Green Extraction of Hodgsonia heteroclita Oilseed Cake Powder to Obtain Optimal Antioxidants and Health Benefits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Optimization of Extraction Conditions Using Box–Behnken Design (BBD) and Response Surface Methodology (RSM)
2.3. Analyses of Phenolic Profile and Total Phenolic Contents
2.4. Determination of Antioxidant Potentials
2.5. In Vitro Enzyme Inhibitory Activities
2.6. Bacterial Reverse Mutation Test (Ames Test)
2.7. Statistical Analysis
3. Results
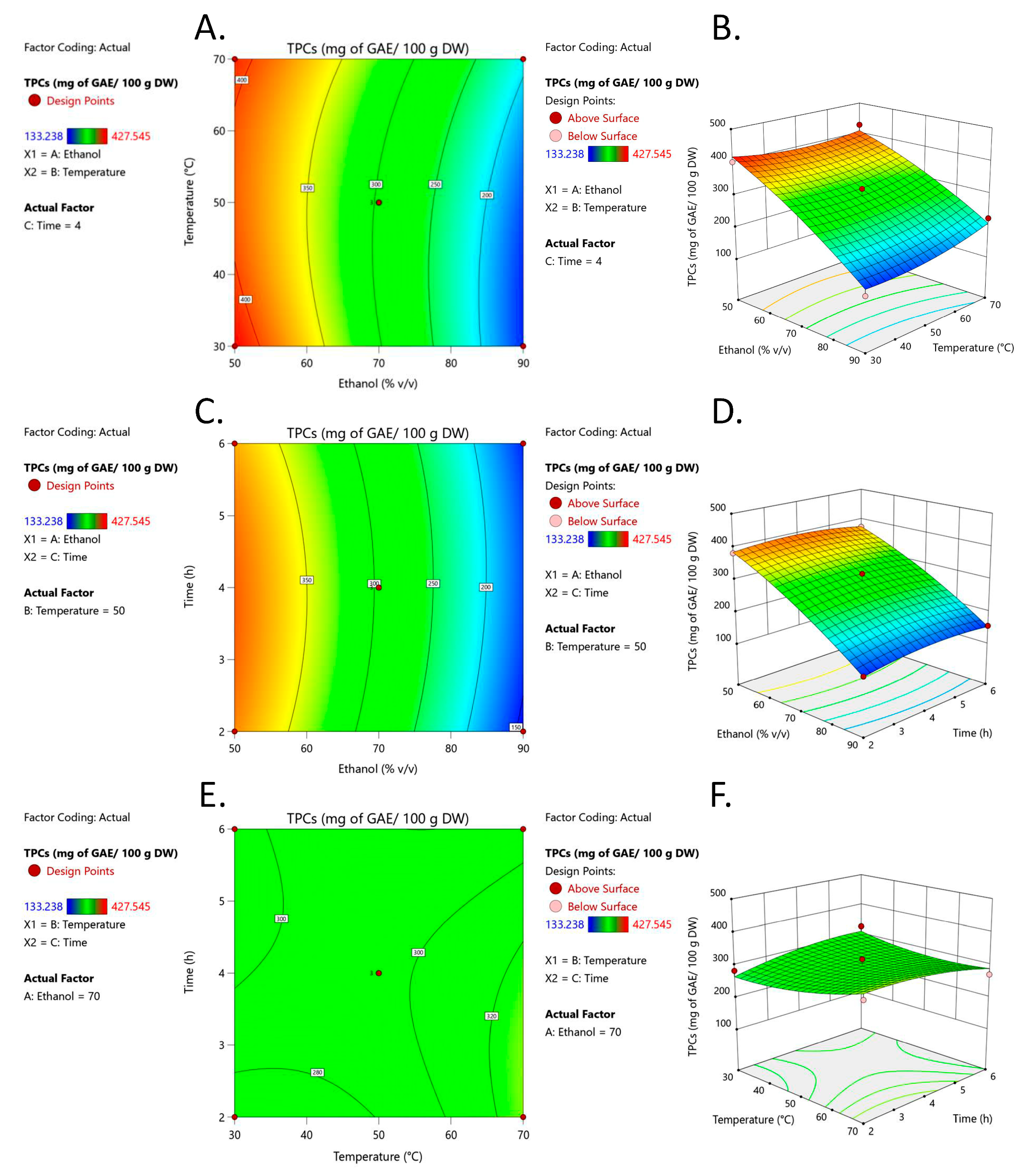
3.1. Optimization of Extraction Conditions
3.1.1. Model Fitting and Variance Analysis
3.1.2. Response Surface Analysis (RSM)
3.2. Phenolic Profiles and Total Phenolic Contents
3.3. Antioxidant Activities
3.4. In Vitro Health-Promoting Activities
3.5. Genotoxicity Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swargiary, A.; Brahma, D. Phytochemical Analysis and Antioxidant Activity of Hodgsonia heteroclita (Roxb). Indian. J. Pharm. Sci. 2017, 79, 212–219. [Google Scholar] [CrossRef]
- Piseskul, J.; Suttisansanee, U.; Chupeerach, C.; Khemthong, C.; Thangsiri, S.; Temviriyanukul, P.; Sahasakul, Y.; Santivarangkna, C.; Chamchan, R.; Aursalung, A.; et al. Optimization of Enzyme-Assisted Mechanical Extraction Process of Hodgsonia heteroclita Oilseeds and Physical, Chemical, and Nutritional Properties of the Oils. Foods 2023, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Khuntaseema, B.; Jomduang, S. Nutritive Values of Inner Pulp from Ma-king Seed (Hodgsonia heteroclite susp. Indochinensis). Agric. Sci. J. 2014, 45, 725–728. [Google Scholar]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Kremmyda, L.S.; Tvrzicka, E.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease: A review. part 2: Fatty acid physiological roles and applications in human health and disease. