Investigation of Advanced Glycation End-Products, α-Dicarbonyl Compounds, and Their Correlations with Chemical Composition and Salt Levels in Commercial Fish Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Chemical Composition Analysis
2.4. Extraction and Detection of GO and MGO
2.5. Determination of Free and Protein-Bound CML and CEL
2.6. Statistic Analysis
3. Results and Discussion
3.1. Chemical Composition of Commercially Prepared and Prefabricated Fish Products
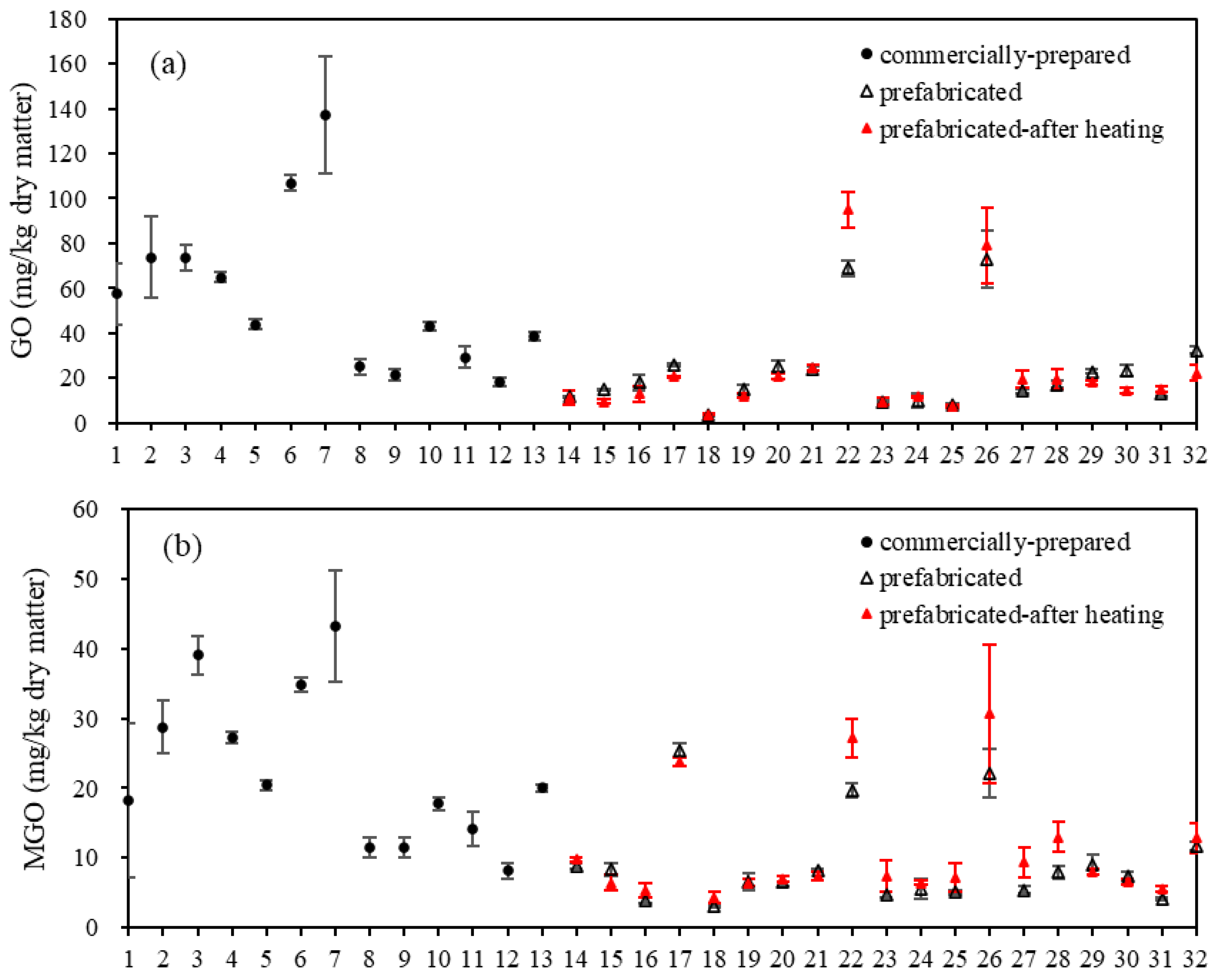
3.2. Contents of GO and MGO in Commercially Prepared and Prefabricated Fish Products
3.3. Contents of Free CML and CEL in Commercially Prepared and Prefabricated Fish Products
3.4. Contents of Protein-Bound CML and CEL in Commercially Prepared and Prefabricated Fish Products
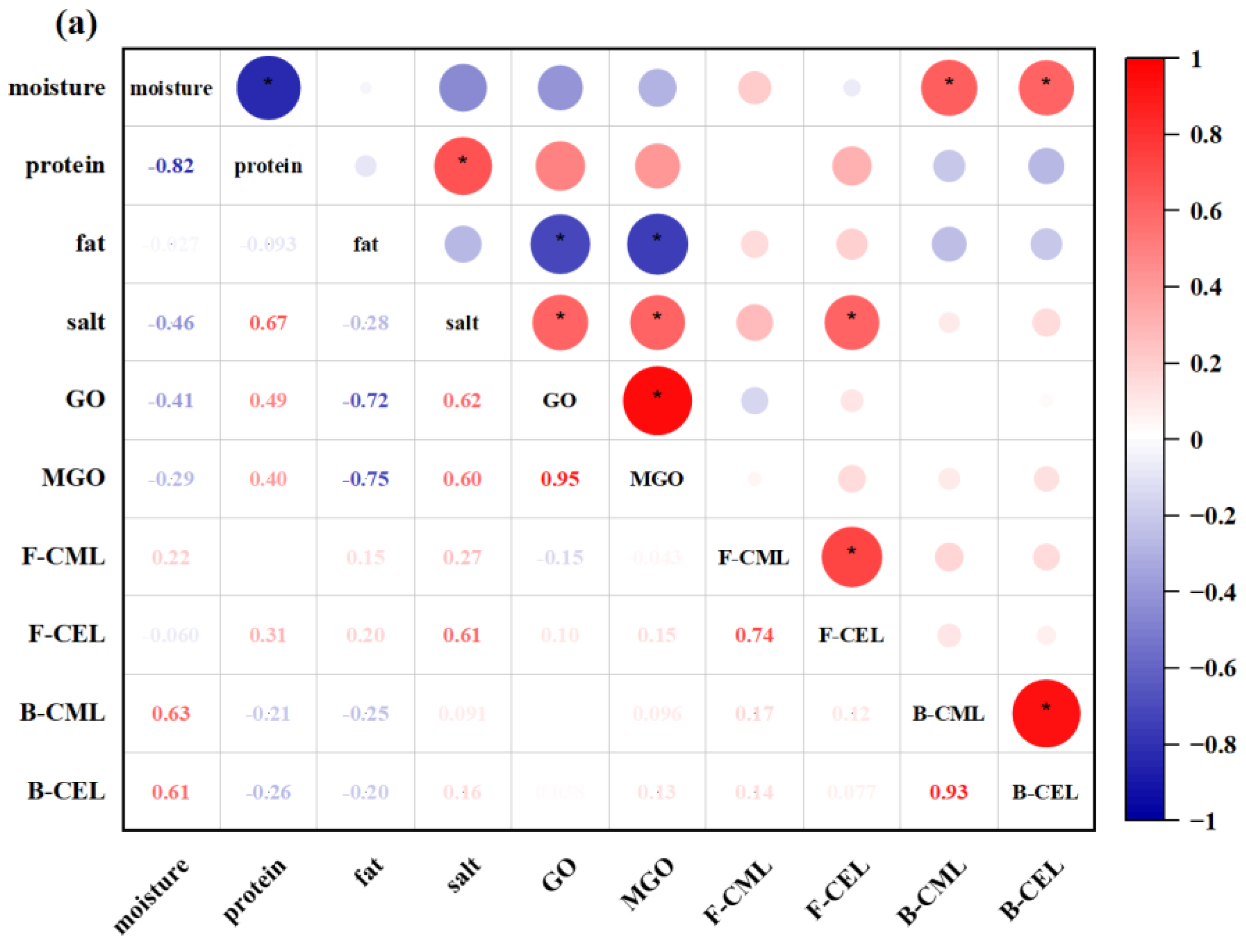
3.5. Correlation Analysis between the Levels of GO, MGO, AGEs, and Chemical Composition in Commercially Prepared and Prefabricated Fish Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, R.; Jiang, Y.; Qin, R.; Shi, H.; Jia, C.; Rong, J.; Liu, R. Study of the formation of food hazard factors in fried fish nuggets. Food Chem. 2022, 373, 131562. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Chu, F.; Huang, Y.; Niu, L.; Lai, K. Effects of salt concentrations on the advanced glycation end-products in dried salted spanish mackerel fillets during storage. J. Food Meas. Charact. 2022, 16, 3469–3476. [Google Scholar] [CrossRef]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine in fish muscle: Biological variation and effects of heat treatment. J. Food Compos. Anal. 2017, 57, 56–63. [Google Scholar] [CrossRef]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Fan, Y.; Huang, Y. Formation of advanced glycation end-products in fish muscle during heating: Relationship with fish freshness. J. Food Compos. Anal. 2017, 63, 133–138. [Google Scholar] [CrossRef]
- Alnajwal, A.M.; Alam, I.; Abulmeaty, M.; Razak, S.; Pawelec, G.; Alam, W. Intake of dietary advanced glycation end products influences inflammatory makers, immune phenotypes, and antiradical capacity of healthy elderly in a little-studied population. Food Sci. Nutr. 2020, 8, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Stolzenberg-Solomon, R.; Zimmerman, T.P.; Duan, Z.; Chen, L.; Kahle, L.; Risch, A.; Subar, A.F.; Cross, A.J.; Hollenbeck, A.; et al. