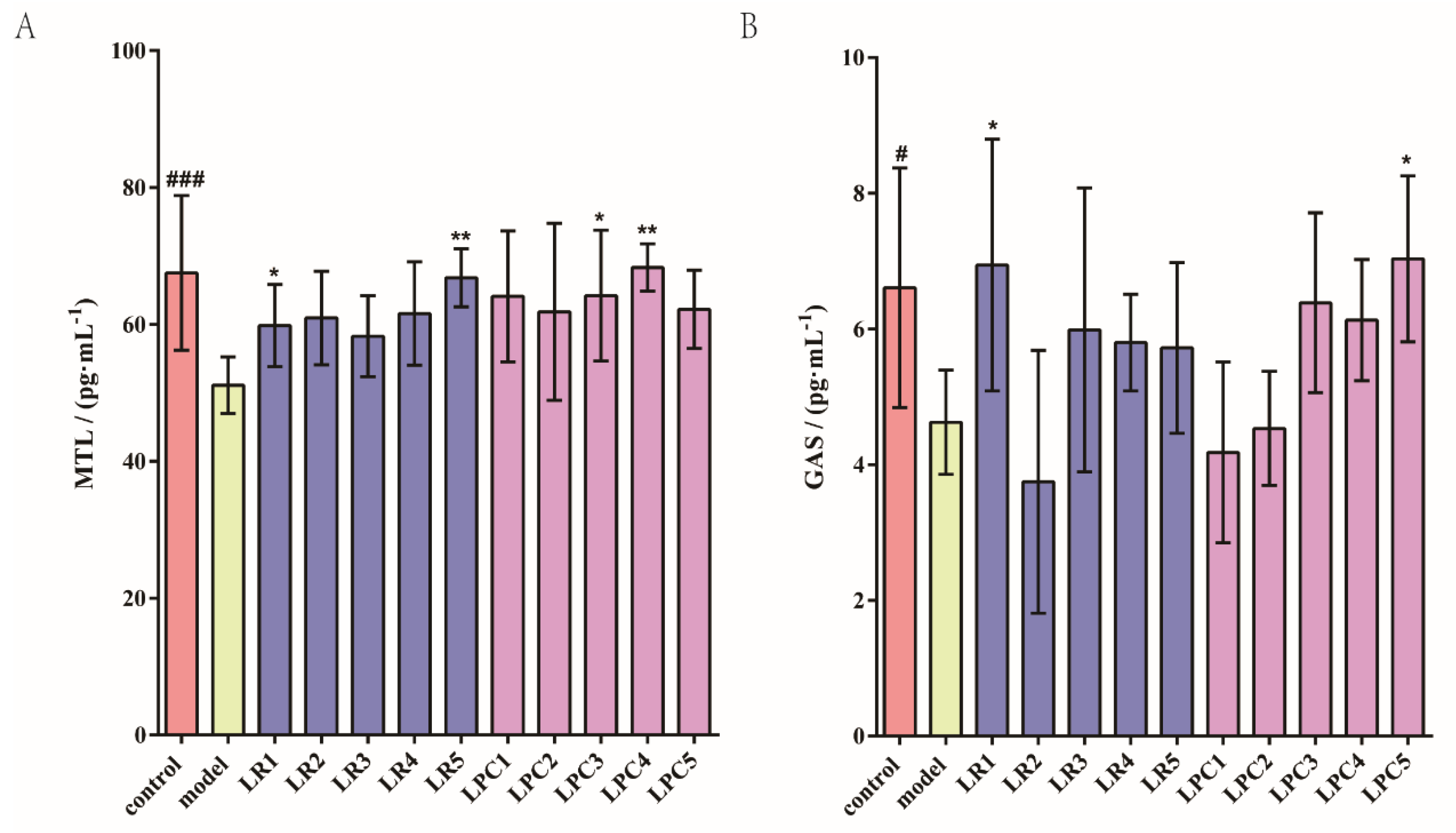
3.2. L. paracasei Had No Effect on MTL and Gas
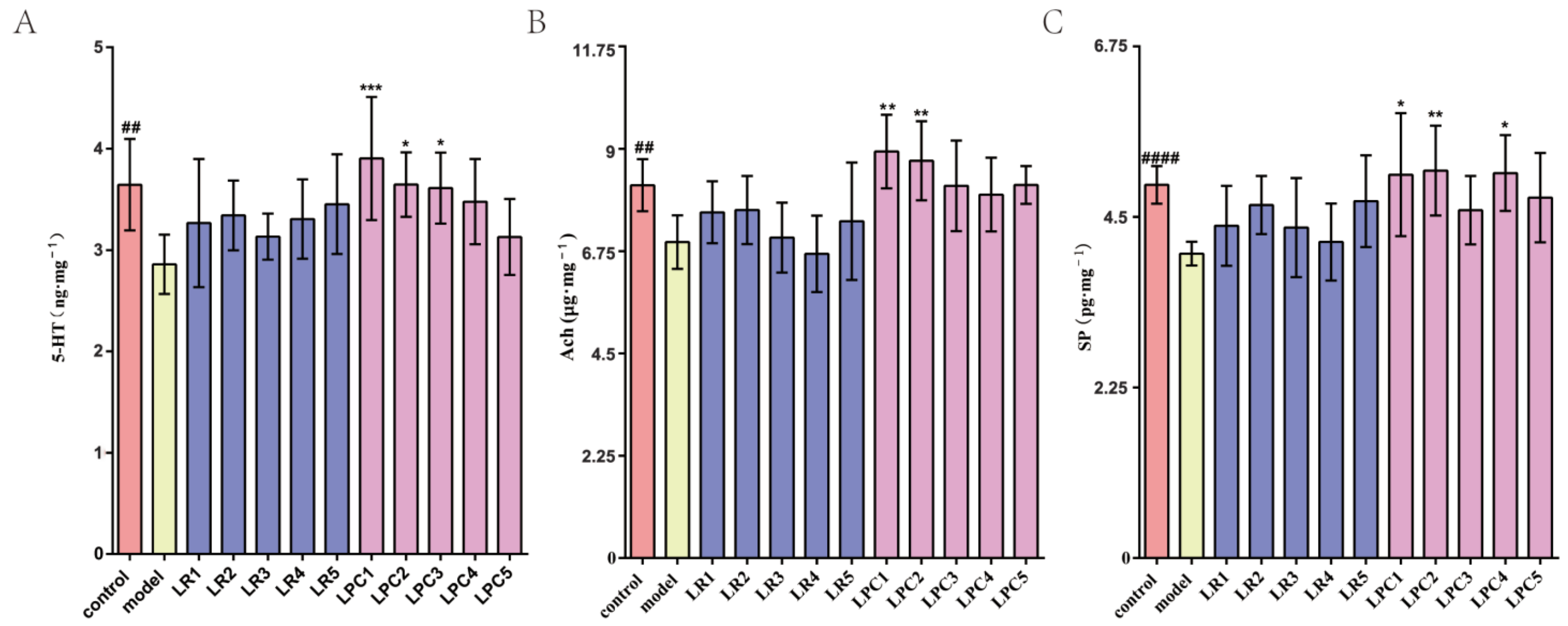
3.3. L. paracasei Up-Regulated the Level of Constipation-Related Gastrointestinal Neurotransmitters in the Colon of Mice
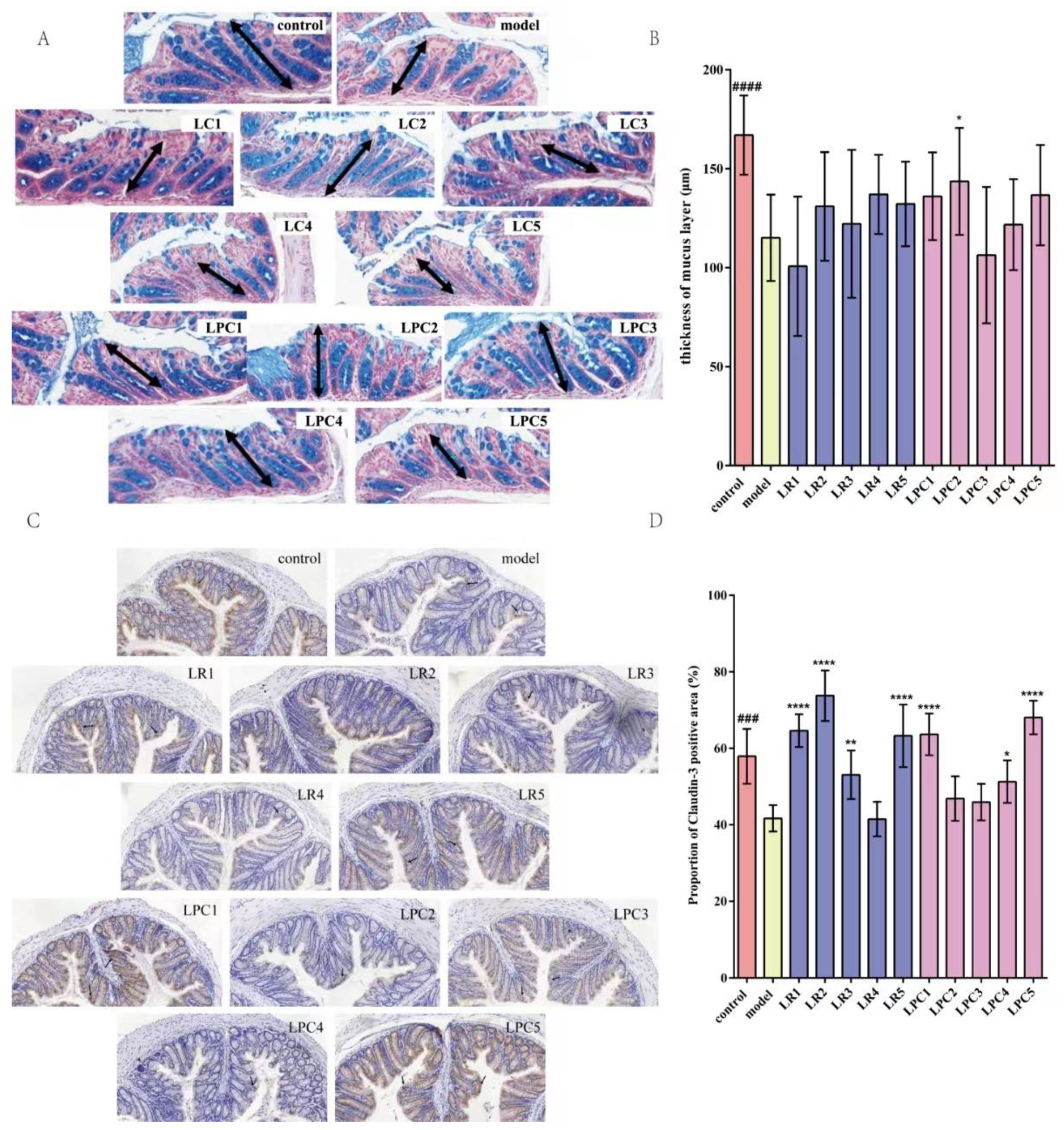
3.4. L. paracasei Up-Regulated Percentage of Positive Signal Area of Claudin-3 Protein
3.5. L. paracasei Increased the Level of Acetic Acid in Feces
3.6. L. paracasei Had a Greater Impact on 5-HT, SP and Ach Indices
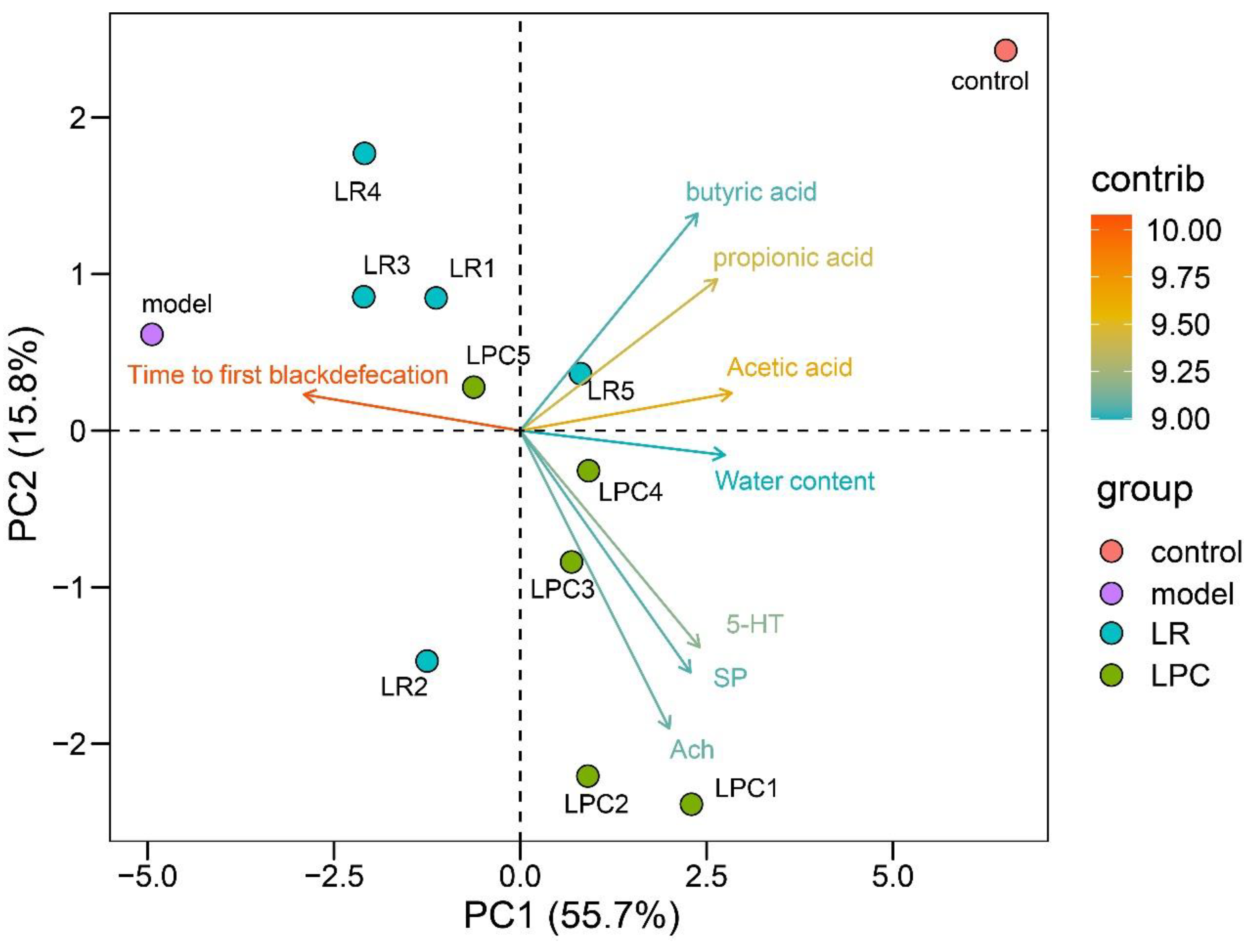
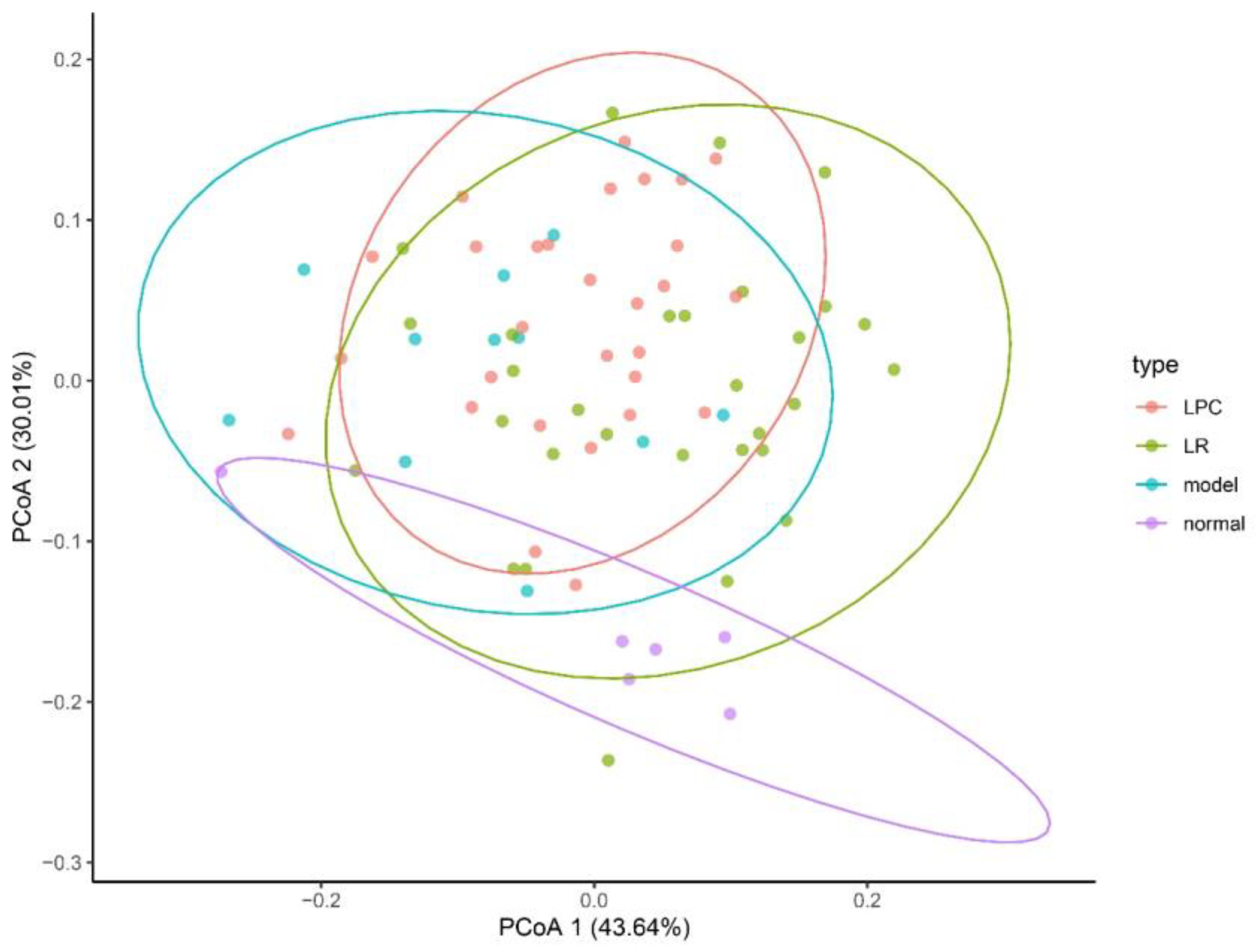
3.7. L. paracasei and L. reuteri Affected the Diversity of the Intestinal Microbiota in Constipated Mice
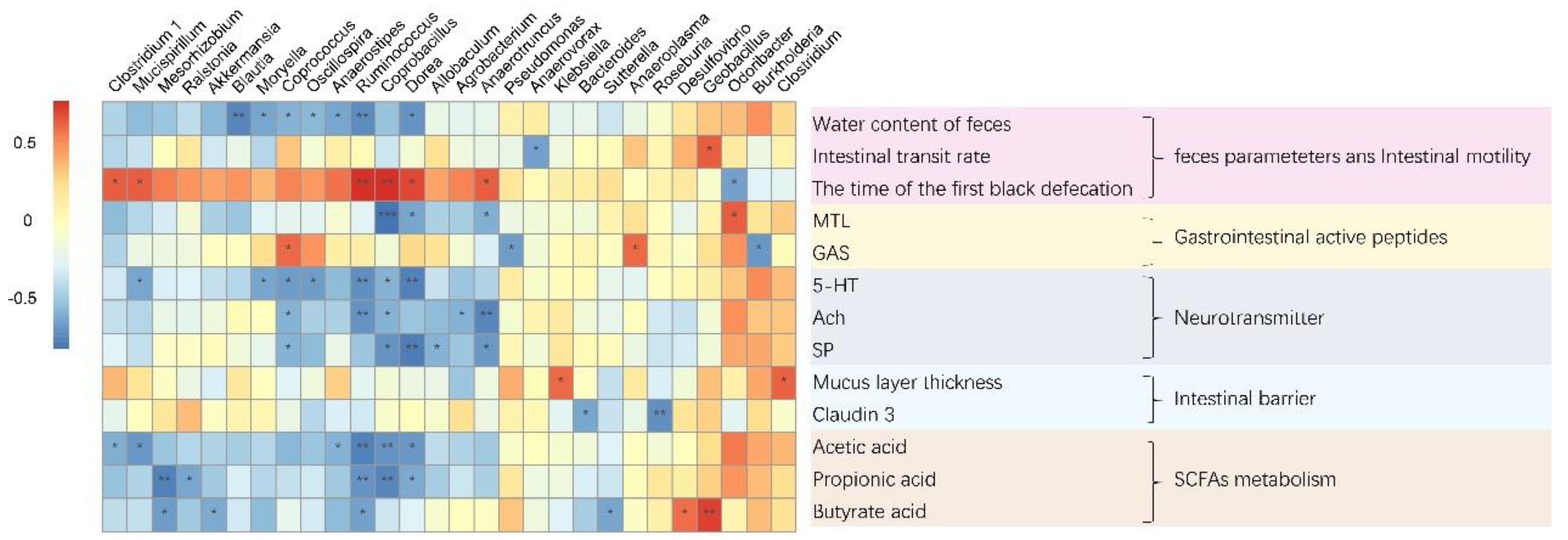
3.8. The Mechanism of L. paracasei to Relieve Constipation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global prevalence of functional constipation according to the Rome criteria: A systematic review and meta-analysis. Lancet. Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Werth, B. Epidemiology of constipation in adults: Why estimates of prevalence differ. J. Epidemiol. Res. 2019, 5, 37–41. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Fukui, H.; Eda, H.; Xu, X.; Kitayama, Y.; Hara, K.; Kodani, M.; Tomita, T.; Oshima, T.; Watari, J.; et al. Involvement of gut microbiota in the association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fukui, H.; Eda, H.; Kitayama, Y.; Hara, K.; Kodani, M.; Tomita, T.; Oshima, T.; Watari, J.; Miwa, H. Involvement of gut microbiota in the association between gastrointestinal motility and 5HT expression/M2 macrophage abundance in the gastrointestinal tract. Mol. Med. Rep. 2017, 16, 3482–3488. [Google Scholar] [CrossRef]
- Ge, X.; Zhao, W.; Ding, C.; Tian, H.; Xu, L.; Wang, H.; Ni, L.; Jiang, J.; Gong, J.; Zhu, W.; et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci. Rep. 2017, 7, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Inman, M.D.; Bienenstock, J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. J. Allergy Clin. Immunol. 2006, 117, S315. [Google Scholar] [CrossRef]
- Karimi, K.; Inman, M.D.; Bienenstock, J.; Forsythe, P. Lactobacillus reuteri induced regulatory T cells protect against an allergic airway response in mice. Am. J. Respir. Crit. Care Med. 2009, 179, 186–193. [Google Scholar] [CrossRef]