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2011, 155, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Usha, T.; Goyal, A.K.; Narzary, D.; Prakash, L.; Wadhwa, G.; Babu, D.; Shanmugarajan, D.; Middha, S.K. Identification of bioactive glucose-lowering compounds of methanolic extract of Hodgsonia heteroclita fruit pulp. Front. Biosci.-Landmark 2018, 23, 875–888. [Google Scholar] [CrossRef]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Ye, L.; Hu, P.; Feng, L.P.; Huang, L.L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.L. Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules 2022, 28, 281. [Google Scholar] [CrossRef]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. WIREs Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- What is Bioethanol? Available online: https://www.esru.strath.ac.uk/EandE/Web_sites/02-03/biofuels/what_bioethanol.htm (accessed on 20 October 2023).
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Zarina, Z.; Tan, S.Y. Determination of flavonoids in Citrus grandis (Pomelo) peels and their inhibition activity on lipid peroxidation in fish tissue. Int. Food Res. J. 2013, 20, 313–317. [Google Scholar]
- Gertenbach, D.D. Solid–Liquid Extraction Technologies for Manufacturing Nutraceuticals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Wang, L.; Cheng, L.; Liu, F.; Li, T.; Yu, Z.; Xu, Y.; Yang, Y. Optimization of Ultrasound-Assisted Extraction and Structural Characterization of the Polysaccharide from Pumpkin (Cucurbita moschata) Seeds. Molecules 2018, 23, 1207. [Google Scholar] [CrossRef]
- Ahamad, J.; Amin, S.; Mir, S.R. Optimization of ultrasound-assisted extraction of charantin from Momordica charantia fruits using response surface methodology. J. Pharm. Bioallied Sci. 2015, 7, 304–307. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, W.J.J.O.; Duyfjes, B.E.E. Cucurbitaceae; Forest Herbarium, Royal Forest Department: Bangkok, Thailand, 2008; Volume 9/4, p. 148.