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP diet and health study. Am. J. Clin. Nutr. 2015, 101, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Hull, G.L.J.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Huang, S.; Dong, X.; Zhang, Y.; Chen, Y.; Yu, Y.; Huang, M.; Zheng, Y. Formation of advanced glycation end products in raw and subsequently boiled broiler muscle: Biological variation and effects of postmortem aging and storage. Food Sci. Hum. Well. 2022, 11, 255–262. [Google Scholar] [CrossRef]
- Nguyen, H.T.; van der Fels-Klerx, H.J.; van Boekel, M.A.J.S. Nε-(carboxymethyl)lysine: A review on analytical methods, formation, and occurrence in processed food, and health impact. Food Rev. Int. 2013, 30, 36–52. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hu, H.; Xie, J.; Shen, M. Investigation into the contents of nutrients, Nε-carboxymethyllysine and Nε-carboxyethyllysine in various commercially canned fishes to find the correlation between them. J. Food Compos. Anal. 2021, 96, 103737. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Jaffe, R. Lipid-peroxidation of muscle food as affected by NaCl. J. Agric. Food Chem. 1991, 39, 1017–1021. [Google Scholar] [CrossRef]
- Sharedeh, D.; Gatellier, P.; Astruc, T.; Daudin, J.D. Effect of pH and NaCl levels in a beef marinade on physicochemical states of lipids and proteins and tissue microstructure. Meat Sci. 2015, 110, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Fan, Y.; Huang, Y. Combination effects of salts and cold storage on the formation of protein-bound Nε-(carboxymethyl)lysine and Nε-(carboxyethyl)lysine in raw and subsequently commercially sterilized ground pork. Food Chem. 2018, 264, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, C.; Quan, W.; Qin, F.; Wang, Z.; He, Z.; Zeng, M. Assessment the influence of salt and polyphosphate on protein oxidation and Nε-(carboxymethyl)lysine and Nε-(carboxyethyl)lysine formation in roasted beef patties. Meat Sci. 2021, 177, 108489. [Google Scholar] [CrossRef] [PubMed]
- Kul, D.B.; Anlar, P.; Oral, Z.F.Y.; Kaya, M.; Kaban, G. Furosine and Nε-carboxymethyl-lysine in cooked meat product (kavurma): Effects of salt and fat levels during storage. J. Stored Prod. Res. 2021, 93, 101856. [Google Scholar] [CrossRef]
