Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Preparation and Extraction
2.3. Chemical Compounds Analysis
2.3.1. Determination of Total Phenolic Content
2.3.2. Determination of Total Flavonoid Content
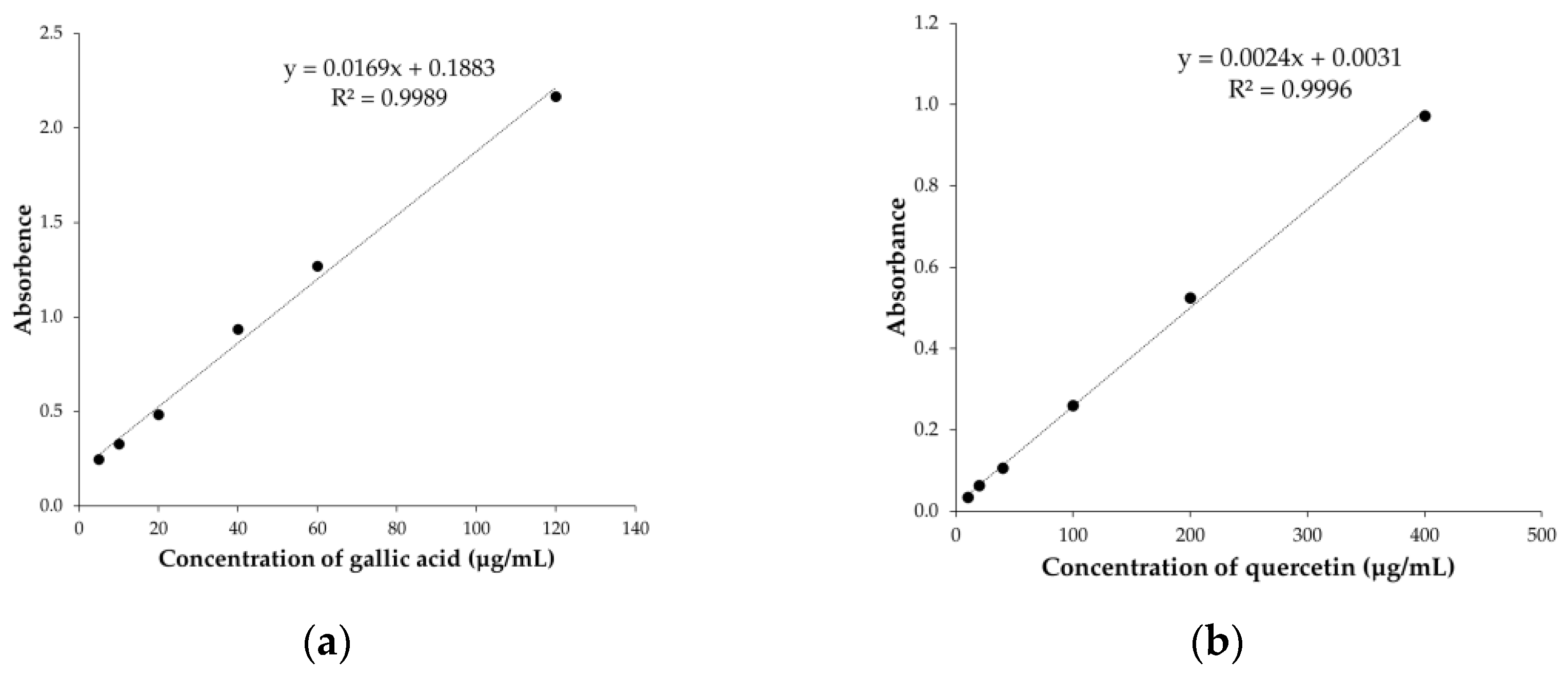
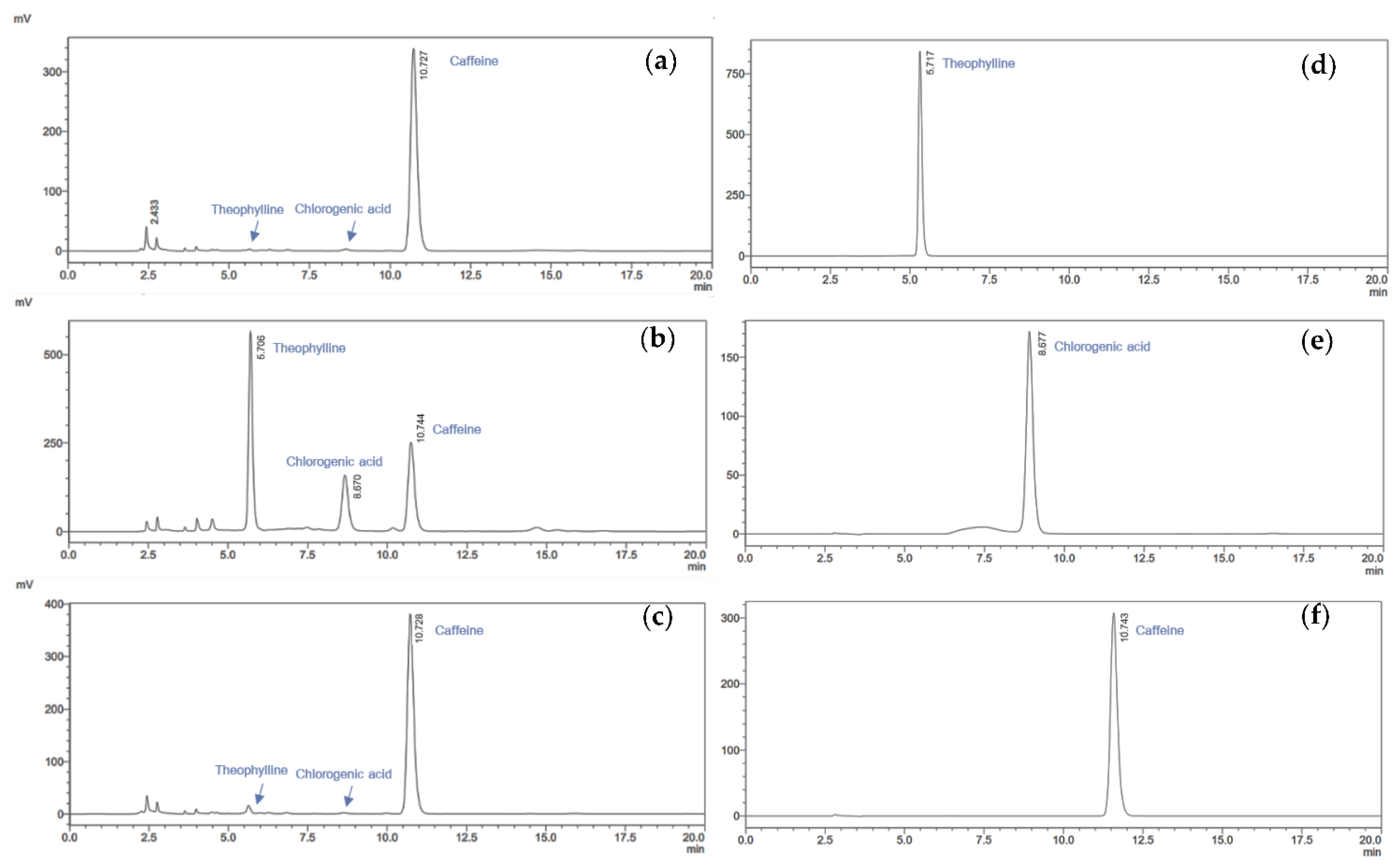
2.3.3. Phytochemical Profile Analysis by HPLC
2.4. Anti-Oxidant Activity Tests
2.4.1. DPPH Radical Scavenging Assay
2.4.2. Lipid Peroxidation Inhibition Using Ferric Thiocyanate Assay
2.5. Enzymes Inhibitory Tests
2.5.1. Collagenase Inhibitory Assay
2.5.2. Elastase Inhibitory Assay
2.5.3. Hyaluronidase Inhibitory Assay
2.5.4. Anti-Tyrosinase Activity
2.6. Cell Culture
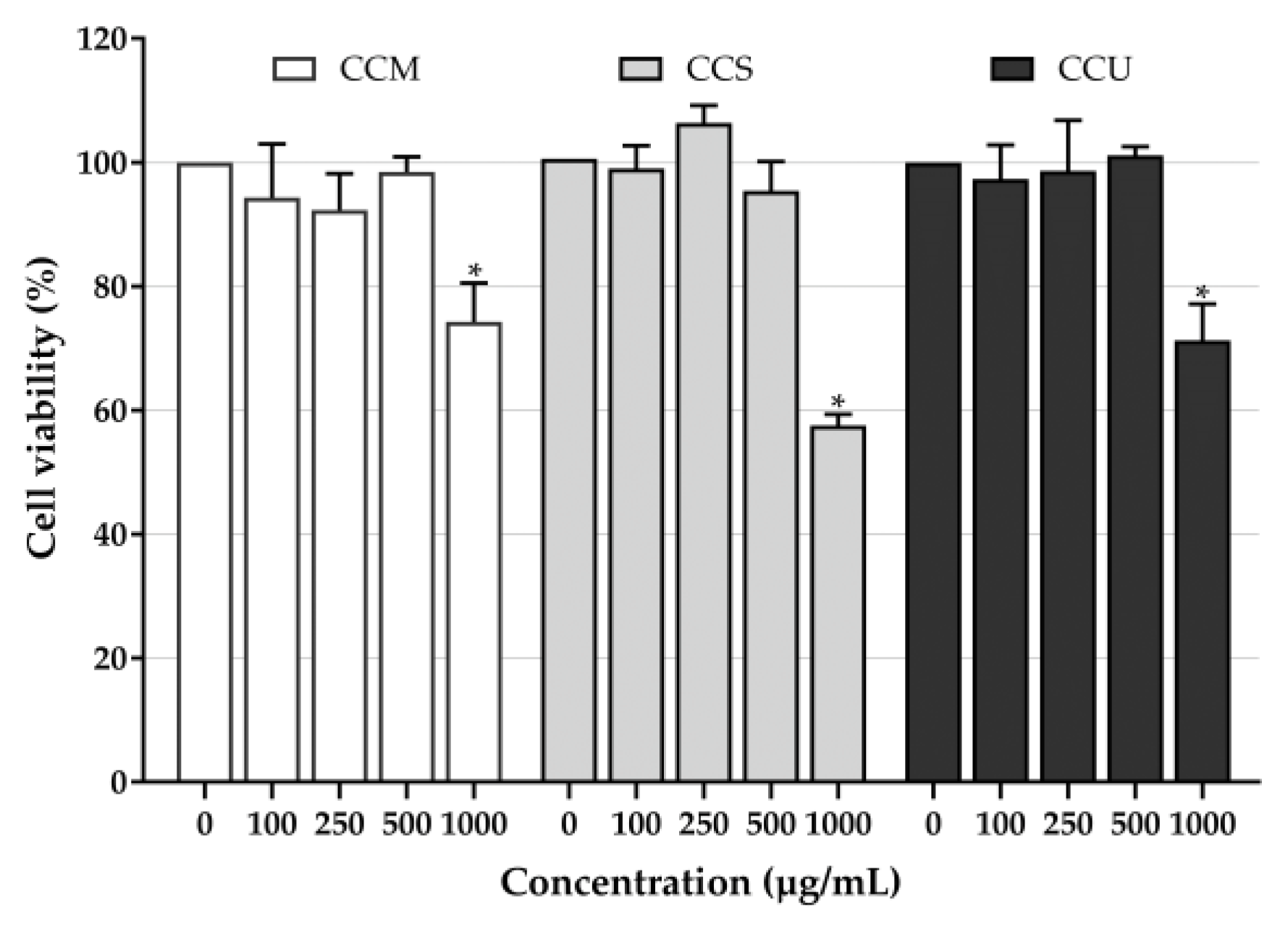
2.7. Cytotoxicity Test
2.8. ROS Production Inhibition (H2DCFDA Assay)
2.9. Statistic Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Total Flavonoid Contents
3.2. HPLC Phytochemicals Profiles of Coffee Cherry Pulp Extracts
3.3. Anti-Oxidant Activities
3.4. The Inhibitory Effects on Collagenase, Elastase, Hyaluronidase, and Tyrosinase Enzymes
3.5. Pearson’s Correlation of Total Phenolic Content, Total Flavonoid Content, and Phytochemicals Content with Anti-Oxidant Activities and Anti-Aging Activities of Coffee Cherry Pulp Extracts
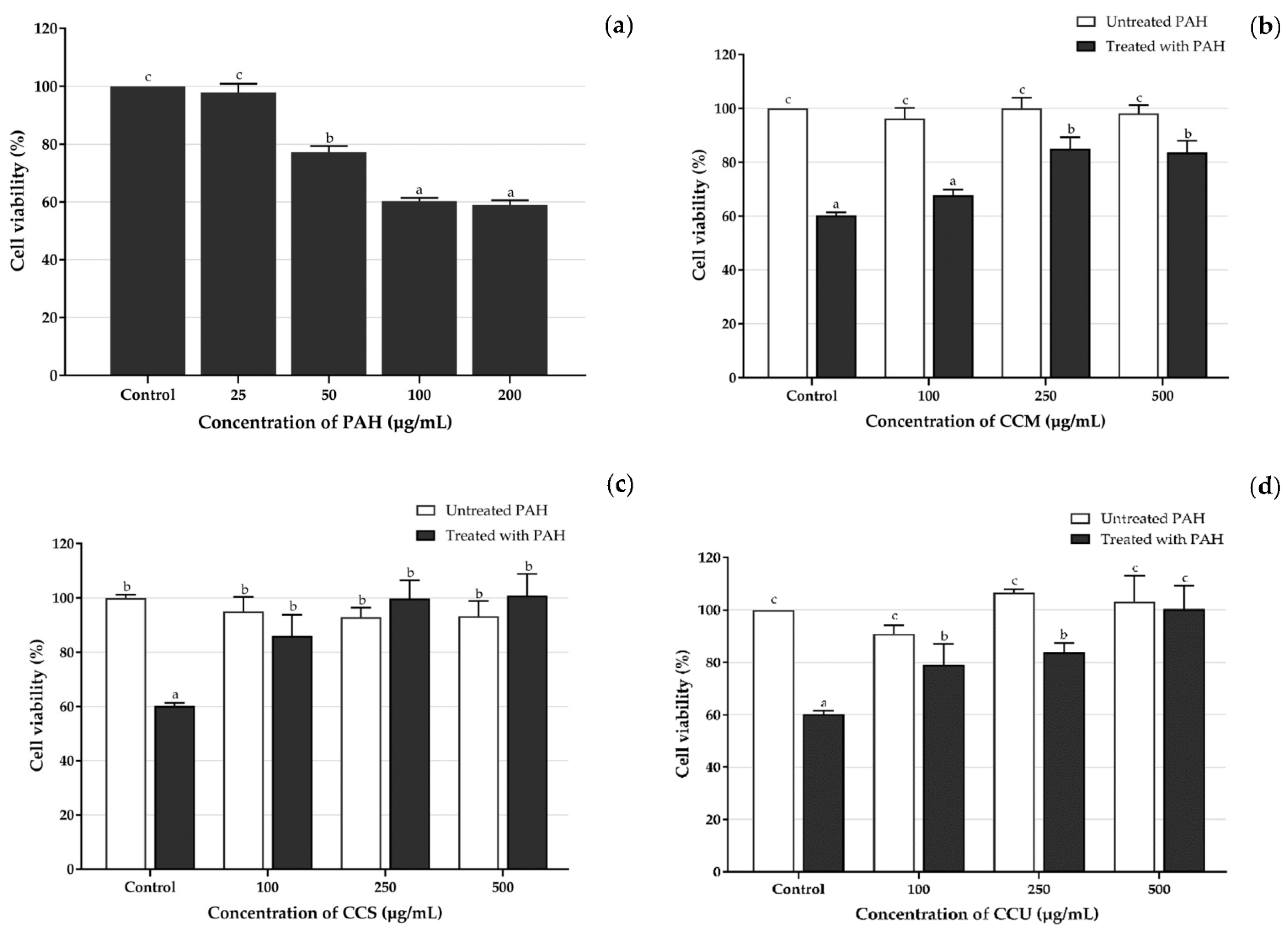
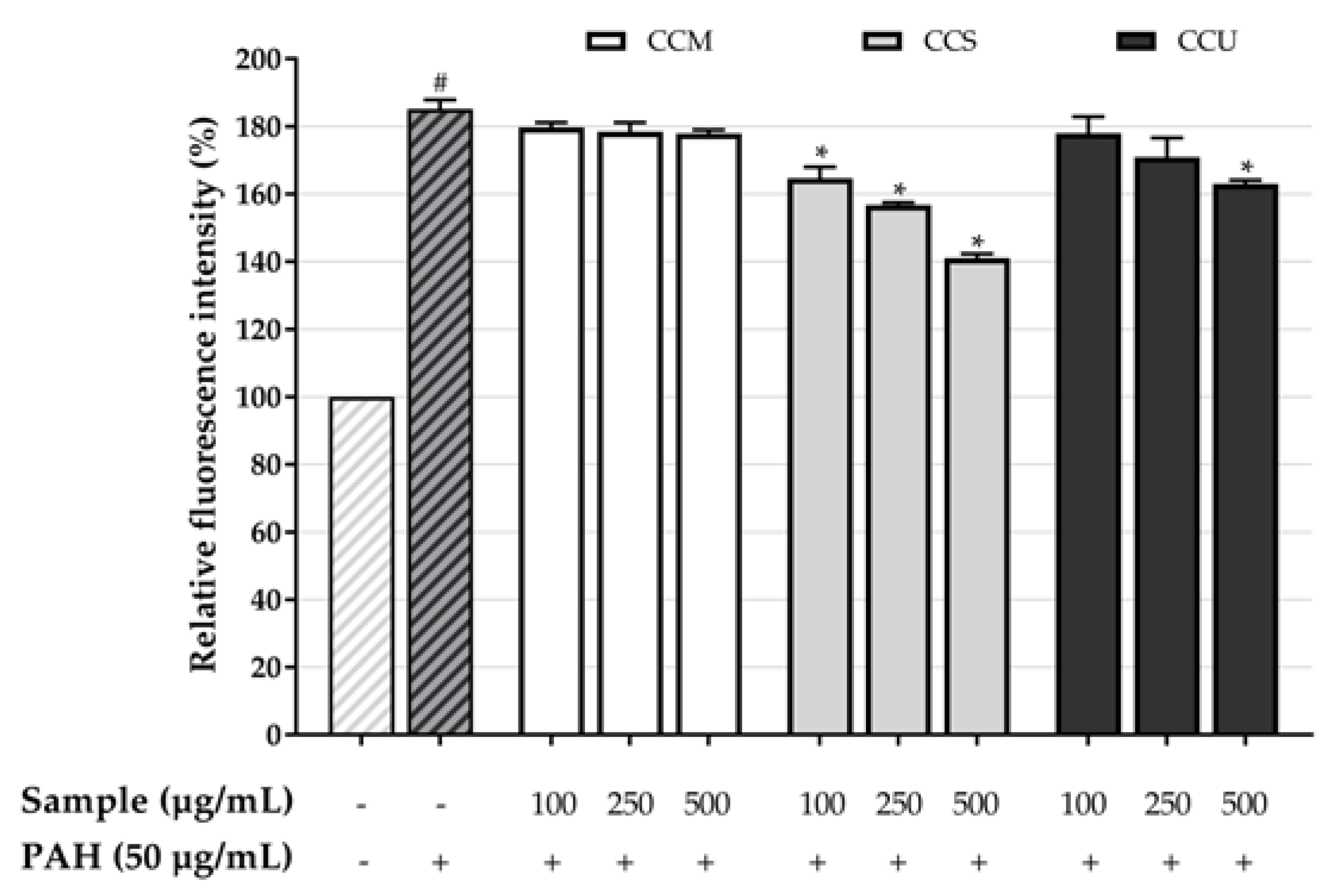
3.6. Effect of Coffee Cherry Pulp Extracts on PAH-Induced Toxicity in HaCaT Cells
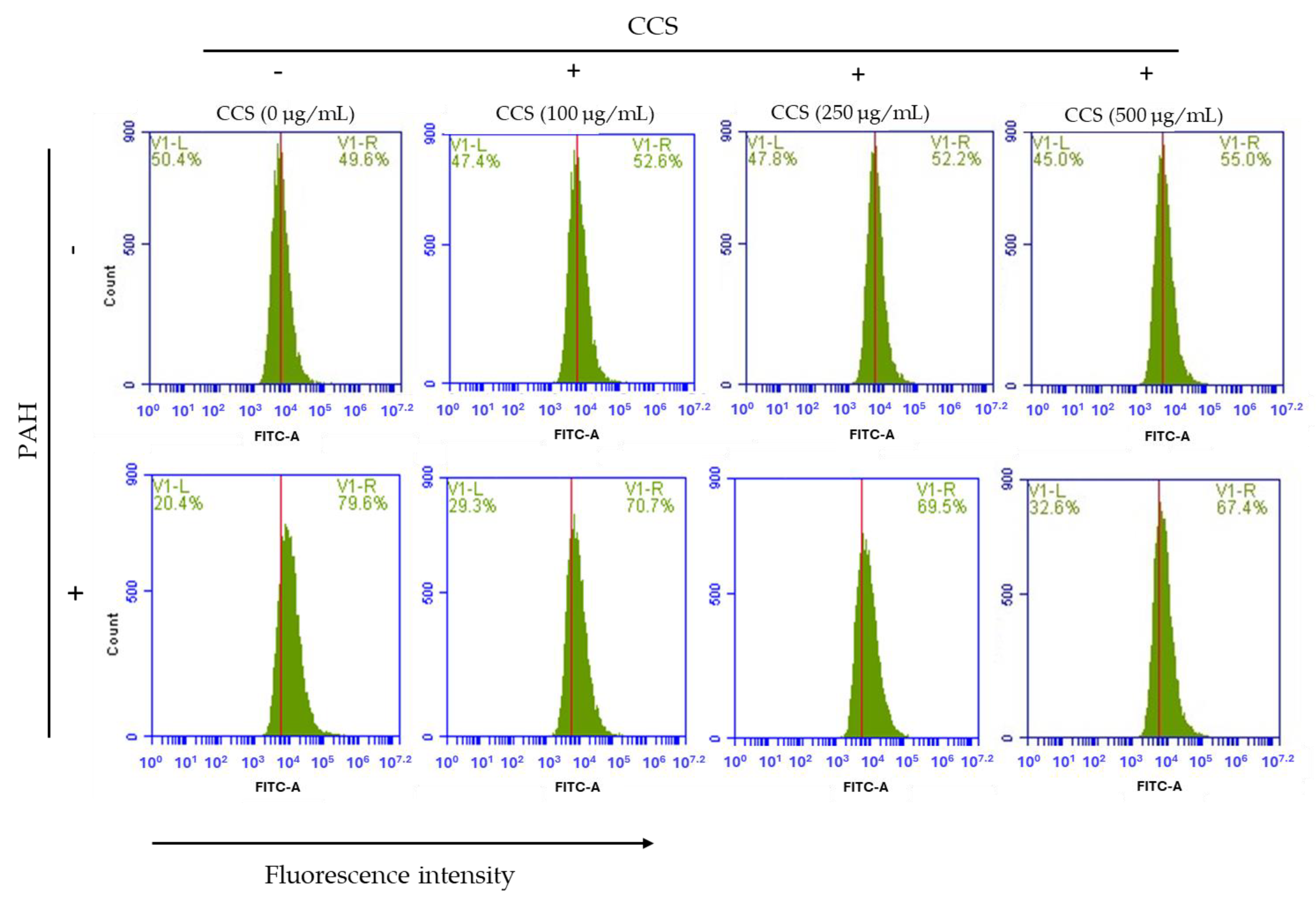
3.7. Effect of Coffee Cherry Pulp Extracts on Intracellular ROS Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulze, C.; Wetzel, F.; Kueper, T.; Malsen, A.; Muhr, G.; Jaspers, S.; Blatt, T.; Wittern, K.-P.; Wenck, H.; Käs, J.A. Stiffening of Human Skin Fibroblasts with Age. Biophys. J. 2010, 99, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Sun, X.; Yu, H. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)-light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J. Environ. Sci. Health Part C 2012, 30, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Ernster, V.L.; Grady, D.; Miike, R.; Black, D.; Selby, J.; Kerlikowske, K. Facial wrinkling in men and women, by smoking status. Am. J. Public Health 1995, 85, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Menichini, E. Urban air pollution by polycyclic aromatic hydrocarbons: Levels and sources of variability. Sci. Total Environ. 