- Chupeerach, C.; Temviriyanukul, P.; Thangsiri, S.; Inthachat, W.; Sahasakul, Y.; Aursalung, A.; Wongchang, P.; Sangkasa-Ad, P.; Wongpia, A.; Polpanit, A.; et al. Phenolic Profiles and Bioactivities of Ten Original Lineage Beans in Thailand. Foods 2022, 11, 3905. [Google Scholar] [CrossRef]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y.; et al. Impact of Drying Processes on Phenolics and In Vitro Health-Related Activities of Indigenous Plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef]
- Luu, L.K.; Thangsiri, S.; Sahasakul, Y.; Aursalung, A.; Inthachat, W.; Temviriyanukul, P.; On-Nom, N.; Chupeerach, C.; Suttisansanee, U. Nutrients, Phytochemicals and In Vitro Disease Prevention of Nephelium hypoleucum Kurz Fruit. Nutrients 2023, 15, 950. [Google Scholar] [CrossRef]
- Wannasaksri, W.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Charoenkiatkul, S.; Suttisansanee, U. In Vitro Phytotherapeutic Properties of Aqueous Extracted Adenia viridiflora Craib. towards Civilization Diseases. Molecules 2021, 26, 1082. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zalazar-García, D.; Torres, E.; Rodriguez-Ortiz, L.; Deng, Y.; Soria, J.; Bucalá, V.; Rodriguez, R.; Mazza, G. Cleaner and sustainable processes for extracting phenolic compounds from bio-waste. J. Environ. Manag. 2020, 273, 111154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Xiang, J.; Johnson, J.B.; Zheng, B.; Luo, L.; Beta, T. From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and α-Glucosidase Inhibitory Activities. Foods 2023, 12, 1144. [Google Scholar] [CrossRef] [PubMed]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Michel, I.; Tessaro, I.C.; Marczak, L.D.F. Optimization of phenolics extraction from sesame seed cake. Sep. Purif. Technol. 2014, 122, 506–514. [Google Scholar] [CrossRef]
- Mansour, R.B.; Falleh, H.; Hammami, M.; Barros, L.; Petropoulos, S.A.; Tarchoun, N.; Ksouri, R. The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita maxima Fruit By-Products. Processes 2023, 11, 1726. [Google Scholar] [CrossRef]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Narzary, D.; Middha, S.K.; Usha, T.; Brahma, B.K.; Goyal, A.K. Comparative evaluation of