The Effect of Temperature and Storage Duration on the Quality and Attributes of the Breast Meat of Hens after Their Laying Periods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Evaluation of the Physicochemical Characteristics of Meat
2.3. Microbiological Analysis
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, H.; Wu, J. Conventional use and sustainable valorization of spent egg-laying hens as functional foods and biomaterials: A review. Bioresour. Bioprocess. 2022, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Kim, H.Y. Physicochemical characteristics of breast and thigh meats from old broiler breeder hen and old laying hen and their effects on quality properties of pressed ham. Poult. Sci. 2020, 99, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Sokołowicz, Z.; Augustyńska-Prejsnar, A.; Krawczyk, J.; Kačániová, M.; Kluz, M.; Hanus, P.; Topczewska, J. Technological and sensory quality and microbiological safety of RIR chicken breast meat marinated with fermented milk products. Animals 2021, 1111, 3282. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F. The European Union and the fight against food waste and losses: From policy to practice. In Food Loss and Waste Policy; Routledge: London, UK, 2022; pp. 92–105. [Google Scholar]
- Laaninen, T. Reducing Food Waste in the European Union, EPRS: European Parliamentary Research Service. 2020. Available online: https://policycommons.net/artifacts/1426904/reducing-food-waste-in-the-european-union/2041486/ (accessed on 6 November 2023).
- Chmiel, M.; Słowiński, M. Effect of Storage in Display Cases on the Sensory Quality of Chicken Breast Meat (M. Pectoralis). Braz. J. Poult. Sci. 2018, 20, 91–98. [Google Scholar] [CrossRef]
- Sujiwo, J.; Kim, D.; Jang, A. Relation among quality traits of chicken breast meat during cold storage: Correlations between freshness traits and torrymeter values. Poult. Sci. 2018, 97, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Kaewthong, P.; Pomponio, L.; Carrascal, J.R.; Knøchel, S.; Wattanachant, S.; Karlsson, A.H. Changes in the Quality of Chicken Breast Meat due to Super chilling and Temperature Fluctuations during Storage. J. Poult. Sci. 2019, 56, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Jeon, J.; Nam, K.C.; Shim, K.S.; Jung, J.H.; Kim, K.S.; Choi, Y.; Kim, S.H.; Jang, A. Comparison of the quality characteristics of chicken breast meat from conventional and animal welfare farms under refrigerated storage. Poult. Sci. 2020, 99, 1788–1796. [Google Scholar] [CrossRef]
- Marmion, M.; Ferone, M.T.; Whyte, P.; Scannell, A.G.M. The changing microbiome of poultry meat; from farm to fridge. Food Microbiol. 2021, 99, 103823. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Carrión-Granda, X.; Mate, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Katiyo, W.; de Kock, H.L.; Coorey, R.; Buys, E.M. Sensory implications of chicken meat spoilage in relation to microbial and physicochemical characteristics during refrigerated storage. LWT-Food Sci. Technol. 2020, 128, 109468. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of modified atmosphere packaging and active/smart technologies to red meat and poultry: A review. Food Bioprocess Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Hinton, A., Jr. Preventing spoilage of poultry meat. In Achieving Sustainable Production of Poultry Meat; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 1, pp. 343–358. [Google Scholar]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Garavito, J.; Moncayo-Martínez, D.; Castellanos, D.A. Evaluation of antimicrobial coatings on preservation and shelf life of fresh chicken breast fillets under cold storage. Foods 2020, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ntzimani, A.; Kalamaras, A.; Tsironi, T.; Taoukis, P. Shelf Life Extension of Chicken Cuts Packed under Modified Atmospheres and Edible Antimicrobial Coatings. Appl. Sci. 2023, 13, 4025. [Google Scholar] [CrossRef]