1992, 116, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne Particle Exposure and Extrinsic Skin Aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Frenis, K.; Kuntic, M.; Daiber, A.; Münzel, T. Accelerated Aging and Age-Related Diseases (CVD and Neurological) Due to Air Pollution and Traffic Noise Exposure. Int. J. Mol. Sci. 2021, 22, 2419. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Byun, E.J.; Lee, J.D.; Kim, S.; Kim, H.S. Air pollution, autophagy, and skin aging: Impact of particulate matter (PM10) on human dermal fibroblasts. Int. J. Mol. Sci. 2018, 19, 2727. [Google Scholar] [CrossRef]
- Shin, K.O.; Uchida, Y.; Park, K. Diesel Particulate Extract Accelerates Premature Skin Aging in Human Fibroblasts via Ceramide-1-Phosphate-Mediated Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2691. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Hopke, P.K.; Rossner, A. Exposure to airborne particulate matter in the ambient, indoor, and occupational environments. Clin. Occup. Environ. Med. 2006, 5, 747–771. [Google Scholar]
- Yu, H.; Xia, Q.; Yan, J.; Herreno-Saenz, D.; Wu, Y.-S.; Tang, I.W.; Fu, P. Photoirradiation of Polycyclic Aromatic Hydrocarbons with UVA Light—A Pathway Leading to the Generation of Reactive Oxygen Species, Lipid Peroxidation, and DNA Damage. Int. J. Environ. Res. Public Health 2006, 3, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, E.; Schäfer, C.; Calles, C.; Bernsmann, T.; Bernshausen, T.; Wurm, M.; Hübenthal, U.; Cline, J.E.; Hajimiragha, H.; Schroeder, P.; et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 8851–8856. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Yang, Y.; Zhao, Z.; Hüls, A.; Vierkötter, A.; Yuan, Z.; Cai, J.; Zhang, J.; Gao, W.; Li, J.; et al. Indoor PM2.5 exposure affects skin aging manifestation in a Chinese population. Sci. Rep. 2017, 7, 15329. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Marini, A.; Jaenicke, T.; Aue, N.; Welss, T.; Uthe, I.; Krutmann, J. Air pollution-induced tanning of human skin. Br. J. Dermatol. 2021, 185, 1026–1034. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement. Altern. Med. 2017, 17, 487. [Google Scholar] [CrossRef]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A. Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum teretifolium. Molecules 2015, 20, 7143–7155. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling-Novel Cues from the Matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef]