- Kong, F.; Tang, J.; Rasco, B.; Crapo, C.; Smiley, S. Quality changes of salmon (Oncorhynchus gorbuscha) muscle during thermal processing. J. Food Sci. 2007, 72, S103–S111. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington VA, USA, 2005. [Google Scholar]
- Qiu, X.; Wu, Y.; Chen, S.; Sun, L.; Liu, G.; Lin, H. Oxidative stability and browning development of semi-dried Shrimp (Acetes chinensis) with different salt contents and packaging methods stored at refrigerated temperature. Food Sci. Technol. Res. 2020, 26, 239–245. [Google Scholar] [CrossRef]
- Li, L.; Kong, S.; Liu, Y.; Huang, Y.; Li, Y.; Lai, K. Effects of acetic acid, ethanol, and sodium chloride on the formation of Nε-carboxymethyllysine, Nε-carboxyethyllysine and their precursors in commercially sterilized pork. J. Food Meas. Charact. 2021, 15, 5337–5344. [Google Scholar] [CrossRef]
- Chu, F.; Lin, Y.; Huang, Y.; Niu, L.; Lai, K. Effect of sucrose on the formation of advanced glycation end-products of ground pork during freeze–thaw cycles and subsequent heat treatment. Foods 2023, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Formation of advanced glycation endproducts in ground beef under pasteurization conditions. Food Chem. 2015, 172, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Yu, H.; Zhang, L.; Zhao, Q.; Lai, K.; Liu, Y.; Huang, Y. Advanced glycation end-products in raw and commercially sterilized pork tenderloin and offal. J. Food Compos. Anal. 2022, 112, 104681. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Chen, M.; Li, X.; Xiang, X.; Lai, K. Protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine in raw and heat treated whites and yolks of hen eggs. J. Food Compos. Anal. 2020, 90, 103491. [Google Scholar] [CrossRef]
- Duman, E.; Kurban, H. Investigation of in vitro formation of advanced lipoxidation end products and advanced glycation end products precursors in high-fat processed meat products. Food Sci. Technol. 2022, 42, e110921. [Google Scholar] [CrossRef]
- Yan, S.; Wu, L.; Xue, X. α-Dicarbonyl compounds in food products: Comprehensively understanding their occurrence, analysis, and control. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1387–1417. [Google Scholar] [CrossRef]