- Dourou, D.; Spyrelli, E.D.; Doulgeraki, A.I.; Argyri, A.A.; Grounta, A.; Nychas, G.J.E.; Chorianopoulos, N.G.; Tassou, C.C. Microbiota of chicken breast and thigh fillets stored under different refrigeration temperatures assessed by next-generation sequencing. Foods 2021, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Sivarajan, M.; Lalithapriya, U.; Mariajenita, P.; Vajiha, B.A.; Harini, K.; Madhushalini, D.; Sukumar, M. Synergistic effect of spice extracts and modified atmospheric packaging towards non-thermal preservation of chicken meat under refrigerated storage. Poult. Sci. 2017, 96, 2839–2844. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Figueroa, J.; Cerda-Leal, F.; Alejandro-Martín, S.; Gacitúa, W. Evaluation of the PLA-nZH-Cu Nanocomposite Film on the Micro-Biological, Organoleptic and Physicochemical Qualities of Packed Chicken Meat. Foods 2022, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.N.; Csehi, B.; József, S.; Ferenc, H.; Kiskó, G.; Dalmadi, I.; Friedrich, L. Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions. Foods 2021, 10, 1855. [Google Scholar] [CrossRef]
- da Rocha, T.C.; Costa Filho, D.V.; de Carvalho, L.M.; de Carvalho, J.M.; Estevez, M.; Madruga, M.S. Effect of refrigeration and freezing on the oxidative stability of WB chicken breast. LWT-Food Sci. Technol. 2022, 171, 114108. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.C.; Huang, M.; Xu, B.C.; Zhou, G.H. The effects of different chilling methods on meat quality and calpain activity of pork muscle longissimus dorsi. J. Food Sci. 2012, 77, C27–C32. [Google Scholar] [CrossRef]
- Latou, E.; Mexis, S.F.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Combined effect of chitosan and modified atmosphere packaging for shelf life extension of chicken breast fillets. LWT-Food Sci. Technol. 2014, 55, 263–268. [Google Scholar] [CrossRef]
- Saleh, E.; Morshdy, A.E.; El-Manakhly, E.; Al-Rashed, S.F.; Hetta, H.; Jeandet, P.; Yahia, R.; Batiha, G.; Ali, E. Effects of olive leaf extracts as natural preservative on retailed poultry meat quality. Foods 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Al-jasser, M.S. Effect of cooling and freezing temperatures on microbial and chemical properties of chicken meat during storage. J. Food Agric. Environ. 2012, 10, 113–116. [Google Scholar]
- Stonehouse, G.G.; Evans, J.A. The use of supercooling for fresh foods: A review. J. Food Eng. 2015, 148, 74–79. [Google Scholar] [CrossRef]
- Bruckner, S.; Albrecht, A.; Petersen, B.; Kreyenschmidt, J. Characterization and comparison of spoilage processes in fresh pork and poultry. J. Food Qual. 2012, 35, 372–382. [Google Scholar] [CrossRef]
- Andreani, N.A.; Fasolato, L. Pseudomonas and related genera. In The Microbiological Quality of Food; Woodhead Publishing: Cambridge, UK, 2017; pp. 25–59. [Google Scholar] [CrossRef]
- Kondratowicz, J.; Chwastowska-Siwiecka, I.; Burczyk, E.; Piekarska, J.; Kuldo, Z. Sensory & microbiological assessment of turkey hens breast muscles depending on method and time of cold storage. Żywność Nauka Technol. Jakość 2011, 18, 143–152. [Google Scholar] [CrossRef]
- Tuncer, B.; Sireli, U.T. Microbial growth on broiler carcasses stored at different temperatures after air- or water-chilling. Poult. Sci. 2008, 87, 793–799. [Google Scholar] [CrossRef]
- Gratta, F.; Fasolato, L.; Birolo, M.; Zomeño, C.; Novelli, E.; Petracci, M.; Pascual, A.; Xiccato, G.; Trocino, A. Effect of breast myopathies on quality and microbial shelf life of broiler meat. Poult. Sci. 2019, 98, 2641–2651. [Google Scholar] [CrossRef]
- Patsias, A.; Badeka, A.V.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of freeze chilling and MAP on quality parameters of raw chicken fillets. Food Microbiol. 2008, 25, 575–581. [Google Scholar] [CrossRef]
- Dawson, P.L.; Chaves, B.D.; Northcutt, J.K.; Han, I.Y. Quality and shelf life of fresh chicken breasts subjected to crust freezing with and without skin. J. Food Qual. 2013, 36, 361–368. [Google Scholar] [CrossRef]
- Galarz, L.A.; Fonseca, G.G.; Prentice, C. Predicting bacterial growth in raw, salted, and cooked chicken breast fillets during storage. Food Sci. Technol. Int. 2016, 22, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Yimenu, S.M.; Koo, J.; Kim, B.S.; Kim, J.H.; Kim, J.Y. Freshness-based real-time shelf-life estimation of packaged chicken meat under dynamic storage conditions. Poult. Sci. 2019, 98, 6921–6930. [Google Scholar] [CrossRef]