- Ojetti, V.; Ianiro, G.; Tortora, A.; D‘angelo, G.; Di Rienzo, T.A.; Bibbò, S.; Migneco, A.; Gasbarrini, A. The effect of Lactobacillus reuteri supplementation in adults with chronic functional constipation: A randomized, double-blind, placebo-controlled trial. J. Gastrointest. Liver Dis. 2014, 23, 387–391. [Google Scholar] [CrossRef]
- Riezzo, G.; Orlando, A.; D’attoma, B.; Linsalata, M.; Martulli, M.; Russo, F. Randomised double-blind placebo-controlled trial on Lactobacillus reuteri DSM 17938: Improvement in symptoms and bowel habit in functional constipation. Benef. Microbes 2018, 9, 51–59. [Google Scholar] [CrossRef]
- Ocaña, V.S.; Pesce de Ruiz Holgado, A.A.; Nader-Macías, M.E. Growth inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus paracasei subsp. paracasei isolated from the human vagina. FEMS Immunol. Med. Microbiol. 1999, 23, 87–92. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, R.; Liu, Y.; He, Y.; Li, S.; Yang, J.; Zhou, J.; Zhang, J. Genomics analysis of Lactobacillus paracasei SLP16. Lett. Appl. Microbiol. 2022, 75, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Lamine, F.; Chabo, C.; Theodorou, V.; Rochat, F.; Bergonzelli, G.E.; Corthésy-Theulaz, I.; Fioramonti, J.; Bueno, L. Synergy between Lactobacillus paracasei and Its Bacterial Products to Counteract Stress-Induced Gut Permeability and Sensitivity Increase in Rats. J. Nutr. 2007, 137, 1901–1907. [Google Scholar] [CrossRef]
- Verdú, E.F.; Bercík, P.; Bergonzelli, G.E.; Huang, X.-X.; Blennerhasset, P.; Rochat, F.; Fiaux, M.; Mansourian, R.; Corthésy-Theulaz, I.; Collins, S.M. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of post-infective gut dysfunction. Gastroenterology 2004, 127, 826–837. [Google Scholar] [CrossRef]
- Chen, C.; Chao, S.; Pan, T. Lactobacillus paracasei subsp. paracasei NTU 101 lyophilized powder improves loperamide-induced constipation in rats. Heliyon 2020, 6, e03804. [Google Scholar] [CrossRef]
- Valerio, F.; Russo, F.; de Candia, S.; Riezzo, G.; Orlando, A.; Lonigro, S.L.; Lavermicocca, P. Effects of probiotic Lactobacillus paracasei-enriched artichokes on constipated patients: A pilot study. J. Clin. Gastroenterol. 2010, 44, S49–S53. [Google Scholar] [CrossRef]