- Meng, R.; Li, L.; Huang, Y.; Liu, Y.; Ma, X.; Xiang, X. Effects of sodium chloride and cold storage on the amounts of glyoxal, methylglyoxal in raw and cooked white meat of grass carp (Ctenopharyngodon idellus). J. Food Meas. Charact. 2021, 15, 5599–5606. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Gökmen, V. Effects of sodium chloride, potassium chloride, and calcium chloride on the formation of α-dicarbonyl compounds and furfurals and the development of browning in cookies during baking. J. Agric. Food Chem. 2016, 64, 7838–7848. [Google Scholar] [CrossRef]
- Zhu, Z.; Fang, R.; Huang, M.; Wei, Y.; Zhou, G. Oxidation combined with Maillard reaction induced free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine formation during braised chicken processing. Food Sci. Hum. Well. 2020, 9, 383–393. [Google Scholar] [CrossRef]
- Homoki-Farkas, P.; Örsi, F.; Kroh, L.W. Methylglyoxal determination from different carbohydrates during heat processing. Food Chem. 1997, 59, 157–163. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Huang, S.-F.; Liao, K.-W.; Ho, C.-T.; Hung, W.-L. Quantitation of α-dicarbonyls, lysine- and arginine-derived advanced glycation end products, in commercial canned meat and seafood products. J. Agric. Food Chem. 2023, 71, 6727–6737. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, L.; Yuan, S.; Guo, Y.; Yao, W.; Zhou, W.; Yu, H. Formation of advanced glycation end-products and α-dicarbonyl compounds through Maillard reaction: Solutions from natural polyphenols. J. Food Compos. Anal. 2023, 120, 105350. [Google Scholar] [CrossRef]
- Xu, L.; Du, B.; Xu, B. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015, 174, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Tavares, W.P.S.; Dong, S.; Jin, W.; Yang, Y.; Han, K.; Zha, F.; Zhao, Y.; Zeng, M. Effect of different cooking conditions on the profiles of Maillard reaction products and nutrient composition of hairtail (Thichiurus lepturus) fillets. Food Res. Int. 2018, 103, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chai, M.; Zeng, M.; He, Z.; Chen, J. Effect of lipid oxidation on the formation of Nε-carboxymethyl-lysine and Nε-carboxyethyl-lysine in Chinese-style sausage during storage. Food Chem. 2018, 269, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Chen, G.; Smith, J.S. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015, 168, 190–195. [Google Scholar] [CrossRef]