- Meredith, H.; Valdramidis, V.; Rotabakk, B.T.; Sivertsvik, M.; McDowell, D.; Bolton, D.J. Effect of different modified atmospheric packaging (MAP) gaseous combinations on Campylobacter and the shelf-life of chilled poultry fillets. Food Microbiol. 2014, 44, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Vaikousi, H.; Biliaderis, C.G.; Koutsoumanis, K.P. Applicability of a microbial time-temperature indicator (TTI) for monitoring spoilage of modified atmosphere packed minced meat. Int. J. Food Microbiol. 2009, 133, 272–278. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.B.; Longhia, D.A.; Martinsa, W.F.; de Aragaoa, G.M.F.; Carciofia, B.A.M. Mathematical modeling of Lactobacillus viridescens growth in vacuum packed sliced ham under non isothermal conditions. Procedia Food Sci. 2016, 7, 33–36. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 834/2007 of 28 June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No 2092/91. 2007. Available online: https://eur-lex.europa.eu/eli/reg/2007/834/oj (accessed on 10 October 2023).
- Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/889/oj (accessed on 10 October 2023).
- Law of June 23, 2022 on organic farming and production. J. Laws 2023, 2023, 1235.
- Ziołecki, J.; Doruchowski, W. Method of Assessing the Slaughter Value of the Poultry; COBRD Publishing: Poznań, Poland, 1989. [Google Scholar]
- Grau, R.; Hamm, R. Eine einfache Methode zur Bestimmung der Wasserbindung im Muskel. Naturwiss 1953, 40, 29–30. [Google Scholar] [CrossRef]
- PN-EN ISO 11133:2014-07; Quality-Assured Culture Media for Food and Water Testing to Enhance Consumer Safety. ISO: Geneva, Switzerland, 2014.
- ISO 9308-1:2014; Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora. ISO: Geneva, Switzerland, 2014.
- ISO 13720:2010; Meat and Meat Products—Enumeration of Presumptive Pseudomonas spp. ISO: Geneva, Switzerland, 2010.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- Shell, W.S.; Sayed, M.L.; Allah, F.M.G.; Gamal, F.E.M.; Khder, A.A.; Samy, A.A.; Ali, A.H.M. Matrix-assisted laser desorption-ionisation-time-of-flight mass spectrometry as a reliable proteomic method for characterisation of Escherichia coli and Salmonella isolates. Vet. World 2017, 10, 1083–1093. [Google Scholar] [CrossRef]
- Kosikowska, U.; Stepień-Pysniak, D.; Pietras-Ozga, D.; Andrzejczuk, S.; Juda, M.; Malm, A. Application of MALDI-TOF MS for identification of clinical isolates of bacteria from humans and animals. J. Lab. Diag. 2015, 51, 23–30. [Google Scholar] [CrossRef]
- Hess, C.; Alispahic, M.; Hess, M. Application of MALDI-TOF MS in veterinary and food microbiology. In MALDI-TOF Mass Spectrometry in Microbiology; Kostrzewa, M., Schubert, S., Eds.; Max Von Pettenkofer-Institut: Munich, Germany, 2016; pp. 109–126. [Google Scholar]
- Peruzy, M.F.; Murru, N.; Yu, Z.; Cnockaert, M.; Joossens, M.; Proroga, Y.T.R.; Houf, K. Determination of the microbiological contamination in minced pork by culture dependent and 16S amplicon sequencing analysis. Int. J. Food Microbiol. 2019, 290, 27–35. [Google Scholar] [CrossRef]
- Ramatla, T.; Ngoma, L.; Mwanza, M. The Utility of MALDI-TOF-Mass Spectrometry, Analytical Profile Index (API) and Conventional-PCR for the Detection of Foodborne Pathogens from Meat. J. Food Nutr. Res. 2021, 9, 442–448. [Google Scholar] [CrossRef]
- Altakhis, M.; Pillidge, C.J.; Osborn, A.M.; Torley, P.J.; Kaur, M. Assessment of the potential use of MALDI-TOF MS for the identification of bacteria associated with chilled vacuum-packaged lamb meat. Meat Sci. 2021, 177, 108508. [Google Scholar] [CrossRef] [PubMed]
- Kunová, S.; Sendra, E.; Haščík, P.; Vukovic, N.L.; Vukic, M.; Kačániová, M. Influence of essential oils on the microbiological quality of fish meat during storage. Animals 2021, 11, 3145. [Google Scholar] [CrossRef] [PubMed]
- ISO 8586-2; Sensory Analysis—General Guidance for the Selection, Training and Monitoring of Assessors—Part 2: Expert Sensory Assessors. ISO: Geneva, Switzerland, 2008.
