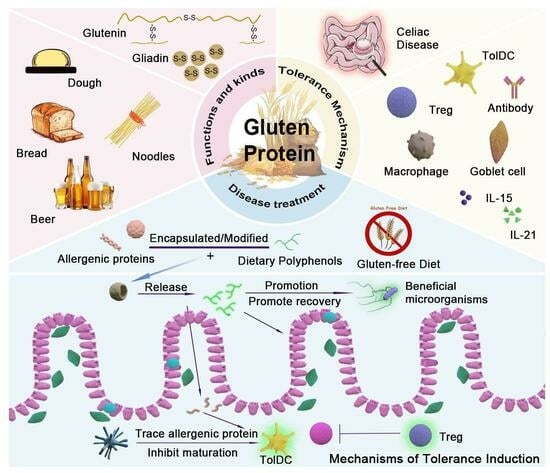
The Role of Gluten in Food Products and Dietary Restriction: Exploring the Potential for Restoring Immune Tolerance
Abstract
1. Introduction
2. Gluten
2.1. Classification of Gluten
2.2. The Role of Gluten in Food
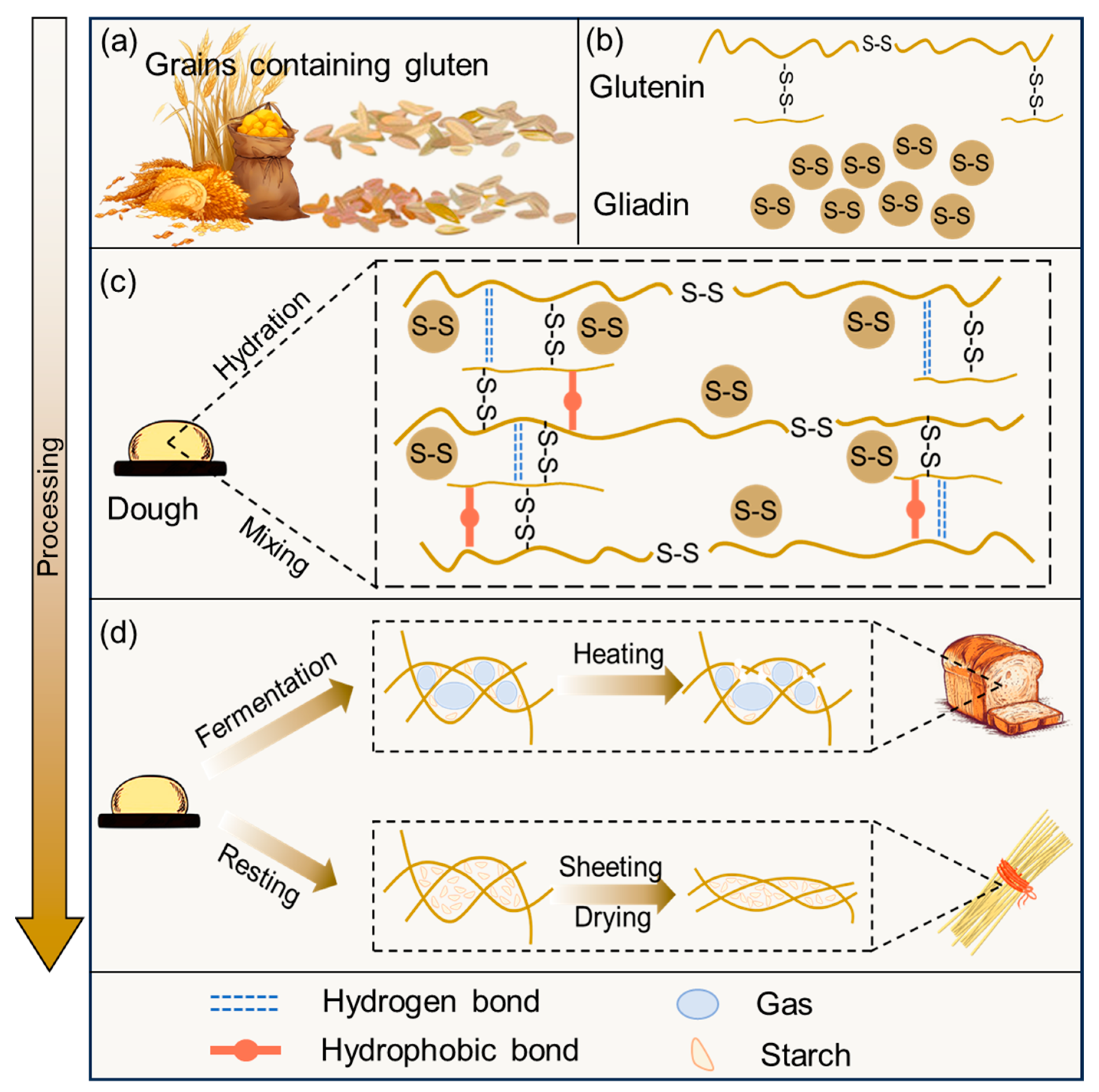
2.2.1. Dough
Formation of the Gluten Network
Rheological Properties of Dough
2.2.2. Bread
2.2.3. Noodles
2.2.4. Beer
2.3. Gluten Digestion
3. Gluten Protein Induces Celiac Disease and Restores Oral Tolerance
3.1. Pathological Mechanisms of CD
3.2. CD-Related Oral Tolerance Mechanisms
3.2.1. Treg
3.2.2. DCs
3.2.3. Goblet Cells
3.3. Reducing Gluten Diet Restrictions
4. Research Progress on Dietary Polyphenols Used in CD Treatment
4.1. Interaction Mechanism of Gluten and Dietary Polyphenol
4.2. Effect of Dietary Polyphenols on Tolerance Mechanism
4.3. The New Situation of the Interaction between Dietary Polyphenols and Gliadin
5. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shewry, P.R.; Halford, N.G. Cereal Seed Storage Proteins: Structures, Properties and Role in Grain Utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- This Vo Kientza, H. Who Discovered the Gluten and Who Discovered Its Production by Lixiviation? N3AF 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of Gluten Proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and Characterization of Gluten Protein Types from Wheat, Rye, Barley and Oats for Use as Reference Materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef] [PubMed]
- Lexhaller, B.; Colgrave, M.L.; Scherf, K.A. Characterization and Relative Quantitation of Wheat, Rye, and Barley Gluten Protein Types by Liquid Chromatography–Tandem Mass Spectrometry. Front. Plant Sci. 2019, 10, 1530. [Google Scholar] [CrossRef]
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-Gluten Uses and Industry Needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Mondoulet, L.; Paty, E.; Drumare, M.F.; Ah-Leung, S.; Scheinmann, P.; Willemot, R.M.; Wal, J.M.; Bernard, H. Influence of Thermal Processing on the Allergenicity of Peanut Proteins. J. Agric. Food Chem. 2005, 53, 4547–4553. [Google Scholar] [CrossRef]
- Gulati, P.; Li, A.; Holding, D.; Santra, D.; Zhang, Y.; Rose, D.J. Heating Reduces Proso Millet Protein Digestibility via Formation of Hydrophobic Aggregates. J. Agric. Food Chem. 2017, 65, 1952–1959. [Google Scholar] [CrossRef]
- Smith, F.; Pan, X.; Bellido, V.; Toole, G.A.; Gates, F.K.; Wickham, M.S.J.; Shewry, P.R.; Bakalis, S.; Padfield, P.; Mills, E.N.C. Digestibility of Gluten Proteins Is Reduced by Baking and Enhanced by Starch Digestion. Mol. Nutr. Food Res. 2015, 59, 2034–2043. [Google Scholar] [CrossRef]
- Kucek, L.K.; Veenstra, L.D.; Amnuaycheewa, P.; Sorrells, M.E. A Grounded Guide to Gluten: How Modern Genotypes and Processing Impact Wheat Sensitivity. Compr. Rev. Food Sci. Food Saf. 2015, 14, 285–302. [Google Scholar] [CrossRef]
- Kaukinen, K.; Peräaho, M.; Collin, P.; Partanen, J.; Woolley, N.; Kaartinen, T.; Nuutinen, T.; Halttunen, T.; Mäki, M.; Korponay-Szabo, I. Small-Bowel Mucosal Transglutaminase 2-Specific IgA Deposits in Coeliac Disease without Villous Atrophy: A Prospective and Randomized Clinical Study. Scand. J. Gastroenterol. 2005, 40, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Mention, J.J.; Ahmed, M.B.; Bègue, B.; Barbe, U.; Verkarre, V.; Asnafi, V.; Colombel, J.F.; Cugnenc, P.H.; Ruemmele, F.M.; McIntyre, E.; et al. Interleukin 15: A Key to Disrupted Intraepithelial Lymphocyte Homeostasis and Lymphomagenesis in Celiac Disease. Gastroenterology 2003, 125, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; Giacomin, P.; Navarro, S.; Clouston, A.; McCann, L.; Dougall, A.; Ferreira, I.; Susianto, A.; O’Rourke, P.; Howlett, M.; et al. Experimental Hookworm Infection and Gluten Microchallenge Promote Tolerance in Celiac Disease. J. Allergy Clin. Immunol. 2015, 135, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jin, Z.; Xu, X. Physicochemical Alterations of Wheat Gluten Proteins upon Dough Formation and Frozen Storage—A Review from Gluten, Glutenin and Gliadin Perspectives. Trends Food Sci. Technol. 2015, 46, 189–198. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, X.-H.; Zhang, Q.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. How Does a Celiac Iceberg Really Float? The Relationship between Celiac Disease and Gluten. Crit. Rev. Food Sci. Nutr. 2022, 63, 9233–9261. [Google Scholar] [CrossRef]
- Fido, R.J.; Békés, F.; Gras, P.W.; Tatham, A.S. Effects of α-, β-, γ- and ω-Gliadins on the Dough Mixing Properties of Wheat Flour. J. Cereal Sci. 1997, 26, 271–277. [Google Scholar] [CrossRef]