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315. [Google Scholar] [CrossRef]
- Wang, W.; Kou, Y.; Du, Y.; Li, M.; Zhang, J.; Yan, A.; Xie, J.; Shen, M. Investigation on the contents of Nε-carboxymethyllysine, Nε-carboxyethyllysine, and N-nitrosamines in commercial sausages on the Chinese market. Foods 2023, 12, 724. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, X.; Yang, Z.; Lai, Y.; Yang, Y.; Lei, C.; Zeng, Y. Pyrraline formation modulated by sodium chloride and controlled by encapsulation with different coating materials in the Maillard reaction. Biomolecules 2019, 9, 721. [Google Scholar] [CrossRef] [PubMed]



| Sample No. | Storage Condition | Package | Heating Method 1 | Shelf Life (Month) | Raw Material | Ingredients |
|---|---|---|---|---|---|---|
| 1 | RT | Regular | Frying | 9 | Yellow croaker | Salt, oil, white granulated sugar, starch, MSG |
| 2 | RT | Regular | Roasting | 12 | Liza haematocheila | Salt, white granulated sugar, trehalose |
| 3 | RT | Vacuum | Roasting | 12 | Yellow croaker | Salt, white granulated sugar, MSG, spices |
| 4 | RT | Regular | Roasting | 9 | Yellow croaker | Salt, white granulated sugar, MSG, spices |
| 5 | RT | Vacuum | CS | 9 | Yellow croaker | Salt, oil, white granulated sugar, soy sauce, MSG, starch, spices |
| 6 | RT | Vacuum | CS | 7 | Yellow trevally | Salt, oil, pepper, spices |
| 7 | RT | Vacuum | CS | 12 | Yellow trevally | Salt, oil, white granulated sugar, pepper, sesame, spices |
| 8 | RT | Vacuum | CS | 12 | Silver carp | Salt, oil, white granulated sugar, pepper, fermented beans, sesame, spices |
| 9 | RT | Canned | CS | 24 | Yellow croaker | Salt, oil, white granulated sugar, fermented beans, soy sauce, rice wine, spices |
| 10 | RT | Canned | CS | 36 | Sardine | Salt, water, tomato juice, xanthan gum |
| 11 | RT | Canned | CS | 36 | Dace | Salt, oil, white granulated sugar, fermented beans, soy sauce, water, spices |
| 12 | RT | Canned | CS | 24 | Anchovy | Salt, oil, white granulated sugar, soy sauce, rice wine, spices |
| 13 | RT | Canned | CS | 36 | Tuna | Salt, water, vegetable juice, MSG |
| 14 | RT | Vacuum | Raw | 6 | Sea eel | Salt |
| 15 | RT | Unpackaged | Raw | 1 | Yellow croaker | Salt |
| 16 | RT | Vacuum | Raw | 6 | Black carp | Salt, Baijiu, MSG, spices |