- PN-EN ISO 8589:2010; General Guidelines for the Design of a Sensory Analysis Laboratory. iTeh Standards: Newark, DE, USA, 2010.
- StatSoft Electronic Statistics Textbook; Data Analysis Software System, Version 13.3; StatSoft, Inc.: Kraków, Poland, 2018.
- Nikmanesh, A.; Baghaei, H.; Mohammadi Nafchi, A. Development and Characterization of Antioxidant and Antibacterial Films Based on Potato Starch Incorporating Viola odorata Extract to Improve the Oxidative and Microbiological Quality of Chicken Fillets during Refrigerated Storage. Foods 2023, 12, 2955. [Google Scholar] [CrossRef] [PubMed]
- Haghighatpanah, N.; Omar-Aziz, M.; Gharaghani, M.; Khodaiyan, F.; Hosseini, S.S.; Kennedy, J.F. Effect of mung bean protein isolate/pullulan films containing marjoram (Origanum majorana L.) essential oil on chemical and microbial properties of minced beef meat. Int. J. Biol. Macromol. 2022, 201, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Surmei, E.; Usturoi, M.G.; Albu, A. Studies on the Evolution of refrigerated poultry meat. Lucr. Stiintifice Ser. Zootehni. 2013, 60, 170–172. [Google Scholar]
- Ruíz-Cruz, S.; Valenzuela-López, C.C.; Chaparro-Hernández, S.; Ornelas-Paz, J.D.J.; Toro-Sánchez, C.L.D.; Márquez-Ríos, E.; Valdez-Hurtado, S. Effects of chitosan-tomato plant extract edible coatings on the quality and shelf life of chicken fillets during refrigerated storage. Food Sci. Technol. 2018, 39, 103–111. [Google Scholar] [CrossRef]
- Surmei, E.; Usturoi, M.G. Considerations regarding quality of Poultry Meat stored in refrigeration conditions. Lucr. Ştiinţifice–Ser. Zooteh. 2012, 58, 199–202. [Google Scholar]
- Sinhamahapatra, M.; Biswas, S.; Das, A.K.; Battacharya, D. Comparative study of different surface decontaminants on chicken quality. Br. Poult. Sci. 2004, 45, 624–630. [Google Scholar] [CrossRef]
- Aziman, N.; Jawaid, M.; Mutalib, N.A.A.; Yusof, N.L.; Nadrah, A.H.; Nazatul, U.K.; Tverezovskiy, V.V.; Tverezovskaya, O.A.; Fouad, H.; Braganca, R.M. Antimicrobial Potential of Plastic Films Incorporated with Sage Extract on Chicken Meat. Foods 2021, 10, 2812. [Google Scholar] [CrossRef]