- David, M.R.; Jennifer, L. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthetic Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am. J. Pathol. 2014, 184, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Itoh, A.; Mishima, Y.; Ichihashi, M. Correlation between the number of melanosomes, tyrosinase mRNA levels, and tyrosinase activity in cultured murine melanoma cells in response to various melanogenesis regulatory agents. J. Cell Physiol. 1995, 163, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Manasa, V.; Padmanabhan, A.; Anu Appaiah, K.A. Utilization of coffee pulp waste for rapid recovery of pectin and polyphenols for sustainable material recycle. Waste Manag. 2021, 120, 762–771. [Google Scholar] [CrossRef]
- Ramirez-Coronel, M.A.; Marnet, N.; Kolli, V.S.; Roussos, S.; Guyot, S.; Augur, C. Characterization and estimation of proanthocyanidins and other phenolics in coffee pulp (Coffea arabica) by thiolysis-high-performance liquid chromatography. J. Agric. Food Chem. 2004, 52, 1344–1349. [Google Scholar] [CrossRef]
- Nemzer, B.; Abshiru, N.; Al-Taher, F. Identification of Phytochemical Compounds in Coffea arabica Whole Coffee Cherries and Their Extracts by LC-MS/MS. J. Agric. Food Chem. 2021, 69, 3430–3438. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and Hydroxycinnamate Contents of Whole Coffee Fruits from China, India, and Mexico. J. Agric. Food Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.D.; Kovtun, S.; Hegde, K. Effectiveness of topical caffeine in cataract prevention: Studies with galactose cataract. Mol. Vis. 2010, 16, 2626–2633. [Google Scholar] [PubMed]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Kieu Tran, T.M.; Kirkman, T.; Nguyen, M.; Van Vuong, Q. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of robusta wet coffee pulp (Coffea canephora). Heliyon 2020, 6, e04498. [Google Scholar] [CrossRef]
- Kiattisin, K.; Intasai, N.; Nitthikan, N.N.; Thananya, N.; Lee, H.K.; Lin, C.W.; Lue, C.S.; Leelapornpisid, P. Antioxidant, anti-tyrosinase, anti-aging potentials and safety of arabica coffee cherry extract. Chiang Mai J. Sci. 2019, 46, 930–945. [Google Scholar]