phytochemical constituents of rind, pulp, and seed of Hodgsonia heteroclita fruit encountered in Kokrajhar District, BTAD, Assam, India. World J. Pharma. Res. 2015, 4, 1629–1636. [Google Scholar]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B.; Kaur, A.; Singh, N. Proximate, mineral, amino acid composition, phenolic profile, antioxidant and functional properties of oilseed cakes. Int. J. Food Sci. Technol. 2021, 56, 6732–6741. [Google Scholar] [CrossRef]
- Teh, S.S.; Bekhit Ael, D.; Birch, J. Antioxidative Polyphenols from Defatted Oilseed Cakes: Effect of Solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.; Bartova, V.; Jarosova, M.; Svajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Bercikova, M.; Zdrahal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef] [PubMed]
- Švarc-Gajić, J.; Rodrigues, F.; Moreira, M.M.; Delerue-Matos, C.; Morais, S.; Dorosh, O.; Silva, A.M.; Bassani, A.; Dzedik, V.; Spigno, G. Chemical composition and bioactivity of oilseed cake extracts obtained by subcritical and modified subcritical water. Bioresour. Bioprocess. 2022, 9, 114. [Google Scholar] [CrossRef]
- Sahasakul, Y.; Aursalung, A.; Thangsiri, S.; Temviriyanukul, P.; Inthachat, W.; Pongwichian, P.; Sasithorn, K.; Suttisansanee, U. Nutritional Compositions, Phenolic Contents and Antioxidant Activities of Rainfed Rice Grown in Different Degrees of Soil Salinity. Foods 2023, 12, 2870. [Google Scholar] [CrossRef]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic Acids as Acetylcholinesterase Inhibitors: Calorimetric and Docking Simulation Studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef]
- Kikugawa, M.; Tsutsuki, H.; Ida, T.; Nakajima, H.; Ihara, H.; Sakamoto, T. Water-soluble ferulic acid derivatives improve amyloid-β-induced neuronal cell death and dysmnesia through inhibition of amyloid-β aggregation. Biosci. Biotechnol. Biochem. 2016, 80, 547–553. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef]
- Kim, J.H.; Wang, Q.; Choi, J.M.; Lee, S.; Cho, E.J. Protective role of caffeic acid in an Aβ25-35-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015, 9, 480–488. [Google Scholar] [CrossRef]