- Mushi, D.E.; Safari, J.; Mtenga, L.A.; Kifaro, G.C.; Eik, L.O. Effects of concentrate levels on fattening performance, carcass and quality attributes of Small East African × Norwegian crossbred goats fed low quality grass hay. Livest. Sci. 2009, 124, 148–155. [Google Scholar] [CrossRef]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.E.; Lee, J.; Kim, Y.J.; Jo, C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Yehia, H.M.; Elkhadragy, M.F.; Al-Megrin, W.A.; Al-Masoud, A.H. Citrox Improves the Quality and Shelf Life of Chicken Fillets Packed under Vacuum and Protects against Some Foodborne Pathogens. Animals 2019, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, S.; Albrecht, A.; Petersen, B.; Kreyenschmidt, J. Influence of cold chain interruptions on the shelf life of fresh pork and poultry. Int. J. Food Sci. Technol. 2012, 47, 1639–1646. [Google Scholar] [CrossRef]
- Rahkila, R.; Johansson, P.; Säde, E.; Björkroth, J. Identification of enterococci from broiler products and a broiler processing plant and description of Enterococcus viikkiensis sp. nov. Appl. Environ. Microbiol. 2011, 77, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun, A.A.M.; Debevere, J.M.; Mossel, D.A.A. Significance of Enterobacteriaceae as index organisms for hygiene on fresh untreated poultry, poultry treated with lactic acid and poultry stored in a modified atmosphere. Food Microbiol. 1994, 11, 169–176. [Google Scholar] [CrossRef]
- Bolton, D.J.; Meredith, H.; Walsh, D.; McDowell, D.A. The effect of chemical treatments in laboratory and broiler plant studies on the microbial status and shelf-life of poultry. Food Control 2014, 36, 230–237. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shel life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef]
- Rodríguez-Calleja, J.M.; Cruz-Romero, M.C.; O’Sullivan, M.G.; García-López, M.L.; Kerry, J.P. High-pressure-based hurdle strategy to extend the shelf-life of fresh chicken breast fillets. Food Control 2012, 25, 516–524. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef]
- Rouger, A.; Moriceau, N.; Prévost, H.; Remenant, B.; Zagorec, M. Diversity of bacterial communities in French chicken cuts stored under modified atmosphere packaging. Food Microbiol. 2018, 70, 7–16. [Google Scholar] [CrossRef]