- Geremu, M.; Tola, Y.B.; Sualeh, A. Extraction and determination of total polyphenols and antioxidant capacity of red coffee (Coffea arabica L.) pulp of wet processing plants. Chem. Biol. Technol. Agric. 2016, 3. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Shih, C.-H.; Lin, T.-C.; Zheng, J.-H.; Hsu, C.-C.; Chen, K.-M.; Lin, Y.-S.; Wu, C.-T. Antioxidation and Tyrosinase Inhibitory Ability of Coffee Pulp Extract by Ethanol. J. Chem. 2021, 2021, 8649618. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Abshiru, N. Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts. Molecules 2021, 26, 4306. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Adhikari, K.; Choi, K.S.; Lee, J. Analysis of Caffeine, Chlorogenic Acid, Trigonelline, and Volatile Compounds in Cold Brew Coffee Using High-Performance Liquid Chromatography and Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. Foods 2020, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, M.; Falguera, V.; Gallego, G.; Peiró, S.; Almajano, M.P. Antioxidant properties of aqueous and ethanolic extracts of tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J. Sci. Food Agric. 2014, 94, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.-M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The Effect of Extraction Methods on Phytochemicals and Biological Activities of Green Coffee Beans Extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef]
- Tunit, P.; Thammarat, P.; Okonogi, S.; Chittasupho, C. Hydrogel Containing Borassus flabellifer L. Male Flower Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Gels 2022, 8, 126. [Google Scholar] [CrossRef]
- Rodríguez Durán, L.V.; Ramírez Coronel, M.A.; Aranda Delgado, E.; Nampoothiri, K.M.; Favela Torres, E.; Aguilar, C.N.; Saucedo Castañeda, G. Soluble and bound hydroxycinnamates in coffee pulp (Coffea arabica) from seven cultivars at three ripening stages. J. Agric. Food Chem. 2014, 62, 7869–7876. [Google Scholar] [CrossRef]