| 17 | RT | Unpackaged | Raw | 1 | Stolephorus commersonnii | Salt |
| 18 | RT | Vacuum | Raw | 6 | Yellow croaker | Salt |
| 19 | RT | Vacuum | Raw | 5 | Black carp | Salt, Baijiu, spices |
| 20 | Frozen | Unpackaged | Raw | -- | Black carp | Salt |
| 21 | Frozen | Unpackaged | Raw | -- | Black carp | Salt |
| 22 | Frozen | Vacuum | Raw | 24 | Sea eel | White granulated sugar, soy sauce, water, glucose syrup, fructose syrup, edible alcohol |
| 23 | Frozen | Vacuum | Raw | 12 | Basa fish | Salt, water |
| 24 | Frozen | Vacuum | Raw | 12 | Snakehead | Salt, white granulated sugar, starch |
| 25 | Frozen | Vacuum | Raw | 12 | Snakehead | Salt, trehalose |
| 26 | Frozen | Vacuum | Raw | 24 | Sea eel | White granulated sugar, soy sauce, glucose syrup, fructose syrup, edible alcohol |
| 27 | Frozen | Vacuum | Raw | 12 | Culter alburnus | Salt, cooking wine, MSG, spices |
| 28 | Frozen | Vacuum | Raw | 18 | Yellow croaker | Salt |
| 29 | Frozen | Vacuum | Raw | 18 | Yellow croaker | Salt, white granulated sugar, MSG |
| 30 | Frozen | Vacuum | Raw | 18 | Yellow croaker | Salt, white granulated sugar, MSG |
| 31 | Frozen | Vacuum | Raw | 12 | Whitefish | Salt, spices |
| 32 | Frozen | Vacuum | Raw | 18 | Bass | Salt, Baijiu, spices |
| Sample No. | Moisture (%) | Protein (%) | Fat (%) | Salt (%) |
|---|---|---|---|---|
| 1 | 6.53 ± 0.02 | 57.15 ± 1.40 | 17.10 ± 0.00 | 2.59 ± 0.17 |
| 2 | 15.92 ± 0.21 | 44.81 ± 0.08 | 1.45 ± 0.05 | 2.91 ± 0.25 |
| 3 | 18.51 ± 0.07 | 45.51 ± 0.82 | 10.15 ± 0.05 | 3.36 ± 0.09 |
| 4 | 12.89 ± 0.07 | 53.86 ± 0.68 | 8.45 ± 0.05 | 3.40 ± 0.37 |
| 5 | 43.53 ± 0.40 | 28.71 ± 0.03 | 14.35 ± 0.15 | 1.39 ± 0.07 |
| 6 | 23.98 ± 0.04 | 37.11 ± 0.05 | 6.35 ± 0.05 | 9.13 ± 0.22 |
| 7 | 25.71 ± 0.05 | 31.19 ± 1.22 | 6.30 ± 0.10 | 8.97 ± 0.70 |
| 8 | 34.67 ± 0.20 | 34.16 ± 0.05 | 21.15 ± 0.05 | 2.96 ± 0.11 |
| 9 | 31.21 ± 0.77 | 32.01 ± 0.35 | 27.40 ± 0.00 | 2.96 ± 0.44 |
| 10 | 67.30 ± 0.65 | 19.35 ± 0.24 | 9.50 ± 0.10 | 0.83 ± 0.12 |
| 11 | 33.70 ± 4.19 | 25.50 ± 1.53 | 35.25 ± 0.05 | 1.43 ± 0.28 |
| 12 | 23.74 ± 0.32 | 24.65 ± 0.07 | 39.65 ± 0.15 | 0.88 ± 0.03 |
| 13 | 76.21 ± 0.04 | 23.48 ± 0.05 | 1.05 ± 0.05 | 1.35 ± 0.25 |
| 14 | 51.43 ± 0.31 | 28.85 ± 0.16 | 4.65 ± 0.05 | 11.52 ± 0.45 |
| 15 | 48.94 ± 0.23 | 31.46 ± 0.66 | 4.35 ± 0.05 | 5.41 ± 0.57 |