- Tatham, A.S.; Shewry, P.R. The Conformation of Wheat Gluten Proteins. The Secondary Structures and Thermal Stabilities of α-, β-, γ- and ω-Gliadins. J. Cereal Sci. 1985, 3, 103–113. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Zhou, J.; Yang, D.; Fu, L. Purification and Immunoglobulin E Epitopes Identification of Low Molecular Weight Glutenin: An Allergen in Chinese Wheat. Food Sci. Hum. Wellness 2023, 12, 720–727. [Google Scholar] [CrossRef]
- Ferranti, P.; Mamone, G.; Picariello, G.; Addeo, F. Mass Spectrometry Analysis of Gliadins in Celiac Disease. J. Mass Spectrom. 2007, 42, 1531–1548. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S. High Molecular Weight Subunits of Wheat Glutenin. J. Cereal Sci. 1992, 15, 105–120. [Google Scholar] [CrossRef]
- Bonilla, J.C.; Erturk, M.Y.; Kokini, J.L. Understanding the Role of Gluten Subunits (LMW, HMW Glutenins and Gliadin) in the Networking Behavior of a Weak Soft Wheat Dough and a Strong Semolina Wheat Flour Dough and the Relationship with Linear and Non-Linear Rheology. Food Hydrocoll. 2020, 108, 106002. [Google Scholar] [CrossRef]
- Hoseney, R.C.; Rogers, D.E. The Formation and Properties of Wheat Flour Doughs. Crit. Rev. Food Sci. Nutr. 1990, 29, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Carini, E.; Vittadini, E.; Curti, E.; Antoniazzi, F.; Viazzani, P. Effect of Different Mixers on Physicochemical Properties and Water Status of Extruded and Laminated Fresh Pasta. Food Chem. 2010, 122, 462–469. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, L.; Hong, Y.; Li, Z.; Li, C.; Gu, Z. An Extensive Review: How Starch and Gluten Impact Dough Machinability and Resultant Bread Qualities. Crit. Rev. Food Sci. Nutr. 2023, 63, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, C.; Békés, F.; Bushuk, W. (Eds.) Gliadin and Glutenin: The Unique Balance of Wheat Quality; AACC International, Inc.: St. Paul, MN, USA, 2006. [Google Scholar]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The Structure and Properties of Gluten: An Elastic Protein from Wheat Grain. Phil. Trans. R. Soc. Lond. B 2002, 357, 133–142. [Google Scholar] [CrossRef]
- Li, W.; Dobraszczyk, B.J.; Dias, A.; Gil, A.M. Polymer Conformation Structure of Wheat Proteins and Gluten Subfractions Revealed by ATR-FTIR. Cereal Chem. 2006, 83, 407–410. [Google Scholar] [CrossRef]
- Almutawah, A.; Barker, S.A.; Belton, P.S. Hydration of Gluten: A Dielectric, Calorimetric, and Fourier Transform Infrared Study. Biomacromolecules 2007, 8, 1601–1606. [Google Scholar] [CrossRef]
- Robertson, G.H.; Gregorski, K.S.; Cao, T.K. Changes in Secondary Protein Structures During Mixing Development of High Absorption (90%) Flour and Water Mixtures. Cereal Chem. 2006, 83, 136–142. [Google Scholar] [CrossRef]
- Georget, D.M.R.; Belton, P.S. Effects of Temperature and Water Content on the Secondary Structure of Wheat Gluten Studied by FTIR Spectroscopy. Biomacromolecules 2006, 7, 469–475. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Microstructure of Hydrated Gluten Network. Food Res. Int. 2011, 44, 2582–2586. [Google Scholar] [CrossRef]
- Edwards, N.M.; Mulvaney, S.J.; Scanlon, M.G.; Dexter, J.E. Role of Gluten and Its Components in Determining Durum Semolina Dough Viscoelastic Properties. Cereal Chem. 2003, 80, 755–763. [Google Scholar] [CrossRef]
- Sissons, M.J.; Soh, H.N.; Turner, M.A. Role of Gluten and Its Components in Influencing Durum Wheat Dough Properties and Spaghetti Cooking Quality. J. Sci. Food Agric. 2007, 87, 1874–1885. [Google Scholar] [CrossRef]
- Liu, R.; Xing, Y.; Zhang, Y.; Zhang, B.; Jiang, X.; Wei, Y. Effect of Mixing Time on the Structural Characteristics of Noodle Dough under Vacuum. Food Chem. 2015, 188, 328–336. [Google Scholar] [CrossRef]
- Gómez, A.; Ferrero, C.; Calvelo, A.; Añón, M.C.; Puppo, M.C. Effect of Mixing Time on Structural and Rheological Properties of Wheat Flour Dough for Breadmaking. Int. J. Food Prop. 2011, 14, 583–598. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, M.; Yang, T.; Ma, M.; Sun, Q.; Li, M. Evolution of the Morphological, Structural, and Molecular Properties of Gluten Protein in Dough with Different Hydration Levels during Mixing. Food Chem. X 2022, 15, 100448. [Google Scholar] [CrossRef]
- Sluková, M.; Levková, J.; Michalcová, A.; Horáčková, Š.; Skřivan, P. Effect of the Dough Mixing Process on the Quality of Wheat and Buckwheat Proteins. Czech J. Food Sci. 2017, 35, 522–531. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Cornillon, P.; Campanella, O.H.; Stroshine, R.L.; Lee, S.; Shim, J.-Y. Small and Large Deformation Rheology for Hard Wheat Flour Dough as Influenced by Mixing and Resting. J. Food Sci. 2008, 73, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cheng, L.; Hong, Y.; Li, Z.; Li, C.; Gu, Z. Combined Effects of Wheat Gluten and Carboxymethylcellulose on Dough Rheological Behaviours and Gluten Network of Potato–Wheat Flour-based Bread. Int. J. Food. Sci. Technol. 2021, 56, 4149–4158. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J.; Yang, S.; Feng, J.; Jia, F.; Zhang, C. Protein Degradation in Wheat Sourdough Fermentation with Lactobacillus Plantarum M616. Interdiscip. Sci. Comput. Life Sci. 2015, 7, 205–210. [Google Scholar] [CrossRef]