- Lee, H.S.; Kwon, M.; Heo, S.; Kim, M.G.; Kim, G.B. Characterization of the biodiversity of the spoilage microbiota in chicken meat using next generation sequencing and culture dependent approach. Korean J. Food Sci. Anim. Resour. 2017, 37, 535–541. [Google Scholar] [CrossRef]
- Morales, P.A.; Aguirre, J.S.; Troncoso, M.R.; Figueroa, G.O. Phenotypic and genotypic characterization of Pseudomonas spp. present in spoiled poultry fillets sold in retail settings. LWT-Food Sci. Technol. 2016, 73, 609–614. [Google Scholar] [CrossRef]
- Stellato, G.; Utter, D.R.; Voorhis, A.; De Angelis, M.; Murat Eren, A.; Ercolini, D. A few Pseudomonas oligotypes dominate in the meat and dairy processing environment. Front. Microbiol. 2017, 8, 264. [Google Scholar] [CrossRef]
- Molenda, J. Microbiology of Food of Animal Origin; Wrocław University of Environmental and Life Sciences Publishing: Wrocław, Poland, 2010. [Google Scholar]
- Herbert, U.; Albrecht, A.; Kreyenschmidt, J. Definition of predictor variables for MAP poultry filets stored under different temperature conditions. Poult. Sci. 2015, 94, 424–432. [Google Scholar] [CrossRef]
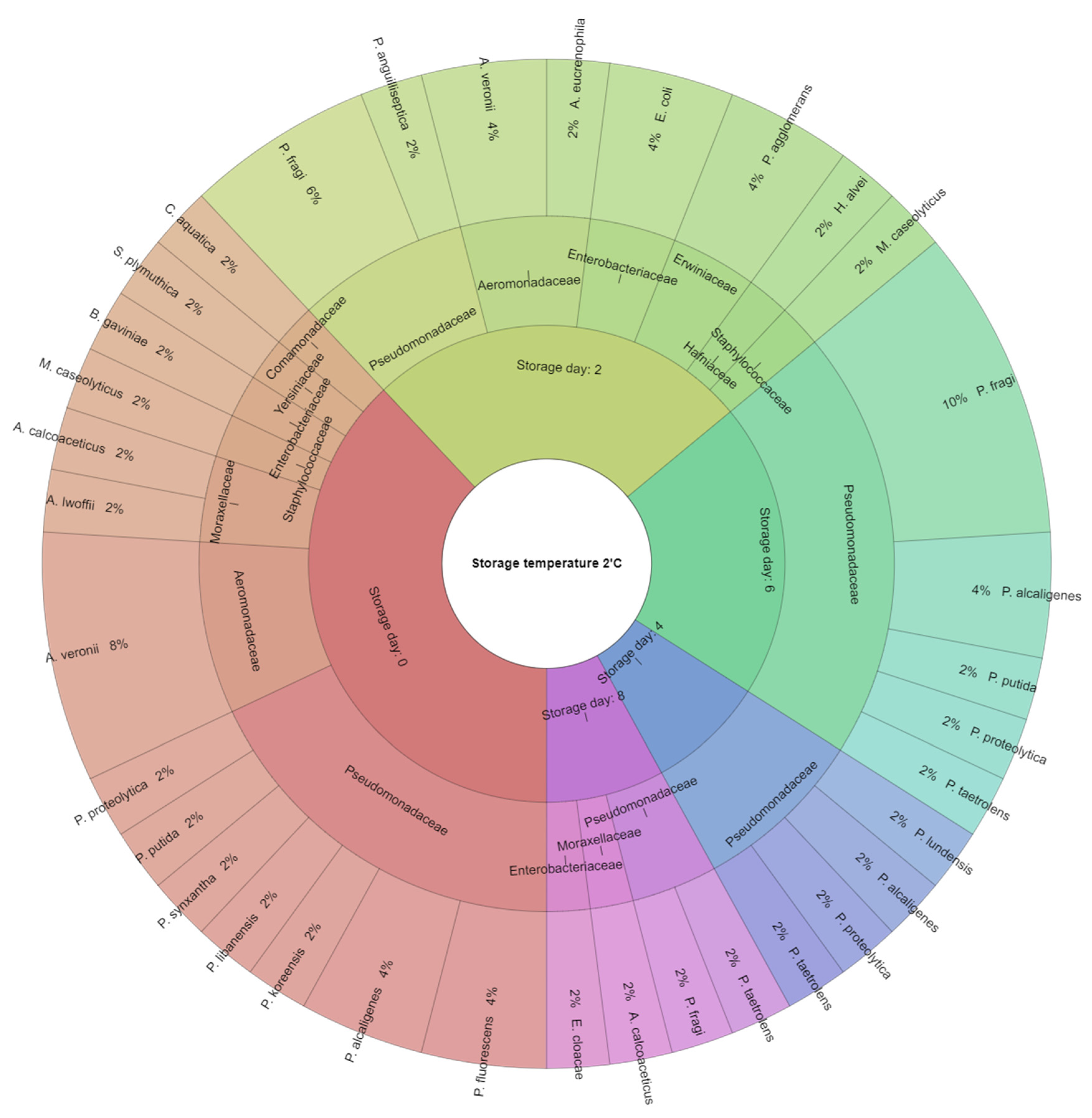

| Tested Parameter | 0 Day | Storage Temp. (°C) | Refrigerated Storage Time (Days) | ||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | p-Value | |||
| pH | 5.62 a ± 0.02 | 2 | 5.66 a ± 0.03 | 5.77 b ± 0.03 | 5.82 b ±0.02 | 5.85 b ± 0.02 | <0.001 |
| 6 | 5.67 a ± 0.04 | 6.20 b ± 0.24 | 6.25 b ± 0.08 | 6.89 c ± 0.10 | <0.001 | ||
| p-value ** | 0.491 | <0.001 | <0.001 | <0.001 | |||
| Drip loss (%) | - | 2 | 0.93 a ± 0.15 | 1.39 a ± 0.15 | 2.01 b ± 0.12 | 2.32 b ± 0.10 | <0.001 |
| 6 | 1.07 a ± 0.10 | 2.39 b ± 0.25 | 2.92 c ± 0.26 | 3.15 c ± 0.23 | <0.001 | ||
| p-value** | 0.026 | <0.001 | <0.001 | <0.001 | |||
| WHC (%) | 24.39 b ± 2.34 | 2 | 31.81 a ± 2.58 | 25.00 b ± 2.61 | 23.33 b ± 2.80 | 28.53 b ± 3.10 | 0.041 |
| 6 | 31.32 ± 2.32 | 30.41 ± 2.56 | 32.25 ± 2.40 | 32.041.12 | 0.066 | ||
| p-value ** | 0.232 | <0.001 | <0.001 | <0.001 | |||
| Colour: L*—lightness | 57.02 a ± 3.20 | 2 | 56.17 a ± 1.78 | 54.62 b ± 1.89 | 54.50 b ± 1.83 | 53.42 b ± 1.88 | <0.001 |