- Riezzo, G.; Orlando, A.; D’Attoma, B.; Guerra, V.; Valerio, F.; Lavermicocca, P.; De Candia, S.; Candia, S.; Russo, F. Randomised clinical trial: Efficacy of Lactobacillus paracaseiaseikes on constikes in the treatment of patients with functional constipation: A double-blind, controlled, crossover study. Aliment. Pharmacol. Ther. 2012, 35, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Tang, X.-D.; Yu, J.; Zhang, H.-Y.; Li, X.-R. Gut microbiota alterations from different Lactobacillus probiotic-fermented yoghurt treatments in slow-transit constipation. J. Funct. Foods 2017, 38, 110–118. [Google Scholar] [CrossRef]
- Chai, M.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Different Bifidobacterium bifidum strains change the intestinal flora composition of mice via different mechanisms to alleviate loperamide-induced constipation. Food Funct. 2021, 12, 6058–6069. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Ross, R.P.; Stanton, C.; Hou, B.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus casei CCFM1074 Alleviates Collagen-Induced Arthritis in Rats via Balancing Treg/Th17 and Modulating the Metabolites and Gut Microbiota. Front. Immunol. 2021, 12, 680073. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Xu, Q.; Yin, B.; Fang, D.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Exerts Strain-Specific Effects on Constipation Induced by Loperamide in BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Ghoshal, U.C. Chronic constipation in Rome IV era: The Indian perspective. Indian J. Gastroenterol. 2017, 36, 163–173. [Google Scholar] [CrossRef]
- Pagnini, C.; Di Paolo, M.C.; Urgesi, R.; Pallotta, L.; Fanello, G.; Graziani, M.G.; Delle Fave, G. Safety and Potential Role of Lactobacillus rhamnosus GG Administration as Monotherapy in Ulcerative Colitis Patients with Mild–Moderate Clinical Activity. Microorganisms 2023, 11, 1381. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.-H.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Kim, H.-S.; Seo, K.-H. Modulation of gut microbiota and increase in fecal water content in mice induced by ad-ministration of Lactobacillus kefiranofaciens DN1. Food Funct. 2017, 8, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Suo, H.; Wang, W.; Wang, H.; Zhang, Y.; Hu, Q.; Zhao, X.; Li, J. Preventive Effects of Different Fermentation Times of Shuidouchi on Diphenoxylate-Induced Constipation in Mice. Foods 2019, 8, 86. [Google Scholar] [CrossRef]