- Yue, Q.; Liu, C.; Li, L.; Zheng, X.; Bian, K. Effects of Fermentation on the Rheological Characteristics of Dough and the Quality of Steamed Bread. J. Food Process. Preserv. 2019, 43, e14115. [Google Scholar] [CrossRef]
- Sun, X.; Wu, S.; Li, W.; Koksel, F.; Du, Y.; Sun, L.; Fang, Y.; Hu, Q.; Pei, F. The Effects of Cooperative Fermentation by Yeast and Lactic Acid Bacteria on the Dough Rheology, Retention and Stabilization of Gas Cells in a Whole Wheat Flour Dough System—A Review. Food Hydrocoll. 2023, 135, 108212. [Google Scholar] [CrossRef]
- Rezaei, M.N.; Jayaram, V.B.; Verstrepen, K.J.; Courtin, C.M. The Impact of Yeast Fermentation on Dough Matrix Properties. J. Sci. Food Agric. 2016, 96, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, Y.; Liu, T.; Zhang, B.; Wang, Z.; Zhang, C. Change in Glutenin Macropolymer Secondary Structure in Wheat Sourdough Fermentation by FTIR. Interdiscip. Sci. Comput. Life Sci. 2017, 9, 247–253. [Google Scholar] [CrossRef]
- Nutter, J.; Saiz, A.I.; Iurlina, M.O. Microstructural and Conformational Changes of Gluten Proteins in Wheat-Rye Sourdough. J. Cereal Sci. 2019, 87, 91–97. [Google Scholar] [CrossRef]
- Zúñiga, R.; Le-Bail, A. Assessment of Thermal Conductivity as a Function of Porosity in Bread Dough during Proving. Food Bioprod. Process. 2009, 87, 17–22. [Google Scholar] [CrossRef]
- Gan, Z.; Ellis, P.R.; Schofield, J.D. Gas Cell Stabilisation and Gas Retention in Wheat Bread Dough. J. Cereal Sci. 1995, 21, 215–230. [Google Scholar] [CrossRef]
- Lefebvre, J.; Popineau, Y.; Deshayes, G.; Lavenant, L. Temperature-Induced Changes in the Dynamic Rheological Behavior and Size Distribution of Polymeric Proteins for Glutens from Wheat Near-Isogenic Lines Differing in HMW Glutenin Subunit Composition. Cereal Chem. 2000, 77, 193–201. [Google Scholar] [CrossRef]
- Weegels, P.L.; De Groot, A.M.G.; Verhoek, J.A.; Hamer, R.J. Effects on Gluten of Heating at Different Moisture Contents. II. Changes in Physico-Chemical Properties and Secondary Structure. J. Cereal Sci. 1994, 19, 39–47. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Deleu, L.J.; Rombouts, I.; Delcour, J.A. Heat-Induced Network Formation between Proteins of Different Sources in Model Systems, Wheat-Based Noodles and Pound Cakes. Food Hydrocoll. 2018, 79, 352–370. [Google Scholar] [CrossRef]
- Gao, X.; Tong, J.; Guo, L.; Yu, L.; Li, S.; Yang, B.; Wang, L.; Liu, Y.; Li, F.; Guo, J.; et al. Influence of Gluten and Starch Granules Interactions on Dough Mixing Properties in Wheat (Triticum aestivum L.). Food Hydrocoll. 2020, 106, 105885. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of Thermal Properties and Behavior of Wheat Starch and Gluten on Their Interaction: A Review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, L.; Liu, Y.; Ma, Y.; Zhu, J.; Yang, Y.; Min, D.; Xie, Y.; Chen, M.; Tong, J.; et al. Novel Parameters Characterizing Size Distribution of A and B Starch Granules in the Gluten Network: Effects on Dough Stability in Bread Wheat. Carbohydr. Polym. 2021, 257, 117623. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Li, X.; Obadi, M.; Jiang, S.; Li, S.; Xu, B. Effects of Dough Resting Time on the Development of Gluten Network in Different Sheeting Directions and the Textural Properties of Noodle Dough. LWT 2021, 141, 110920. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, M.; Yang, T.; Li, M.; Sun, Q. Dynamic Distribution and Transition of Gluten Proteins during Noodle Processing. Food Hydrocoll. 2022, 123, 107114. [Google Scholar] [CrossRef]
- Obadi, M.; Li, Y.; Qi, Y.; Xu, B. The Resting Process: A Comprehensive Review of the Effects on Dough Properties and Resulting Noodles Quality, Including Improvement Strategies. Int. J. Food Sci. Technol. 2023, 58, 5637–5647. [Google Scholar] [CrossRef]
- An, D.; Li, H.; Li, D.; Zhang, D.; Huang, Y.; Obadi, M.; Xu, B. The Relation between Wheat Starch Properties and Noodle Springiness: From the View of Microstructure Quantitative Analysis of Gluten-Based Network. Food Chem. 2022, 393, 133396. [Google Scholar] [CrossRef]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat Gluten Functionality as a Quality Determinant in Cereal-Based Food Products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- Lin, Q.; Shen, H.; Ma, S.; Zhang, Q.; Yu, X.; Jiang, H. Morphological Distribution and Structure Transition of Gluten Induced by Various Drying Technologies and Its Effects on Chinese Dried Noodle Quality Characteristics. Food Bioprocess Technol. 2023, 16, 1374–1387. [Google Scholar] [CrossRef]