| 6 | 56.17 a ± 1.78 | 49.27 b ± 1.33 | 49.30 b ± 1.13 | 45.60 b ± 1.79 | <0.001 | ||
| p-value ** | 0.023 | <0.001 | <0.001 | <0.001 | |||
| a*—redness | 3.69 a ± 0.62 | 2 | 3.73 a ± 0.66 | 3.78 a ± 0.28 | 3.90 a ± 0.36 | 4.22 b ± 0.45 | 0.044 |
| 6 | 3.78 a ± 0.27 | 4.20 b ± 0.70 | 3.98 ab ± 0.25 | 4.28 c ± 0.39 | 0.003 | ||
| p-value ** | 0.051 | 0.030 | 0.535 | 0.902 | |||
| b*—yellowness | 6.78 a ± 0.02 | 2 | 9.76 b ± 0.76 | 11.58 c ± 1.26 | 11.49 c ± 0.49 | 11.98 c ± 1.19 | <0.001 |
| 6 | 10.09 b ± 1.36 | 13.16 d ± 1.30 | 14.02 d ± 0.89 | 13.41 d ± 0.85 | <0.001 | ||
| p-value ** | 0.504 | 0.004 | <0.001 | 0.020 | |||
| Shear force (N) | 31.20 a ± 4.50 | 2 | 31.00 a ± 2.70 | 30.25 a ±3.40 | 28.90 a ± 3.62 | 25.60 b ± 3.80 | 0.038 |
| 6 | 30.68 a ± 4.50 | 25.70 b ± 2.80 | 20.84 c ± 3.20 | 19.90 c ± 2.80 | 0.002 | ||
| p-value ** | 0.502 | 0.032 | <0.001 | <0.001 | |||
| Parameter | 0 Day | Storage Temp. (°C) | Refrigerated Storage Time (Days) | ||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | p-Value | |||
| Total microorganism count | 3.48 a ± 0.39 | 2 | 3.50 a ± 0.05 | 3.72 a ± 0.44 | 3.80 a ± 0.54 | 4.64 b ± 0.38 | <0.001 |
| 3.43–3.57 | 3.30–4.30 | 3.30–4.43 | 4.34–5.34 | ||||
| 6 | 4.11 a ± 0.45 | 6.29 b ± 0.15 | 7.07 c ± 0.37 | nt | <0.001 | ||
| 2.85–3.87 | 3.63–4.66 | 6.13–6.47 | 6.62–7.62 | ||||
| p-value ** | 0.007 | <0.001 | <0.001 | ||||
| Family Enterobacteriaceae | <2 | 2 | 3.61 a ± 0.13 | 3.57 a ± 0.41 | 3.75 a ± 0.13 | 4.16 b ± 0.32 | 0.005 |
| 3.40–3.73 | 3.14–3.99 | 3.59–3.91 | 3.70–4.43 | ||||
| 6 | 3.70 a ± 0.10 | 5.70 b ± 0.54 | 6.76 c ± 0.09 | nt | <0.001 | ||
| 3.53–3.80 | 5.09–6.24 | 6.62–6.88 | |||||
| p-value ** | 0.248 | <0.001 | <0.001 | ||||
| Pseudomonas spp. | 2.87 a ± 0.35 | 2 | 2.85 a ± 0.77 | 3.69 b ± 0.13 | 3.70 b ± 0.14 | 4.48 c ± 0.25 | <0.001 |
| 2.20–3.32 | 3.59–3.83 | 3.51–3.91 | 4.00–4.66 | ||||
| 6 | 3.70 a ± 0.04 | 6.32 b ± 0.12 | 7.11 c ± 0.23 | nt | <0.001 | ||
| 2.41–3.32 | 3.62–3.73 | 6.18–6.51 | 6.73–7.38 | ||||
| p-value ** | 0.023 | 0.020 | 0.007 | ||||
| Salmonella spp. | nd | 2 | nd | nd | nd | nd | |
| 6 | nd | nd | nd | nd | |||