| 16 | 51.56 ± 0.26 | 30.19 ± 0.19 | 3.50 ± 0.00 | 6.19 ± 0.25 |
| 17 | 39.44 ± 0.40 | 44.09 ± 0.72 | 6.65 ± 0.05 | 4.83 ± 0.09 |
| 18 | 58.70 ± 0.44 | 29.91 ± 0.08 | 1.95 ± 0.05 | 4.83 ± 0.09 |
| 19 | 48.21 ± 0.36 | 32.41 ± 0.08 | 8.10 ± 0.00 | 5.36 ± 0.28 |
| 20 | 62.64 ± 1.04 | 25.78 ± 0.17 | 4.30 ± 0.00 | 8.78 ± 0.26 |
| 21 | 70.56 ± 0.00 | 23.50 ± 0.12 | 3.90 ± 0.10 | 8.41 ± 0.03 |
| 22 | 58.47 ± 0.59 | 20.18 ± 0.41 | 18.25 ± 0.15 | 1.01 ± 0.02 |
| 23 | 88.09 ± 0.04 | 12.66 ± 0.11 | 0.60 ± 0.00 | 0.73 ± 0.14 |
| 24 | 84.58 ± 0.26 | 10.41 ± 0.03 | 3.15 ± 0.05 | 1.14 ± 0.06 |
| 25 | 84.14 ± 0.48 | 10.52 ± 1.32 | 3.45 ± 0.05 | 0.63 ± 0.04 |
| 26 | 51.06 ± 0.63 | 16.12 ± 0.26 | 22.70 ± 0.10 | 1.21 ± 0.06 |
| 27 | 80.77 ± 0.04 | 17.56 ± 0.18 | 0.80 ± 0.00 | 1.39 ± 0.19 |
| 28 | 72.18 ± 0.07 | 18.97 ± 0.35 | 10.00 ± 0.00 | 0.13 ± 0.02 |
| 29 | 74.30 ± 0.17 | 19.27 ± 0.15 | 11.40 ± 0.00 | 0.12 ± 0.02 |
| 30 | 65.33 ± 1.44 | 18.53 ± 0.21 | 12.50 ± 0.00 | 1.54 ± 0.06 |
| 31 | 72.87 ± 0.78 | 18.43 ± 0.03 | 11.15 ± 0.05 | 1.51 ± 0.06 |
| 32 | 79.45 ± 0.01 | 17.69 ± 0.36 | 0.90 ± 0.00 | 1.78 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, L.; Kong, S.; Chu, F.; Huang, Y.; Lai, K. Investigation of Advanced Glycation End-Products, α-Dicarbonyl Compounds, and Their Correlations with Chemical Composition and Salt Levels in Commercial Fish Products. Foods 2023, 12, 4324. https://doi.org/10.3390/foods12234324
Niu L, Kong S, Chu F, Huang Y, Lai K. Investigation of Advanced Glycation End-Products, α-Dicarbonyl Compounds, and Their Correlations with Chemical Composition and Salt Levels in Commercial Fish Products. Foods. 2023; 12(23):4324. https://doi.org/10.3390/foods12234324
Chicago/Turabian StyleNiu, Lihong, Shanshan Kong, Fuyu Chu, Yiqun Huang, and Keqiang Lai. 2023. "Investigation of Advanced Glycation End-Products, α-Dicarbonyl Compounds, and Their Correlations with Chemical Composition and Salt Levels in Commercial Fish Products" Foods 12, no. 23: 4324. https://doi.org/10.3390/foods12234324
APA StyleNiu, L., Kong, S., Chu, F., Huang, Y., & Lai, K. (2023). Investigation of Advanced Glycation End-Products, α-Dicarbonyl Compounds, and Their Correlations with Chemical Composition and Salt Levels in Commercial Fish Products. Foods, 12(23), 4324. https://doi.org/10.3390/foods12234324