- Gan, Q.; Howell, J.A.; Field, R.W.; England, R.; Bird, M.R.; O’Shaughnessy, C.L.; MeKechinie, M.T. Beer Clarification by Microfiltration—Product Quality Control and Fractionation of Particles and Macromolecules. J. Membr. Sci. 2001, 194, 185–196. [Google Scholar] [CrossRef]
- Siebert, K.J. Haze Formation in Beverages. LWT—Food Sci. Technol. 2006, 39, 987–994. [Google Scholar] [CrossRef]
- Baiano, A. Craft Beer: An Overview. Comp. Rev. Food Sci. Food Saf. 2021, 20, 1829–1856. [Google Scholar] [CrossRef]
- Watson, H.G.; Decloedt, A.I.; Vanderputten, D.; Van Landschoot, A. Variation in Gluten Protein and Peptide Concentrations in Belgian Barley Malt Beers: Variation in Gluten Protein and Peptide Concentrations in Belgian Beers. J. Inst. Brew. 2018, 124, 148–157. [Google Scholar] [CrossRef]
- Bamforth, C.W. The Relative Significance of Physics and Chemistry for Beer Foam Excellence: Theory and Practice. J. Inst. Brew. 2004, 110, 259–266. [Google Scholar] [CrossRef]
- Evans, D.E.; Sheehan, M.C.; Stewart, D.C. The Impact of Malt Derived Proteins on Beer Foam Quality. Part II: The Influence of Malt Foam-Positive Proteins and Non-Starch Polysaccharides on Beer Foam Quality. J. Inst. Brew. 1999, 105, 171–178. [Google Scholar] [CrossRef]
- Di Ghionno, L.; Marconi, O.; Sileoni, V.; De Francesco, G.; Perretti, G. Brewing with Prolyl Endopeptidase from Aspergillus niger: The Impact of Enzymatic Treatment on Gluten Levels, Quality Attributes and Sensory Profile. Int. J. Food Sci. Technol. 2017, 52, 1367–1374. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-Evaluation of the Mechanisms of Dietary Fibre and Implications for Macronutrient Bioaccessibility, Digestion and Postprandial Metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef]
- Gojković Cvjetković, V.; Marjanović-Balaban, Ž.; Vujadinović, D.; Vukić, M.; Ivanović, M. Research of the Effect of Cold Atmospheric Plasma on Gluten Proteins from Gluten-Free Flour. J. Eng. Process. Manag. 2022, 14, 57–65. [Google Scholar] [CrossRef]
- Gojković Cvjetković, V.; Marjanović-Balaban, Ž.; Vujadinović, D.; Vukić, M.; Rajić, D. Investigation of the Effect of Cold Atmospheric Plasma on Gliadins and Glutenins Extracted from Wheat Flour Samples. Food Process. Preserv. 2022, 46, e15789. [Google Scholar] [CrossRef]
- Xiang, S.; Zou, H.; Liu, Y.; Ruan, R. Effects of Microwave Heating on the Protein Structure, Digestion Properties and Maillard Products of Gluten. J. Food Sci. Technol. 2020, 57, 2139–2149. [Google Scholar] [CrossRef]
- Kuang, J.; Xu, K.; Dang, B.; Zheng, W.; Yang, X.; Zhang, W.; Zhang, J.; Huang, J. Interaction with Wheat Starch Affect the Aggregation Behavior and Digestibility of Gluten Proteins. Int. J. Biol. Macromol. 2023, 253, 127066. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gregorio, M.R.; Días, R.; Mateus, N.; De Freitas, V. Identification and Characterization of Proteolytically Resistant Gluten-Derived Peptides. Food Funct. 2018, 9, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Velasco, A.; Alvarez-Ramirez, J.; Rodríguez-Huezo, E.; Meraz-Rodríguez, M.; Vernon-Carter, E.J.; Lobato-Calleros, C. Effect of the Preparation Method and Storage Time on the in Vitro Protein Digestibility of Maize Tortillas. J. Cereal Sci. 2018, 84, 7–12. [Google Scholar] [CrossRef]
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Wen, W.; Li, S.; Gu, Y.; Wang, S.; Wang, J. Effects of Starch on the Digestibility of Gluten under Different Thermal Processing Conditions. J. Agric. Food Chem. 2019, 67, 7120–7127. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Effects of Edible Plant Polyphenols on Gluten Protein Functionality and Potential Applications of Polyphenol–Gluten Interactions. Comp. Rev. Food Sci. Food Saf. 2020, 19, 2164–2199. [Google Scholar] [CrossRef]
- Wen, W.; Li, S.; Gu, Y.; Wang, S.; Wang, J. Effects of Dietary Fiber on the Digestion and Structure of Gluten under Different Thermal Processing Conditions. Food Hydrocoll. 2020, 108, 106080. [Google Scholar] [CrossRef]
- Kumagai, H.; Suda, A.; Sakurai, H.; Kumagai, H.; Arai, S.; Inomata, N.; Ikezawa, Z. Improvement of Digestibility, Reduction in Allergenicity, and Induction of Oral Tolerance of Wheat Gliadin by Deamidation. Biosci. Biotechnol. Biochem. 2007, 71, 977–985. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of Heat, pH and Shear on Digestibility and Antigenic Characteristics of Wheat Gluten. Eur. Food Res. Technol. 2016, 242, 1829–1836. [Google Scholar] [CrossRef]
