Metabolic Profiling and Molecular Mechanisms Underlying Melatonin-Induced Secondary Metabolism of Postharvest Goji Berry (Lycium barbarum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
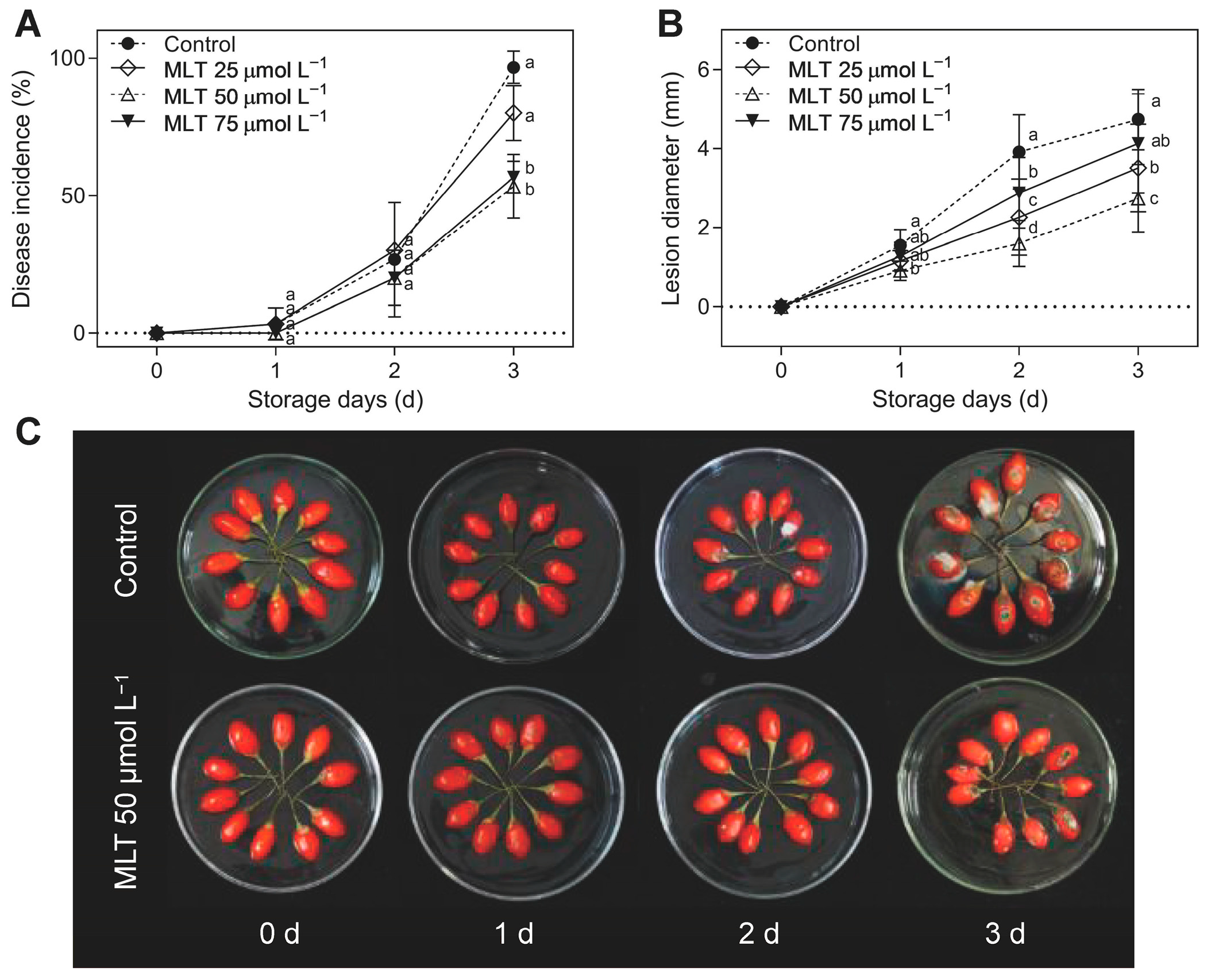
2.2. Injury Inoculation and Investigation of Disease Expansion
2.3. Analysis of Endogenous MLT Levels
2.4. Assay of Activities of Defense-related Enzymes
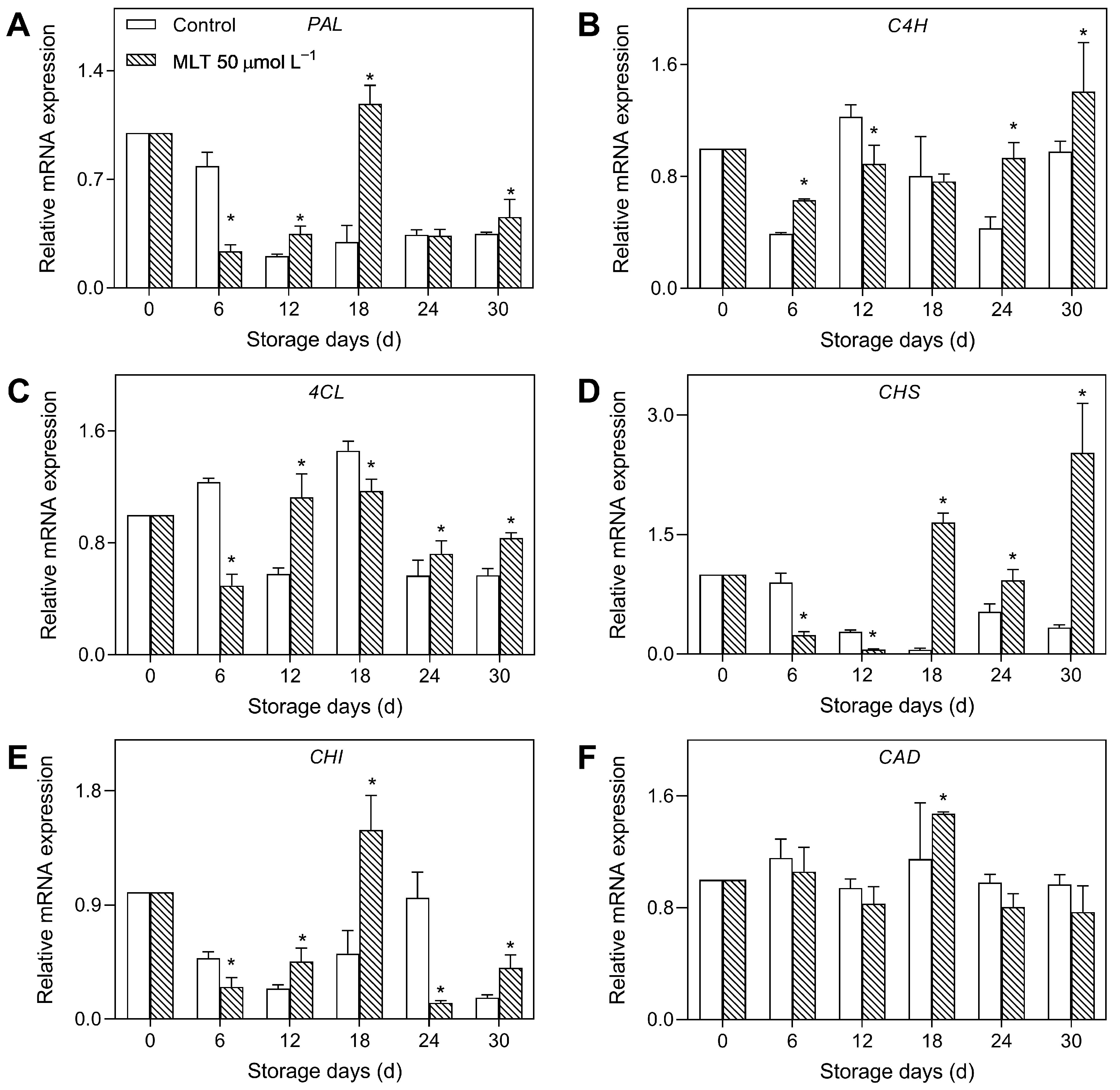
2.5. Expression Analysis of Genes Related to the Phenylpropanoid Pathway
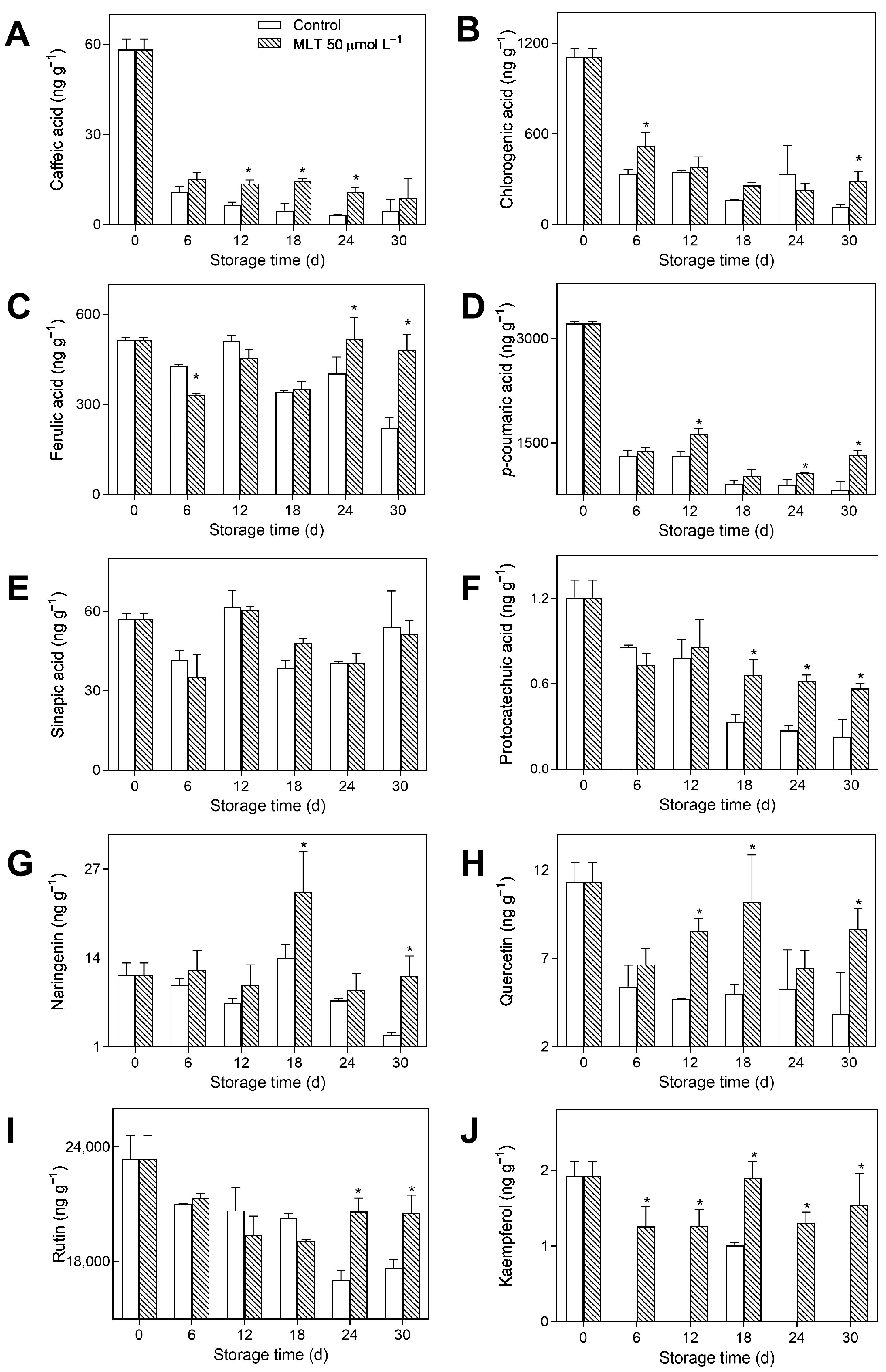
2.6. Analysis of Secondary Metabolites
2.7. Statistical Analysis
3. Results
3.1. Disease Incidence, Lesion Development, and Endogenous MLT Levels
3.2. Activities of Defense-Related Enzymes
3.3. Expressions of Genes Encoding Defense-Related Enzymes
3.4. Metabolic Profiling of Secondary Metabolite Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, R.H.; Zhang, X.X.; Ni, Z.J.; Thakur, K.; Wang, W.; Yan, Y.M.; Cao, Y.L.; Zhang, J.G.; Rengasamy, K.R.R.; Wei, Z.J. Lycium barbarum (Goji) as functional food: A review of its nutrition, phytochemical structure, biological features, and food industry prospects. Crit. Rev. Food Sci. Nutr. 2022, 63, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, M.A.; Jurić, S.; Vidrih, R.; Vinceković, M.; Vuković, M.; Jemrić, T. The effects of postharvest application of lecithin to improve storage potential and quality of fresh goji (Lycium barbarum L.) berries. Food Chem. 2017, 230, 241–249. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Yan, L.; Zheng, H.; Liu, C.; Zheng, L. Effects of postharvest cysteine treatment on sensory quality and contents of bioactive compounds in goji fruit. Food Chem. 2022, 366, 130546. [Google Scholar] [CrossRef] [PubMed]
- Murariu, O.C.; Brezeanu, C.; Jităreanu, C.D.; Robu, T.; Irimia, L.M.; Trofin, A.E.; Popa, L.D.; Stoleru, V.; Murariu, F.; Brezeanu, P.M. Functional quality of improved tomato genotypes grown in open field and in plastic tunnel under organic farming. Agriculture 2021, 11, 609. [Google Scholar] [CrossRef]