- Hellström, P.M.; Rosell, S. Effects of neurotensin, substance P and methionine-enkephalin on colonic motility. Acta Physiol. Scand. 1981, 113, 147–154. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells- A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef]
- Kendig, D.M.; Grider, J.R. Serotonin and colonic motility. Neurogastroenterol. Motil. 2015, 27, 899–905. [Google Scholar] [CrossRef]
- De Vadder, F.; Grasset, E.; Holm, L.M.; Karsenty, G.; MacPherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]


| Strains | Abbreviation | Sources |
|---|---|---|
| Lactobacillus reuteri FYNDL1-3 | LR1 | faeces |
| Lactobacillus reuteri FCQHC8L6 | LR2 | faeces |
| Lactobacillus reuteri FHNXY12L1 | LR3 | faeces |
| Lactobacillus reuteri FZJTZ20M3 | LR4 | faeces |
| Lactobacillus reuteri FCQNA25M2 | LR5 | faeces |
| Lactobacillus paracasei FJFZ2L5 | LPC1 | faeces |
| Lactobacillus paracasei FJSSZ3L1 | LPC2 | faeces |
| Lactobacillus paracasei FXJWS7M1 | LPC3 | faeces |
| Lactobacillus paracasei FNMHLBE11L4 | LPC4 | faeces |