- Ogilvie, O.; Roberts, S.; Sutton, K.; Gerrard, J.; Larsen, N.; Domigan, L. The Effect of Baking Time and Temperature on Gluten Protein Structure and Celiac Peptide Digestibility. Food Res. Int. 2021, 140, 109988. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Wang, B.; Yang, W.; Luo, S.; Zhao, Y.; Jiang, S.; Mu, D.; Zheng, Z. Formation of Macromolecules in Wheat Gluten/Starch Mixtures during Twin-screw Extrusion: Effect of Different Additives. J. Sci. Food Agric. 2017, 97, 5131–5138. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L.E. Effects of Chemical Composition and Baking on in Vitro Digestibility of Proteins in Breads Made from Selected Gluten-Containing and Gluten-Free Flours. Food Chem. 2017, 233, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xie, X.; Zhang, Y.; Duan, J.; Ma, M.; Wang, Y.; Qiu, D.; Lu, X.; Yang, G.; He, G. Effects of Cold Jet Atmospheric Pressure Plasma on the Structural Characteristics and Immunoreactivity of Celiac-Toxic Peptides and Wheat Storage Proteins. Int. J. Mol. Sci. 2020, 21, 1012. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, O.; Roberts, S.; Sutton, K.; Gerrard, J.; Larsen, N.; Domigan, L. The Effect of Dough Mixing Speed and Work Input on the Structure, Digestibility and Celiac Immunogenicity of the Gluten Macropolymer within Bread. Food Chem. 2021, 359, 129841. [Google Scholar] [CrossRef]
- Petitot, M.; Abecassis, J.; Micard, V. Structuring of Pasta Components during Processing: Impact on Starch and Protein Digestibility and Allergenicity. Trends Food Sci. Technol. 2009, 20, 521–532. [Google Scholar] [CrossRef]
- Fernandez-Feo, M.; Wei, G.; Blumenkranz, G.; Dewhirst, F.E.; Schuppan, D.; Oppenheim, F.G.; Helmerhorst, E.J. The Cultivable Human Oral Gluten-Degrading Microbiome and Its Potential Implications in Coeliac Disease and Gluten Sensitivity. Clin. Microbiol. Infect. 2013, 19, E386–E394. [Google Scholar] [CrossRef]
- Leffler, D.; Schuppan, D.; Pallav, K.; Najarian, R.; Goldsmith, J.D.; Hansen, J.; Kabbani, T.; Dennis, M.; Kelly, C.P. Kinetics of the Histological, Serological and Symptomatic Responses to Gluten Challenge in Adults with Coeliac Disease. Gut 2013, 62, 996–1004. [Google Scholar] [CrossRef]
- Abadie, V.; Kim, S.M.; Lejeune, T.; Palanski, B.A.; Ernest, J.D.; Tastet, O.; Voisine, J.; Discepolo, V.; Marietta, E.; Hawash, M.B.F.; et al. IL-15, Gluten and HLA-DQ8 Drive Tissue Destruction in Coeliac Disease. Nature 2020, 578, 600–604. [Google Scholar] [CrossRef]
- Ogilvie, O.; Roberts, S.; Sutton, K.; Domigan, L.; Larsen, N.; Gerrard, J.; Demarais, N. Proteomic Modelling of Gluten Digestion from a Physiologically Relevant Food System: A Focus on the Digestion of Immunogenic Peptides from Wheat Implicated in Celiac Disease. Food Chem. 2020, 333, 127466. [Google Scholar] [CrossRef]
- Sollid, L.M. The Roles of MHC Class II Genes and Post-Translational Modification in Celiac Disease. Immunogenetics 2017, 69, 605–616. [Google Scholar] [CrossRef]
- Bodd, M.; Ráki, M.; Tollefsen, S.; Fallang, L.E.; Bergseng, E.; Lundin, K.E.A.; Sollid, L.M. HLA-DQ2-Restricted Gluten-Reactive T Cells Produce IL-21 but Not IL-17 or IL-22. Mucosal Immunol. 2010, 3, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Frossi, B.; De Carli, M.; Calabrò, A. Coeliac Disease and Mast Cells. Int. J. Mol. Sci. 2019, 20, 3400. [Google Scholar] [CrossRef] [PubMed]
- Asri, N.; Rostami-Nejad, M.; Barzegar, M.; Nikzamir, A.; Rezaei-Tavirani, M.; Razzaghi, M.; Zali, M.R. Suppressive Mechanisms Induced by Tregs in Celiac Disease. Iran Biomed. J. 2020, 24, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Mucida, D. Oral Tolerance in the Absence of Naturally Occurring Tregs. J. Clin. Investig. 2005, 115, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Vorobjova, T.; Uibo, O.; Heilman, K.; Rägo, T.; Honkanen, J.; Vaarala, O.; Tillmann, V.; Ojakivi, I.; Uibo, R. Increased FOXP3 Expression in Small-Bowel Mucosa of Children with Coeliac Disease and Type I Diabetes Mellitus. Scand. J. Gastroenterol. 2009, 44, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Ochi, H.; Abraham, M.; Ishikawa, H.; Frenkel, D.; Yang, K.; Basso, A.S.; Wu, H.; Chen, M.-L.; Gandhi, R.; Miller, A.; et al. Oral CD3-Specific Antibody Suppresses Autoimmune Encephalomyelitis by Inducing CD4+CD25−LAP+ T Cells. Nat. Med. 2006, 12, 627–635. [Google Scholar] [CrossRef]
- Oida, T.; Weiner, H.L. TGF-β Induces Surface LAP Expression on Murine CD4 T Cells Independent of Foxp3 Induction. PLoS ONE 2010, 5, e15523. [Google Scholar] [CrossRef]