- Zhou, Y.; Lai, Y.; Chen, Z.; Qu, H.; Ma, S.; Wang, Y.; Jiang, Y.M. Evolution of physiological characteristics and nutritional quality in fresh goji berry (Lycium barbarum) stored under different temperatures. J. Food Process Preserv. 2020, 44, e14835. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, H.W.; Tian, Y.; Sun, D.W. Effects of salicylic acid combined with gas atmospheric control on postharvest quality and storage stability of wolfberries: Quality attributes and interaction evaluation. J. Food Process Preserv. 2021, 44, e13764. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Wang, J.; Yang, Q.; Wang, P.; Zhao, H.; Wang, J.; Wang, C.; Xu, X.H. Salicylic acid inhibits the postharvest decay of goji berry (Lycium barbarum L. ) by modulating the antioxidant system and phenylpropanoid metabolites. Postharvest Biol. Technol. 2021, 178, 111558. [Google Scholar]
- Zhang, H.; Ma, Z.; Wang, J.; Wang, P.; Lu, D.; Deng, S.; Lei, H.L.; Gao, Y.F.; Tao, Y.Y. Treatment with exogenous salicylic acid maintains quality, increases bioactive compounds, and enhances the antioxidant capacity of fresh goji (Lycium barbarum L.) fruit during storage. LWT Food Sci. Technol. 2021, 140, 110837. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Z.J.; Song, C.B.; Ma, W.P.; Cao, S.Q.; Wei, Z.J. Hydrogen sulfide treatment improves quality attributes via regulating the antioxidant system in goji berry (Lycium barbarum L.). Food Chem. 2022, 405, 134858. [Google Scholar] [CrossRef]
- Cong, K.P.; Li, T.T.; Wu, C.E.; Zeng, K.F.; Zhang, J.H.; Fan, G.J.; Pan, Y.; Wang, J.H.; Suo, A.D. Effects of plasma-activated water on overall quality of fresh goji berries during storage. Sci. Hortic. 2022, 293, 110650. [Google Scholar] [CrossRef]
- Ban, Z.; Wei, W.; Yang, X.; Feng, J.; Guan, J.; Li, L. Combination of heat treatment and chitosan coating to improve postharvest quality of wolfberry (Lycium barbarum). Int. J. Food Sci. Technol. 2015, 50, 1019–1025. [Google Scholar] [CrossRef]