- Osawa, T.; Namiki, M. A Novel Type of Antioxidant Isolated from Leaf Wax of Eucalyptus leaves. Agric. Biol. Chem. 1981, 45, 735–739. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Rani, A.P.; Hamzah, R.A.; Arumwardana, S.; Afifah, E.; Kusuma, H.S.W.; Rihibiha, D.D.; Nufus, H.; Amalia, A. Antioxidant and Antiaging Assays of Hibiscus sabdariffa Extract and Its Compounds. Nat. Prod. Sci. 2017, 23, 192–200. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.; Kim, J.M.; Lee, M.K.; Seo, S.J.; Park, K.Y. Afzelin suppresses proinflammatory responses in particulate matter-exposed human keratinocytes. Int. J. Mol. Med. 2019, 43, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Singh, A.P. In Vitro Antioxidant and Free Radical Scavenging Activity of Nardostachys jatamansi DC. J. Acupunct. Meridian Stud. 2012, 5, 112–118. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Ramírez-Martínez, J.R. Phenolic compounds in coffee pulp: Quantitative determination by HPLC. J. Sci. Food Agric. 1988, 43, 135–144. [Google Scholar] [CrossRef]
- Castro, M.D.L.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstractions. 1. The reactions of phenols with 2,2-diphenyl-1-picrylhydrazyl (dpph*) in alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination; InTech: London, UK, 2012. [Google Scholar]
- Tang, W.; Li, W.; Yang, Y.; Lin, X.; Wang, L.; Li, C.; Yang, R. Phenolic Compounds Profile and Antioxidant Capacity of Pitahaya Fruit Peel from Two Red-Skinned Species (Hylocereus polyrhizus and Hylocereus undatus). Foods 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Wu, L. Effect of chlorogenic acid on antioxidant activity of Flos Lonicerae extracts. J. Zhejiang Univ. Sci. B 2007, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crops Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Ersoy, E.; Eroglu Ozkan, E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Bertolini, M.; Ramot, Y.; Gherardini, J.; Heinen, G.; Chéret, J.; Welss, T.; Giesen, M.; Funk, W.; Paus, R. Theophylline exerts complex anti-ageing and anti-cytotoxicity effects in human skin ex vivo. Int. J. Cosmet. Sci. 2020, 42, 79–88. [Google Scholar] [CrossRef]
- Robert, L.; Jacob, M.P.; Frances, C.; Godeau, G.; Hornebeck, W. Interaction between elastin and elastases and its role in the aging of the arterial wall, skin and other connective tissues. A review. Mech. Ageing Dev. 1984, 28, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Eun Lee, K.; Bharadwaj, S.; Yadava, U.; Gu Kang, S. Evaluation of caffeine as inhibitor against collagenase, elastase and tyrosinase using in silico and in vitro approach. J. Enzym. Inhib. Med. Chem. 2019, 34, 927–936. [Google Scholar] [CrossRef] [PubMed]