- Ardiansyah; Ohsaki, Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Maistro, E.L.; Angeli, J.P.; Andrade, S.F.; Mantovani, M.S. In vitro genotoxicity assessment of caffeic, cinnamic and ferulic acids. Genet. Mol. Res. 2011, 10, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- OECD. SIDS Initial Assessment Profile. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=cd93235e-9715-4766-a0bc-4b219347aef2 (accessed on 24 October 2023).
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant Activity of Phenolic Acids and Their Metabolites: Synthesis and Antioxidant Properties of the Sulfate Derivatives of Ferulic and Caffeic Acids and of the Acyl Glucuronide of Ferulic Acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against Superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Kaur, P.; Purewal, S.S.; Sandhu, K.S.; Kaur, M. DNA damage protection: An excellent application of bioactive compounds. Bioresour. Bioprocess. 2019, 6, 2. [Google Scholar] [CrossRef]
| Coded | Independent Variables | Variable Degree | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Ethanol concentration (% v/v) | 50 | 70 | 90 | |
| Temperature (°C) | 30 | 50 | 70 | |
| X3 | Time (h) | 2 | 4 | 6 |
| Run | Box–Behnken Design of Independent Variables | ||
|---|---|---|---|
| : Ethanol (% v/v) | : Temperature (°C) | : Time (h) | |
| 1 | 0 (70) | −1 (30) | −1 (2) |
| 2 | 0 (70) | 0 (50) | 0 (4) |
| 3 | 1 (90) | 0 (50) | −1 (2) |
| 4 | 0 (70) | 0 (50) | 0 (4) |
| 5 | 0 (70) | 1 (70) | 1 (6) |
| 6 | 1 (90) | 1 (70) | 0 (4) |
| 7 | 1 (90) | −1 (30) | 0 (4) |
| 8 | 0 (70) | −1 (30) | 1 (6) |
| 9 | −1 (50) | −1 (30) | 0 (4) |
| 10 | −1 (50) | 0 (50) | 1 (6) |
| 11 | −1 (50) | 1 (70) | 0 (4) |
| 12 | −1 (50) | 0 (50) | −1 (2) |
| 13 | 0 (70) | 0 (50) | 0 (4) |
| 14 | 0 (70) | 1 (70) | −1 (2) |
| 15 | 1 (90) | 0 (50) | 1 (6) |
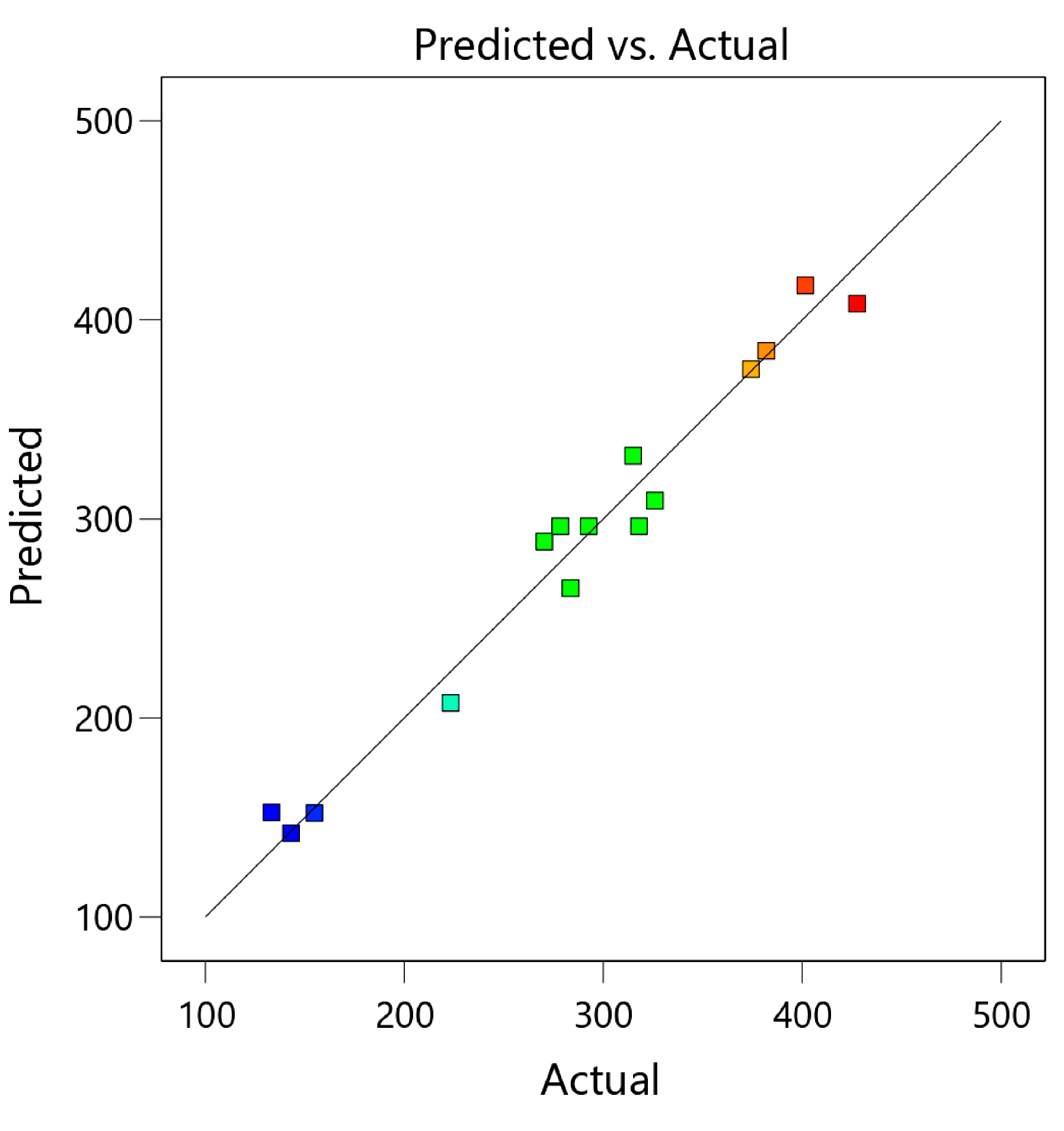
| Run | Box–Behnken Design of Independent Variables | TPCs (mg of GAE/100 g DW) | |||
|---|---|---|---|---|---|
| : Ethanol (% v/v) | : Temperature (°C) | : Time (h) | Experimental | Predicted | |
| 1 | 0 (70) | −1 (30) | −1 (2) | 283.55 ± 8.26 ef | 265.24 |
| 2 | 0 (70) | 0 (50) | 0 (4) | 292.69 ± 7.13 e | 296.39 |
| 3 | 1 (90) | 0 (50) | −1 (2) | 143.14 ± 6.02 hi | 142.14 |