| Parameter | 0 Day | Storage Temp. (°C) | Refrigerated Storage Time (Days) | |||
|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |||
| Intensity and desirability of the odour | 4.90 a ± 0.30 | 2 | 4.62 a ± 0.30 | A 4.10 ab ± 0.30 | A 3.90 ab ± 0.32 | A 3.30 b ± 0.52 |
| 6 | 4.10 a ± 0.42 | B 2.60 b ± 0.63 | B 1.80 c ± 0.42 | B 1.00 c ± 0.48 | ||
| Outside colour | 4.80 a ± 0.41 | 2 | 4.60 a ± 0.50 | A 3.70 ab ± 0.51 | A 3.80 ab ± 0.42 | A 3.31 b ± 0.56 |
| 6 | 4.30 a ± 0.60 | B 2.50 b ± 0.42 | B 2.60 b ± 0.69 | B 1.40 c ± 0.51 | ||
| Consistency | 4.65 a ± 0.50 | 2 | 4.70 a ± 0.48 | A 3.90 b ± 0.30 | A 3.50 b ± 0.52 | A 3.00 c ± 0.46 |
| 6 | 3.50 a ± 0.36 | B 2.20 ab ± 0.48 | B 1.40 c ± 0.52 | B 1.30 c ± 0.48 | ||
| General appearance | 4.85 a ± 0.36 | 2 | 4.60 a ± 0.52 | A 3.90 ab ± 0.44 | A 3.70 ab ± 0.48 | A 3.00 b ± 0.52 |
| 6 | 3.70a ± 0.52 | B 2.30 b ± 0.52 | B 1.60 bc ± 0.52 | B 1.50 c ± 0.52 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augustyńska-Prejsnar, A.; Hanus, P.; Ormian, M.; Kačániová, M.; Sokołowicz, Z.; Topczewska, J. The Effect of Temperature and Storage Duration on the Quality and Attributes of the Breast Meat of Hens after Their Laying Periods. Foods 2023, 12, 4340. https://doi.org/10.3390/foods12234340
Augustyńska-Prejsnar A, Hanus P, Ormian M, Kačániová M, Sokołowicz Z, Topczewska J. The Effect of Temperature and Storage Duration on the Quality and Attributes of the Breast Meat of Hens after Their Laying Periods. Foods. 2023; 12(23):4340. https://doi.org/10.3390/foods12234340
Chicago/Turabian StyleAugustyńska-Prejsnar, Anna, Paweł Hanus, Małgorzata Ormian, Miroslava Kačániová, Zofia Sokołowicz, and Jadwiga Topczewska. 2023. "The Effect of Temperature and Storage Duration on the Quality and Attributes of the Breast Meat of Hens after Their Laying Periods" Foods 12, no. 23: 4340. https://doi.org/10.3390/foods12234340
APA StyleAugustyńska-Prejsnar, A., Hanus, P., Ormian, M., Kačániová, M., Sokołowicz, Z., & Topczewska, J. (2023). The Effect of Temperature and Storage Duration on the Quality and Attributes of the Breast Meat of Hens after Their Laying Periods. Foods, 12(23), 4340. https://doi.org/10.3390/foods12234340