- McCook, J.P.; Dorogi, P.L.; Vasily, D.B.; Cefalo, D.R. In vitro inhibition of hyaluronidase by sodium copper chlorophyllin complex and chlorophyllin analogs. Clin. Cosmet. Investig. Dermatol. 2015, 8, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Pietrzak, W.; Klimek, K.; Miazga-Karska, M.; Firlej, A.; Flisiński, M.; Grzywa-Celińska, A. Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. Int. J. Mol. Sci. 2021, 22, 6219. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, H.; Tokeshi, I.; Nakamura, S.; Muto, N.; Nakada, T. Inhibition of boar sperm hyaluronidase activity by tannic acid reduces polyspermy during in vitro fertilization of porcine oocytes. Zygote 2006, 14, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, D.S.; Kim, W.G.; Ryoo, I.J.; Lee, D.H.; Huh, C.H.; Youn, S.W.; Yoo, I.D.; Park, K.C. Terrein: A new melanogenesis inhibitor and its mechanism. Cell Mol. Life Sci. 2004, 61, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X.; Ni, H.; Du, X.; Chen, F.; Jiang, Z.; Li, Q. Identification and Characterization of the Tyrosinase Inhibitory Activity of Caffeine from Camellia Pollen. J. Agric. Food Chem. 2019, 67, 12741–12751. [Google Scholar] [CrossRef]
- Duangjai, A.; Utsintong, M.; Saokaew, S. Coffee (Coffea arabica L.) Pulp Extracts as a Potential Source of Whitening Agents. Int. J. Pharm. Sci. Rev. Res. 2020, 64, 123–1227. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Deiana, L.; Carru, C.; Pintus, G. Coumaric acid induces mitochondrial damage and oxidative-mediated cell death of human endothelial cells. Cardiovasc. Toxicol. 2013, 13, 301–306. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Nguyen le, H.V.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gu, X.; Gao, J.; Wang, Z.; Liu, G.; Barkema, H.W.; Han, B. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21Waf1/Cip1 to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radic. Biol. Med. 2019, 137, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Boo, Y.C. Siegesbeckiae herba extract and chlorogenic acid ameliorate the death of HaCaT keratinocytes exposed to airborne particulate matter by mitigating oxidative stress. Antioxidants 2021, 10, 1762. [Google Scholar] [CrossRef]