| Lactobacillus paracasei VCQWX3-102L7 | LPC5 | pickled vegetable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, S.; Mei, C.; Tang, N.; Wang, J.; Yu, Q.; Wang, G.; Wu, G.; Zhao, J.; Chen, W. Lactobacillus paracasei Relieves Constipation by Acting on the Acetic Acid-5-HT-Intestinal Motility Pathway. Foods 2023, 12, 4176. https://doi.org/10.3390/foods12224176
Wang L, Yang S, Mei C, Tang N, Wang J, Yu Q, Wang G, Wu G, Zhao J, Chen W. Lactobacillus paracasei Relieves Constipation by Acting on the Acetic Acid-5-HT-Intestinal Motility Pathway. Foods. 2023; 12(22):4176. https://doi.org/10.3390/foods12224176
Chicago/Turabian StyleWang, Linlin, Shurong Yang, Chunxia Mei, Nan Tang, Jialiang Wang, Qiangqing Yu, Gang Wang, Gaojue Wu, Jianxin Zhao, and Wei Chen. 2023. "Lactobacillus paracasei Relieves Constipation by Acting on the Acetic Acid-5-HT-Intestinal Motility Pathway" Foods 12, no. 22: 4176. https://doi.org/10.3390/foods12224176
APA StyleWang, L., Yang, S., Mei, C., Tang, N., Wang, J., Yu, Q., Wang, G., Wu, G., Zhao, J., & Chen, W. (2023). Lactobacillus paracasei Relieves Constipation by Acting on the Acetic Acid-5-HT-Intestinal Motility Pathway. Foods, 12(22), 4176. https://doi.org/10.3390/foods12224176