- He, X.; Wu, C.; Lu, L.; Yan, X.; Yu, H.; Kang, N. Influence of acidic electrolyzed water combined with vacuum precooling treatment on quality and antioxidant performance of fresh Lycium barbarum L. J. Food Process Preserv. 2022, 46, e17149. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R. Melatonin: A blooming biomolecule for postharvest management of perishable fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, T.; Liu, G.; Hu, M.; Yun, Z.; Duan, X.; Cai, K.; Jiang, G.X. Inhibition of downy blight and enhancement of resistance in litchi fruit by postharvest application of melatonin. Food Chem. 2021, 347, 129009. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X.L. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Madebo, M.P.; Zheng, Y.; Jin, P. Melatonin-mediated postharvest quality and antioxidant properties of fresh fruits: A comprehensive meta-analysis. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3205–3226. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, L.; Li, K.; Cao, J.; Zhao, Z. Integrative transcriptomic and metabolomic alterations unravel the effect of melatonin on mitigating postharvest chilling injury upon plum (cv.Friar) fruit. Postharvest Biol. Technol. 2022, 186, 111819. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Liu, J.; Jiang, Y.; Zhang, Z.K. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods 2020, 9, 454. [Google Scholar] [CrossRef]
- Qu, G.; Wu, W.; Ba, L.; Ma, C.; Ji, N.; Cao, S. Melatonin enhances the postharvest disease resistance of blueberries fruit by modulating the jasmonic acid signaling pathway and phenylpropanoid metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Deng, S.; Wang, P.; Zhang, H.; Lei, H.; Cui, M.; Ma, W.; Wang, J. Effects of melatonin treatment on postharvest physiology and storage quality of Goji berry. Food Ferment. Ind. 2022, 48, 198–204, (In Chinese with