- Gherardini, J.; Wegner, J.; Chéret, J.; Ghatak, S.; Lehmann, J.; Alam, M.; Jimenez, F.; Funk, W.; Böhm, M.; Botchkareva, N.V.; et al. Transepidermal UV radiation of scalp skin ex vivo induces hair follicle damage that is alleviated by the topical treatment with caffeine. Int. J. Cosmet. Sci. 2019, 41, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Potratz, S.; Jungnickel, H.; Grabiger, S.; Tarnow, P.; Otto, W.; Fritsche, E.; von Bergen, M.; Luch, A. Differential cellular metabolite alterations in HaCaT cells caused by exposure to the aryl hydrocarbon receptor-binding polycyclic aromatic hydrocarbons chrysene, benzo[a]pyrene and dibenzo[a,l]pyrene. Toxicol. Rep. 2016, 3, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, Z.; Jiang, S.; Zhao, J.; Yan, S.; Xu, F.; Xu, J. Effects of Ambient Fine Particles PM2.5 on Human HaCaT Cells. Int. J. Environ. Res. Public Health 2017, 14, 72. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Zhen, A.X.; Cho, S.J.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Kim, T.H.; et al. 3,4-Dicaffeoylquinic acid protects human keratinocytes against environmental oxidative damage. J. Funct. Foods 2019, 52, 430–441. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Patel, M.; Brody, N.; Jagdeo, J. Caffeine protects human skin fibroblasts from acute reactive oxygen species-induced necrosis. J. Drugs Dermatol. 2012, 11, 1342–1346. [Google Scholar]
| Test Material | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) | Anti-Oxidant Activity TEAC (µM Trolox/g) | ||
|---|---|---|---|---|---|
| DPPH | Lipid Peroxidation Inhibition | ||||
| Coffee cherry pulp extract | CCM | 110.0 ± 0.8 a | 136.8 ± 3.7 a | 46.2 ± 2.2 a | 44.0 ± 3.5 a |
| CCS | 324.6 ± 1.2 c | 296.8 ± 1.2 c | 98.2 ± 0.8 b | 136.6 ± 6.2 b | |
| CCU | 127.2 ± 0.8 b | 151.0 ± 1.2 b | 52.3 ± 4.3 a | 60.3 ± 4.6 a | |
| Standard | Chlorogenic acid | NT | NT | 104.2 ± 0.6 b | 1283.8 ± 37.5 e |
| Caffeine | NT | NT | 100.6 ± 0.9 b | 556.5 ± 25.8 d | |
| Theophylline | NT | NT | 97.5 ± 3.1 b | 240.3 ± 8.6 c | |
| Test Material | Amount (mg/g Extract) | ||
|---|---|---|---|
| Theophylline | Chlorogenic Acid | Caffeine | |