- Chen, M.-L.; Yan, B.-S.; Bando, Y.; Kuchroo, V.K.; Weiner, H.L. Latency-Associated Peptide Identifies a Novel CD4+CD25+ Regulatory T Cell Subset with TGFβ-Mediated Function and Enhanced Suppression of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2008, 180, 7327–7337. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Awasthi, A.; Carrier, Y.; Peron, J.P.S.; Bettelli, E.; Kamanaka, M.; Flavell, R.A.; Kuchroo, V.K.; Oukka, M.; Weiner, H.L. A Dominant Function for Interleukin 27 in Generating Interleukin 10–Producing Anti-Inflammatory T Cells. Nat. Immunol. 2007, 8, 1380–1389. [Google Scholar] [CrossRef]
- Ruane, D.T.; Lavelle, E.C. The Role of CD103+ Dendritic Cells in the Intestinal Mucosal Immune System. Front. Immun. 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Palová-Jelínková, L.; Rožková, D.; Pecharová, B.; Bártová, J.; Šedivá, A.; Tlaskalová-Hogenová, H.; Spíšek, R.; Tučková, L. Gliadin Fragments Induce Phenotypic and Functional Maturation of Human Dendritic Cells. J. Immunol. 2005, 175, 7038–7045. [Google Scholar] [CrossRef] [PubMed]
- Vorobjova, T. Increased Density of Tolerogenic Dendritic Cells in the Small Bowel Mucosa of Celiac Patients. WJG 2015, 21, 439. [Google Scholar] [CrossRef]
- Kheiri, F.; Rostami-Nejad, M.; Amani, D.; Sadeghi, A.; Moradi, A.; Aghamohammadi, E.; Sahebkar, A.; Zali, M.R. Expression of Tolerogenic Dendritic Cells in the Small Intestinal Tissue of Patients with Celiac Disease. Heliyon 2022, 8, e12273. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Nejad, M.; Kheiri, F.; Amani, D.; Ehsani-Ardakani, M. Tolerogenic Dendritic Cell, an Unknown Cell in Celiac Disease. EC Gastroenterol. Dig. Syst. 2020, 7, 1–7. [Google Scholar]
- Popat, S.; Hearle, N.; Wixey, J.; Hogberg, L.; Bevan, S.; Lim, W.; Stenhammar, L.; Houlston, R.S. Analysis of the CTLA4 Gene in Swedish Coeliac Disease Patients. Scand. J. Gastroenterol. 2002, 37, 28–31. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Crespo, J.F.; Cabanillas, B. Recent Advances in Cellular and Molecular Mechanisms of IgE-Mediated Food Allergy. Food Chem. 2023, 411, 135500. [Google Scholar] [CrossRef]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet Cells Deliver Luminal Antigen to CD103+ Dendritic Cells in the Small Intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Gustafsson, J.K.; Knoop, K.A.; McDonald, K.G.; Bidani, S.S.; Davis, J.E.; Floyd, A.N.; Hogan, S.P.; Hsieh, C.-S.; Newberry, R.D. Goblet Cell Associated Antigen Passages Support the Induction and Maintenance of Oral Tolerance. Mucosal Immunol. 2020, 13, 271–282. [Google Scholar] [CrossRef]
- Troncone, R.; Ferguson, A. Gliadin Presented via the Gut Induces Oral Tolerance in Mice. Clin. Exp. Immunol. 1988, 72, 284–287. [Google Scholar] [PubMed]
- Freitag, T.L.; Podojil, J.R.; Pearson, R.M.; Fokta, F.J.; Sahl, C.; Messing, M.; Andersson, L.C.; Leskinen, K.; Saavalainen, P.; Hoover, L.I.; et al. Gliadin Nanoparticles Induce Immune Tolerance to Gliadin in Mouse Models of Celiac Disease. Gastroenterology 2020, 158, 1667–1681.e12. [Google Scholar] [CrossRef]
- Ribeiro, M.; de Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Review of Structural Features and Binding Capacity of Polyphenols to Gluten Proteins and Peptides In Vitro: Relevance to Celiac Disease. Antioxidants 2020, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.L.; Bean, S.R.; Tilley, M.; Adrianos, S.L.; Awika, J.M. Interaction Mechanisms of Condensed Tannins (Proanthocyanidins) with Wheat Gluten Proteins. Food Chem. 2018, 245, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, J.; Zheng, B.; Lu, Q.; Chen, L. Effects of Matcha and Its Active Components on the Structure and Rheological Properties of Gluten. LWT 2020, 124, 109197. [Google Scholar] [CrossRef]
- Han, C.-W.; Ma, M.; Zhang, H.-H.; Li, M.; Sun, Q.-J. Progressive Study of the Effect of Superfine Green Tea, Soluble Tea, and Tea Polyphenols on the Physico-Chemical and Structural Properties of Wheat Gluten in Noodle System. Food Chem. 2020, 308, 125676. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, Y.; Yang, Y.; Zhao, J.; Zhang, Y.; Li, L.; Wang, Q.; Ming, J. Interaction between Wheat Gliadin and Quercetin under Different pH Conditions Analyzed by Multi-Spectroscopy Methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117937. [Google Scholar] [CrossRef]
- Mazzaracchio, P.; Tozzi, S.; Boga, C.; Forlani, L.; Pifferi, P.G.; Barbiroli, G. Interaction between Gliadins and Anthocyan Derivatives. Food Chem. 2011, 129, 1100–1107. [Google Scholar] [CrossRef]
- Dias, R.; Perez-Gregorio, M.R.; Mateus, N.; De Freitas, V. Interaction Study between Wheat-Derived Peptides and Procyanidin B3 by Mass Spectrometry. Food Chem. 2016, 194, 1304–1312. [Google Scholar] [CrossRef]
- Dias, R.; Brás, N.F.; Fernandes, I.; Pérez-Gregorio, M.; Mateus, N.; Freitas, V. Molecular Insights on the Interaction and Preventive Potential of Epigallocatechin-3-Gallate in Celiac Disease. Int. J. Biol. Macromol. 2018, 112, 1029–1037. [Google Scholar] [CrossRef]