| 4 | 0 (70) | 0 (50) | 0 (4) | 318.05 ± 7.21 d | 296.39 |
| 5 | 0 (70) | 1 (70) | 1 (6) | 270.34 ± 18.32 f | 288.65 |
| 6 | 1 (90) | 1 (70) | 0 (4) | 223.33 ± 6.37 g | 207.60 |
| 7 | 1 (90) | −1 (30) | 0 (4) | 133.24 ± 13.27 i | 152.55 |
| 8 | 0 (70) | −1 (30) | 1 (6) | 325.99 ± 5.23 d | 309.25 |
| 9 | −1 (50) | −1 (30) | 0 (4) | 401.60 ± 14.88 b | 417.34 |
| 10 | −1 (50) | 0 (50) | 1 (6) | 374.29 ± 7.11 c | 375.28 |
| 11 | −1 (50) | 1 (70) | 0 (4) | 427.55 ± 14.20 a | 408.24 |
| 12 | −1 (50) | 0 (50) | −1 (2) | 381.98 ± 10.16 c | 384.55 |
| 13 | 0 (70) | 0 (50) | 0 (4) | 278.42 ± 11.37 ef | 296.39 |
| 14 | 0 (70) | 1 (70) | −1 (2) | 315.07 ± 5.42 d | 331.80 |
| 15 | 1 (90) | 0 (50) | 1 (6) | 154.84 ± 7.39 h | 152.26 |
| Dependent Variables | Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|---|
| TPCs | Model | 1.16 × 105 | 9 | 12,859.56 | 19.53 | 0.0022 | ** |
| 1.08 × 105 | 1 | 1.08 × 105 | 164.50 | <0.0001 | *** | ||
| 1055.63 | 1 | 1055.63 | 1.60 | 0.2613 | |||
| 0.3672 | 1 | 0.3672 | 0.0006 | 0.9821 | |||
| 1028.9 | 1 | 1028.9 | 1.56 | 0.2666 | |||
| 94.01 | 1 | 94.01 | 0.1428 | 0.7210 | |||
| 1899.3 | 1 | 1899.3 | 2.88 | 0.1502 | |||
| 1139.32 | 1 | 1139.32 | 1.73 | 0.2455 | |||
| 1145.09 | 1 | 1145.09 | 1.74 | 0.2444 | |||
| 859.91 | 1 | 859.91 | 1.31 | 0.3049 | |||
| Residual | 3292.39 | 5 | 658.48 | ||||
| Lack of Fit | 2486.71 | 3 | 828.9 | 2.06 | 0.3436 | NS | |
| Pure Error | 805.69 | 2 | 402.84 | ||||
| Cor Total | 1.19 × 105 | 14 | |||||
| R2 | 0.9723 | ||||||
| Adjusted R2 | 0.9226 |
| Samples | Phenolic Profile (mg/100 g DW) | TPCs (mg GAE/100 g DW) | ||
|---|---|---|---|---|
| 4-Hydroxybenzoic Acid | Caffeic Acid | Ferulic Acid | ||
| Fresh | 727.68 ± 0.58 a | 501.72 ± 1.33 a | 896.31 ± 5.34 a | 43.87 ± 1.92 d |
| NP-OCP | 571.77 ± 27.80 b | ND | 580.45 ± 10.88 c | 117.00 ± 9.79 c |
| HP-OCP | 550.52 ± 21.59 b | 369.89 ± 2.74 b | 660.58 ± 8.75 b | 138.00 ± 3.81 b |
| HEP-OCP | 397.64 ± 3.78 c | ND | 298.79 ± 0.98 d | 413.96 ± 12.00 a |
| Samples | Antioxidant Activities | ||
|---|---|---|---|
| DPPH Radical Scavenging Assay (µmol TE/100 g DW) | FRAP Assay (µmol TE/g DW) | ORAC Assay (µmol TE/g DW) | |