English Abstract). [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Zhang, H.; Wang, P.; Wang, C.; Zhou, Y.; Li, H.; Yu, S.; Wu, R. Inhibitory effect of carvacrol against Alternaria alternata causing goji fruit rot by disrupting the integrity and composition of cell wall. Front. Microbiol. 2023, 14, 1139749. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, H.; Chen, S.; Yu, D.; Reiter, R.J. Phytomelatonin: An emerging regulator of plant biotic stress resistance. Trends Plant Sci. 2021, 26, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, S.; Xue, J.; Mu, B.; Song, H.; Liu, Y. Exogenous melatonin treatment induces disease resistance against Botrytis cinerea on post-harvest grapes by activating defence responses. Foods 2022, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, Q.; Feng, S.; Lei, Q.; Abbas, F.; Yao, Y.; Chen, W.; Li, X.; Zhu, X. Melatonin maintains fruit quality and reduces anthracnose in postharvest papaya via enhancement of antioxidants and inhibition of pathogen development. Antioxidants 2022, 11, 804. [Google Scholar] [CrossRef]
- Yan, R.; Li, S.; Cheng, Y.; Kebbeh, M.; Huan, C.; Zheng, X. Melatonin treatment maintains the quality of cherry tomato by regulating endogenous melatonin and ascorbate-glutathione cycle during room temperature. J. Food Biochem. 2022, 46, e14285. [Google Scholar] [CrossRef]
- Sun, C.; Huang, Y.; Lian, S.; Saleem, M.; Li, B.; Wang, C. Improving the biocontrol efficacy of Meyerozyma guilliermondii Y-1 with melatonin against postharvest gray mold in apple fruit. Postharvest Biol. Technol. 2021, 171, 111351. [Google Scholar] [CrossRef]
- Yan, R.; Xu, Q.; Dong, J.; Kebbeh, M.; Shen, S.; Huan, C.; Zheng, X. Effects of exogenous melatonin on ripening and decay incidence in plums (Prunus salicina L. cv. Taoxingli) during storage at room temperature. Sci. Hortic. 2022, 292, 110655. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Zhang, Y.; Butelli, E.; Alseekh, S.; Tohge, T.; Rallapalli, G.; Luo, J.; Kawar, P.; Hill, L.; Santino, A.; Fernie, A.R.; et al. Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat. Commun. 2015, 6, 8635. [Google Scholar] [CrossRef]