- Dias, R.; Brás, N.F.; Pérez-Gregorio, M.; Fernandes, I.; Mateus, N.; Freitas, V. A Multi-Spectroscopic Study on the Interaction of Food Polyphenols with a Bioactive Gluten Peptide: From Chemistry to Biological Implications. Food Chem. 2019, 299, 125051. [Google Scholar] [CrossRef]
- Van Buiten, C.B.; Lambert, J.D.; Elias, R.J. Green Tea Polyphenols Mitigate Gliadin-Mediated Inflammation and Permeability in Vitro. Mol. Nutr. Food Res. 2018, 62, e1700879. [Google Scholar] [CrossRef]
- Rohn, S. Possibilities and Limitations in the Analysis of Covalent Interactions between Phenolic Compounds and Proteins. Food Res. Int. 2014, 65, 13–19. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, L.; Konrad, A.; Schoeb, T.; Elson, C.O. Curcumin Induces the Tolerogenic Dendritic Cell That Promotes Differentiation of Intestine-Protective Regulatory T Cells. Eur. J. Immunol. 2009, 39, 3134–3146. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Kawai, K.; Tsuno, N.H.; Okaji, Y.; Asakage, M.; Tsuchiya, T.; Yamada, J.; Sunami, E.; Osada, T.; Kitayama, J.; et al. Epigallocatechin Gallate Affects Human Dendritic Cell Differentiation and Maturation. J. Allergy Clin. Immunol. 2008, 121, 209–214. [Google Scholar] [CrossRef]
- Rahman, S.U.; Li, Y.; Huang, Y.; Zhu, L.; Feng, S.; Wu, J.; Wang, X. Treatment of Inflammatory Bowel Disease via Green Tea Polyphenols: Possible Application and Protective Approaches. Inflammopharmacology 2018, 26, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Svajger, U.; Obermajer, N.; Jeras, M. Dendritic Cells Treated with Resveratrol during Differentiation from Monocytes Gain Substantial Tolerogenic Properties upon Activation. Immunology 2010, 129, 525–535. [Google Scholar] [CrossRef] [PubMed]
- del Cornò, M.; Scazzocchio, B.; Masella, R.; Gessani, S. Regulation of Dendritic Cell Function by Dietary Polyphenols. Crit. Rev. Food Sci. Nutr. 2016, 56, 737–747. [Google Scholar] [CrossRef]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of Regulatory T Cells by Green Tea Polyphenol EGCG. Immunol. Lett. 2011, 139, 7–13. [Google Scholar] [CrossRef]
- De Santis, S.; Kunde, D.; Serino, G.; Galleggiante, V.; Caruso, M.L.; Mastronardi, M.; Cavalcanti, E.; Ranson, N.; Pinto, A.; Campiglia, P.; et al. Secretory Leukoprotease Inhibitor Is Required for Efficient Quercetin-Mediated Suppression of TNFα Secretion. Oncotarget 2016, 7, 75800–75809. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Malara, A.; Hambly, R.; Sweeney, C.M.; Kirby, B.; Fletcher, J.M.; Dunne, A. Naturally Derived Heme-Oxygenase 1 Inducers Attenuate Inflammatory Responses in Human Dendritic Cells and T Cells: Relevance for Psoriasis Treatment. Sci. Rep. 2018, 8, 10287. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Fletcher, J.M.; Dunne, A. Plant-Derived Polyphenols Modulate Human Dendritic Cell Metabolism and Immune Function via AMPK-Dependent Induction of Heme Oxygenase-1. Front. Immunol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Wang, H.-K.; Yeh, C.-H.; Iwamoto, T.; Satsu, H.; Shimizu, M.; Totsuka, M. Dietary Flavonoid Naringenin Induces Regulatory T Cells via an Aryl Hydrocarbon Receptor Mediated Pathway. J. Agric. Food Chem. 2012, 60, 2171–2178. [Google Scholar] [CrossRef]
- Banc, A.; Desbat, B.; Renard, D.; Popineau, Y.; Mangavel, C.; Navailles, L. Structure and Orientation Changes of Omega- and Gamma-Gliadins at the Air-Water Interface: A PM-IRRAS Spectroscopy and Brewster Angle Microscopy Study. Langmuir Acs J. Surf. Colloids 2007, 23, 13066–13075. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Li, M.; Yang, J.; Xiong, L.; Sun, Q. Fabrication and Characterization of Biocompatible Hybrid Nanoparticles from Spontaneous Co-Assembly of Casein/Gliadin and Proanthocyanidin. Food Hydrocoll. 2017, 73, 74–89. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, Y.; Luo, S.; Mu, D.; Li, X.; Zhong, X.; Jiang, S.; Zheng, Z. Encapsulation of Curcumin in Soluble Soybean Polysaccharide-Coated Gliadin Nanoparticles: Interaction, Stability, Antioxidant Capacity, and Bioaccessibility. J. Sci. Food Agric. 2022, 102, 5121–5131. [Google Scholar] [CrossRef]
- Wu, W.; Kong, X.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Fabrication and Characterization of Resveratrol-Loaded Gliadin Nanoparticles Stabilized by Gum Arabic and Chitosan Hydrochloride. LWT 2020, 129, 109532. [Google Scholar] [CrossRef]
- Ma, L.; Zou, L.; McClements, D.J.; Liu, W. One-Step Preparation of High Internal Phase Emulsions Using Natural Edible Pickering Stabilizers: Gliadin Nanoparticles/Gum Arabic. Food Hydrocoll. 2020, 100, 105381. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-Carotene in Wheat Gluten Nanoparticle-Xanthan Gum-Stabilized Pickering Emulsions: Enhancement of Carotenoid Stability and Bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (−)-Epigallocatechin-3-Gallate and Quercetin in Particle-Stabilized W/O/W Emulsion Gels: Controlled Release and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]