| Fresh | 0.04 ± 0.00 d | 0.88 ± 0.09 d | 9.65 ± 0.91 d |
| NP-OCP | 0.10 ± 0.01 c | 2.14 ± 0.14 c | 25.32 ± 2.32 c |
| HP-OCP | 0.13 ± 0.01 b | 2.85 ± 0.09 b | 33.19 ± 2.04 b |
| HEP-OCP | 0.56 ± 0.05 a | 7.78 ± 0.56 a | 68.72 ± 3.59 a |
| Enzyme Inhibition (% Inhibition) | Samples * | |||
|---|---|---|---|---|
| Fresh | NP-OCP | HP-OCP | HEP-OCP | |
| Lipase | 10.63 ± 0.72 | ND | ND | ND |
| α-Amylase | ND | ND | ND | 3.72 ± 0.25 |
| α-Glucosidase | 11.56 ± 0.58 b | 11.31 ± 0.61 b | 9.42 ± 0.85 c | 15.81 ± 1.33 a |
| DPP-IV | ND | ND | ND | 16.66 ± 0.76 |
| AChE | ND | ND | ND | ND |
| BChE | ND | ND | ND | ND |
| BACE-1 | 39.80 ± 3.16 d | 65.40 ± 2.40 c | 71.71 ± 0.92 b | 76.60 ± 2.98 a |
| ACE | 25.77 ± 2.11 c | 68.47 ± 3.49 b | 74.82 ± 2.99 b | 93.52 ± 2.90 a |
| Doses (µg/Plate) | TA98 | TA100 | TA102 | TA1535 | TA1537 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | |
| Neg | 82.50 ± 2.36 | 1.00 (−) | 75.67 ± 2.81 | 1.00 (−) | 371.00 ± 13.33 | 1.00 (−) | 10.17 ± 0.69 | 1.00 (−) | 9.50 ± 0.96 | 1.00 (−) |
| 10 | 82.17 ± 1.77 | 1.00 (−) | 75.50 ± 1.89 | 1.00 (−) | 369.67 ± 7.20 | 1.00 (−) | 10.33 ± 0.75 | 1.02 (−) | 9.50 ± 0.76 | 1.00 (−) |
| 100 | 82.00 ± 1.63 | 0.99 (−) | 74.83 ± 2.11 | 0.99 (−) | 367.50 ± 12.20 | 0.99 (−) | 9.50 ± 0.50 | 0.93 (−) | 10.17 ± 0.69 | 1.07 (−) |
| 500 | 80.83 ± 0.69 | 0.98 (−) | 73.67 ± 1.97 | 0.97 (−) | 365.00 ± 6.93 | 0.98 (−) | 10.33 ± 0.94 | 0.98 (−) | 9.17 ± 0.37 | 0.96 (−) |
| 1000 | 81.00 ± 0.82 | 0.98 (−) | 75.33 ± 2.21 | 1.00 (−) | 363.83 ± 9.32 | 0.98 (−) | 10.33 ± 1.11 | 1.02 (−) | 9.33 ± 0.75 | 0.98 (−) |
| 2000 | 81.83 ± 1.57 | 0.99 (−) | 74.50 ± 1.71 | 0.98 (−) | 363.00 ± 6.86 | 0.98 (−) | 10.83 ± 0.90 | 1.07 (−) | 9.67 ± 0.75 | 1.02 (−) |
| 4-NQO | 1152.67 ± 32.78 | 13.97 (+) | ||||||||
| NaN3 | 1060.00 ± 31.58 | 14.01 (+) | 295.00 ± 6.95 | 29.02 (+) | ||||||
| MMC | 1056.33 ± 30.32 | 2.86 (+) | ||||||||
| 9-AA | 788.67 ± 8.06 | 83.02 (+) | ||||||||
| Doses (µg/Plate) | TA98 | TA100 | TA102 | TA1535 | TA1537 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | Revertant Colonies | MR | |
| Neg | 83.83 ± 2.79 | 1.00 (−) | 83.50 ± 2.22 | 1.00 (−) | 359.33 ± 13.83 | 1.00 (−) | 12.50 ± 0.96 | 1.00 (−) | 9.67 ± 0.75 | 1.00 (−) |