| CCM | 4.2 ± 0.2 a | 6.2 ± 0.1 a | 26.8 ± 0.0 a |
| CCS | 45.9 ± 1.0 b | 17.3 ± 0.0 c | 45.0 ± 0.8 c |
| CCU | 3.6 ± 0.0 a | 7.3 ± 0.3 b | 30.1 ± 0.2 b |
| Test Material | Inhibition (%) | Tyrosinase (IC50; µg/mL) | |||
|---|---|---|---|---|---|
| Collagenase | Elastase | Hyaluronidase | |||
| Coffee cherry pulp extract | CCM | 68.9 ± 3.2 b | 26.4 ± 2.4 a | 31.1 ± 3.3 b | 898.0 ± 8.4 e |
| CCS | 89.4 ± 3.8 c | 56.1 ± 1.4 c | 32.5 ± 1.7 b | 523.8 ± 5.1 d | |
| CCU | 75.8 ± 3.7 b | 21.3 ± 2.7 a | 35.9 ± 0.9 b | 890.5 ± 2.4 e | |
| Positive control | EGCG | 95.9 ± 3.8 c | 71.1 ± 1.7 d | NT | NT |
| Tannic acid | NT | NT | 98.8 ± 1.8 d | NT | |
| Kojic acid | NT | NT | NT | 25.5 ± 0.5 a | |
| Standard | Chlorogenic acid | 85.4 ± 5.2 c | 69.7 ± 6.6 d | 50.1 ± 1.0 c | 121.0 ± 1.4 b |
| Caffeine | 39.0 ± 1.3 a | 34.0 ± 3.2 b | 19.1 ± 1.4 a | 130.0 ± 3.6 b | |
| Theophylline | 37.6 ± 1.0 a | 35.4 ± 3.6 b | 19.7 ± 2.1 a | 165.9 ± 5.5 c | |
| Variable | Anti-Oxidant Activity | Enzyme Inhibitory Activity | |||||
|---|---|---|---|---|---|---|---|
| DPPH | Lipid | Collagenase | Elastase | Hyaluronidase | Tyrosinase | ||
| Coffee cherry pulp extracts | TPC | 0.900 ** | 0.915 * | 0.790 * | 0.906 * | 0.552 | 0.565 |
| TFC | 0.930 * | 0.881 * | 0.898 * | 0.977 ** | 0.746 | 0.723 | |
| The detected amount of compound in the extracts | |||||||
| Chlorogenic acid | HPLC | 0.958 ** | 0.891 * | 0.969 ** | 0.975 ** | 0.309 | 1.000 ** |
| Caffeine | HPLC | 0.934 * | 0.922 * | 0.985 ** | 0.956 ** | 0.379 | 0.998 ** |
| Theophylline | HPLC | 0.982 ** | 0.842* | 0.940 * | 0.992 ** | 0.214 | 0.994 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Potprommanee, S.; Kiattisin, K. Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts. Foods 2023, 12, 4292. https://doi.org/10.3390/foods12234292
Preedalikit W, Chittasupho C, Leelapornpisid P, Potprommanee S, Kiattisin K. Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts. Foods. 2023; 12(23):4292. https://doi.org/10.3390/foods12234292
Chicago/Turabian StylePreedalikit, Weeraya, Chuda Chittasupho, Pimporn Leelapornpisid, Siriporn Potprommanee, and Kanokwan Kiattisin. 2023. "Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts" Foods 12, no. 23: 4292. https://doi.org/10.3390/foods12234292