| Factors | Digestibility of Gluten | Mechanism of Influence | References | |
|---|---|---|---|---|
| Internal | The amino acid sequence of the proteins | The higher the proline content, the lower the digestibility | Gluten is rich in proline, making it difficult for enzymes to break down. | [73] |
| Protein folding and cross-linking | Reduce digestion | Tight protein folding or protein aggregation limits enzyme cleavage sites and affects gluten digestibility. | [74] | |
| External | Protease inhibitors | Reduce digestion | Protease inhibitors decrease protein digestibility by inactivating digestive proteases. | [75] |
| Starch | Improve digestion | Starch protects gluten from aggregation in water, disrupts the spatial structure of gluten, exposes more cleavage sites, and facilitates gluten digestion. | [76] | |
| Tannin | Reduce digestion | Tannins reduce gluten digestibility by denaturizing proteases, inhibiting intestinal amino acid transporters, and complex glutens. | [77] | |
| Dietary fiber | Reduce digestion | Dietary fiber surrounds gluten, creates a steric hindrance between gluten and proteases, and compresses gluten conformation, inhibiting proteolysis by proteases. | [78] | |
| Low pH | Improve digestion | Acidic deamidation of gluten occurs at low pH and is accompanied by partial hydrolysis of peptide bonds. | [79] | |
| Processing | Grind | Improve digestion | Cellular structures are split in grinding and the gluten matrix is exposed to the environment and hydrolases. | [68] |
| Shear | Unchanged | - | [80] | |
| Heat | Reduce digestion | Heating changes the degree of network interconnection within the thiol-rich gliadin and thus the structure of gluten in bread. | [81] | |
| Extrusion | Improve digestion | Extrusion treatment increases the structural flexibility of wheat proteins and exposes more restriction sites. | [82] | |
| Fermentation | Improve digestion | Gas production and capture during fermentation maximize the separation of parallel protein chains and limit gluten cross-linking during baking. | [83] | |
| Cold atmospheric plasma | Improve digestion | Generating numerous high-energy excited atoms, photons, electrons, and reactive oxygen and nitrogen species modifies gluten to depolymerize gluten proteins, reducing the amount of large-sized protein polymers and decreasing immunoreactivity. | [84] |
| System | Polyphenol | Particle Size | Activity (Details of Research) | References |
|---|---|---|---|---|
| Nano-encapsulated particles | Proanthocyanidins | Around 30 nm | No cytotoxicity for normal liver cells Exhibited clear cytotoxicity against liver hepatocellular carcinoma | [122] |
| Curcumin | 196.66 nm | Increase bioavailability | [123] | |
| Resveratrol | Around 300 nm | Improve bioavailability Improve chemical stability Improve dissolution Improve antioxidant activity | [124] | |
| Micron emulsions | β-carotene | 251.3 ± 5.1 nm | Improve stability No effect on lipid digestion or carotenoid bioaccessibility | [125] |
| Wheat gluten nanoparticle–xanthan gum: 23.9 μm; wheat gluten nanoparticles: 9.4 μm | Effective protection from chemical degradation Increase bioavailability | [126] | ||
| Quercetin | - | Enhance solubility Bioavailability increased 4 times | [127] | |
| EGCG | Improve chemical stability Bioavailability increased 2 times |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Zheng, W.; Li, X.; Han, W.; Shen, J.; Lin, Q.; Hou, L.; Liao, L.; Zeng, X. The Role of Gluten in Food Products and Dietary Restriction: Exploring the Potential for Restoring Immune Tolerance. Foods 2023, 12, 4179. https://doi.org/10.3390/foods12224179
Ye L, Zheng W, Li X, Han W, Shen J, Lin Q, Hou L, Liao L, Zeng X. The Role of Gluten in Food Products and Dietary Restriction: Exploring the Potential for Restoring Immune Tolerance. Foods. 2023; 12(22):4179. https://doi.org/10.3390/foods12224179
Chicago/Turabian StyleYe, Li, Wenyu Zheng, Xue Li, Wenmin Han, Jialing Shen, Qiuya Lin, Liyan Hou, Lan Liao, and Xin’an Zeng. 2023. "The Role of Gluten in Food Products and Dietary Restriction: Exploring the Potential for Restoring Immune Tolerance" Foods 12, no. 22: 4179. https://doi.org/10.3390/foods12224179
APA StyleYe, L., Zheng, W., Li, X., Han, W., Shen, J., Lin, Q., Hou, L., Liao, L., & Zeng, X. (2023). The Role of Gluten in Food Products and Dietary Restriction: Exploring the Potential for Restoring Immune Tolerance. Foods, 12(22), 4179. https://doi.org/10.3390/foods12224179