| 10 | 81.50 ± 1.38 | 0.97 (−) | 81.33 ± 1.49 | 0.97 (−) | 363.83 ± 11.47 | 1.01 (−) | 12.83 ± 1.07 | 1.03 (−) | 10.60 ± 1.26 | 1.09 (−) |
| 100 | 82.33 ± 1.60 | 0.98 (−) | 82.67 ± 2.05 | 0.99 (−) | 363.67 ± 9.48 | 1.01 (−) | 12.67 ± 1.37 | 1.01 (−) | 9.67 ± 0.94 | 1.00 (−) |
| 500 | 82.83 ± 0.90 | 0.99 (−) | 83.67 ± 1.80 | 1.00 (−) | 365.33 ± 16.70 | 1.02 (−) | 13.00 ± 0.82 | 1.04 (−) | 9.50 ± 0.96 | 0.98 (−) |
| 1000 | 82.33 ± 1.11 | 0.98 (−) | 83.83 ± 1.34 | 1.00 (−) | 356.83 ± 6.44 | 0.99 (−) | 12.33 ± 1.25 | 0.99 (−) | 9.83 ± 0.69 | 1.02 (−) |
| 2000 | 82.50 ± 1.26 | 0.98 (−) | 84.83 ± 1.77 | 1.02 (−) | 358.67 ± 7.18 | 1.00 (−) | 13.33 ± 0.47 | 1.07 (−) | 9.50 ± 0.76 | 0.98 (−) |
| 2-AA | 1086.67 ± 17.07 | 12.96 (+) | 1113.33 ± 47.31 | 13.33 (+) | 1100.00 ± 48.88 | 3.06 (+) | 299.67 ± 13.03 | 23.97 (+) | 194.33 ± 6.21 | 20.10 (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inthachat, W.; Thangsiri, S.; Khemthong, C.; On-Nom, N.; Chupeerach, C.; Sahasakul, Y.; Temviriyanukul, P.; Suttisansanee, U. Green Extraction of Hodgsonia heteroclita Oilseed Cake Powder to Obtain Optimal Antioxidants and Health Benefits. Foods 2023, 12, 4281. https://doi.org/10.3390/foods12234281
Inthachat W, Thangsiri S, Khemthong C, On-Nom N, Chupeerach C, Sahasakul Y, Temviriyanukul P, Suttisansanee U. Green Extraction of Hodgsonia heteroclita Oilseed Cake Powder to Obtain Optimal Antioxidants and Health Benefits. Foods. 2023; 12(23):4281. https://doi.org/10.3390/foods12234281
Chicago/Turabian StyleInthachat, Woorawee, Sirinapa Thangsiri, Chanakan Khemthong, Nattira On-Nom, Chaowanee Chupeerach, Yuraporn Sahasakul, Piya Temviriyanukul, and Uthaiwan Suttisansanee. 2023. "Green Extraction of Hodgsonia heteroclita Oilseed Cake Powder to Obtain Optimal Antioxidants and Health Benefits" Foods 12, no. 23: 4281. https://doi.org/10.3390/foods12234281
APA StyleInthachat, W., Thangsiri, S., Khemthong, C., On-Nom, N., Chupeerach, C., Sahasakul, Y., Temviriyanukul, P., & Suttisansanee, U. (2023). Green Extraction of Hodgsonia heteroclita Oilseed Cake Powder to Obtain Optimal Antioxidants and Health Benefits. Foods, 12(23), 4281. https://doi.org/10.3390/foods12234281