- Shang, F.; Liu, R.; Wu, W.; Han, Y.; Fang, X.; Chen, H.; Gao, H. Effects of melatonin on the components, quality and antioxidant activities of blueberry fruits. LWT Food Sci. Technol. 2021, 147, 111582. [Google Scholar] [CrossRef]



| Gene Name | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Product (bp) |
|---|---|---|---|
| PAL | CGCCACGAGATAGGTTGATGACAT | GGCTTCTTACTGCTCGGAACTTCA | 169 |
| C4H | GCAAGTGACCGAGCCAGACA | AGCCACCAAGCATTAACCAAGATC | 177 |
| 4CL | TGAGAGTCGGAGCAGCGATATTGA | TTCCTAATGGAGCACCACCAGACA | 195 |
| CHS | CACTGATAGCGAGCACAAGACTGA | AGGCACCTCAACAACCACTATGTC | 180 |
| CHI | CTGCCACTGCAAAGATTTCATCCA | CCCTGCTTCCACCTAAGTACCATT | 114 |
| CAD | ACCAGAACAGGCAGCACCACTA | CTCCCATGTGTCCAACTCCTCCT | 128 |
| Actin | CTCAGCACCTTCCAGCAGAT | TAACACTGCAACCGCATTTC | 162 |
| EF1α | GAAGGGTGTCCCTCAGATCA | CCGTCCATGTCGTCTCTTTT | 180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, H.; Hou, J.; Yang, E.; Zhao, L.; Zhou, Y.; Ma, W.; Ma, D.; Li, J. Metabolic Profiling and Molecular Mechanisms Underlying Melatonin-Induced Secondary Metabolism of Postharvest Goji Berry (Lycium barbarum L.). Foods 2023, 12, 4326. https://doi.org/10.3390/foods12234326
Wang J, Zhang H, Hou J, Yang E, Zhao L, Zhou Y, Ma W, Ma D, Li J. Metabolic Profiling and Molecular Mechanisms Underlying Melatonin-Induced Secondary Metabolism of Postharvest Goji Berry (Lycium barbarum L.). Foods. 2023; 12(23):4326. https://doi.org/10.3390/foods12234326
Chicago/Turabian StyleWang, Junjie, Huaiyu Zhang, Jie Hou, En Yang, Lunaike Zhao, Yueli Zhou, Wenping Ma, Danmei Ma, and Jiayi Li. 2023. "Metabolic Profiling and Molecular Mechanisms Underlying Melatonin-Induced Secondary Metabolism of Postharvest Goji Berry (Lycium barbarum L.)" Foods 12, no. 23: 4326. https://doi.org/10.3390/foods12234326






