Nutritional and Technological Properties of Albino Peach Palm (Bactris gasipaes) from the Amazon: Influence of Cooking and Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruits
2.2. Sample Preparation
2.3. Physicochemical Characterization
2.4. Mineral Composition
2.5. Ascorbic Acid, Phenolic Compound, Flavonoid, and Carotenoid Content
2.6. Granulometric Analysis
2.7. Thermogravimetric and Absorption Spectroscopy Analysis
2.8. Scanning Electron Microscopy
2.9. Functional Technological Properties
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Pulp and Flour
3.2. Color Parameters
3.3. Mineral Composition
| Elements (mg/kg dm) | Pulp | Flour | Reference Values a (mg/day) | ||
|---|---|---|---|---|---|
| Raw | Cooked | Raw | Cooked | ||
| Major minerals | |||||
| Ca | 1068.32 ± 137.44 b | 3278.34 ± 60.05 a | 891.70 ± 82.89 b | 1046.88 ± 116.06 b | 1000 |
| Mg | 1443.48 ± 132.16 b | 3632.41 ± 283.22 a | 1921.23 ± 192.58 b | 1669.45 ± 172.22 b | 400 |
| P | 1811.35 ± 198.27 a | 2237.89 ± 266.32 a | 1993.78 ± 19.35 a | 1647.83 ± 238.29 a | 1000 |
| Minor minerals | |||||
| Al | 23.59 ± 1.23 c | 24.50 ± 2.12 c | 89.31 ± 1.07 b | 127.91 ± 13.59 a | |
| As | 4.46 ± 0.20 a | 6.64 ± 0.67 a | <LOD | <LOD | |
| Ba | 1.11 ± 0.08 b | 2.39 ± 0.75 ab | 3.2 ± 0.14 a | 2.16 ± 0.16 ab | |
| Cd | <LOD | 0.09 ± <0.01 | <LOD | <LOD | |
| Cr | 20.15 ± 0.53 b | 21.36 ± 0.16 b | 1.62 ± 0.12 c | 32.85 ± 0.47 a | |
| Cu | 18.96 ± 0.38 a | 5.56 ± 1.38 b | 13.89 ± 0.74 a | 16.18 ± 1.77 a | 2 |
| Fe | 66.28 ± 2.30 a | 43.08 ± 3.96 b | 17.80 ± 4.49 b | 52.53 ± 4.49 ab | 18 |
| Mn | 19.34 ± 0.27 b | 49.61 ± 3.27 a | 15.35 ± 0.81 b | 22.17 ± 1.44 b | 2 |
| Se | 4.08 ± 0.57 a | 4.90 ± 0.24 a | 4.79 ± 1.40 a | 5.26 ± 1.15 a | 70 b |
| Zn | 9.04 ± 0.48 b | 22.40 ± 0.30 a | 16.33 ± 2.30 ab | 10.47 ± 1.15 b | 15 |
3.4. Bioactive Compounds Contents
3.5. Thermogravimetric Analysis
3.6. Absorption Spectroscopy in the Infrared Region
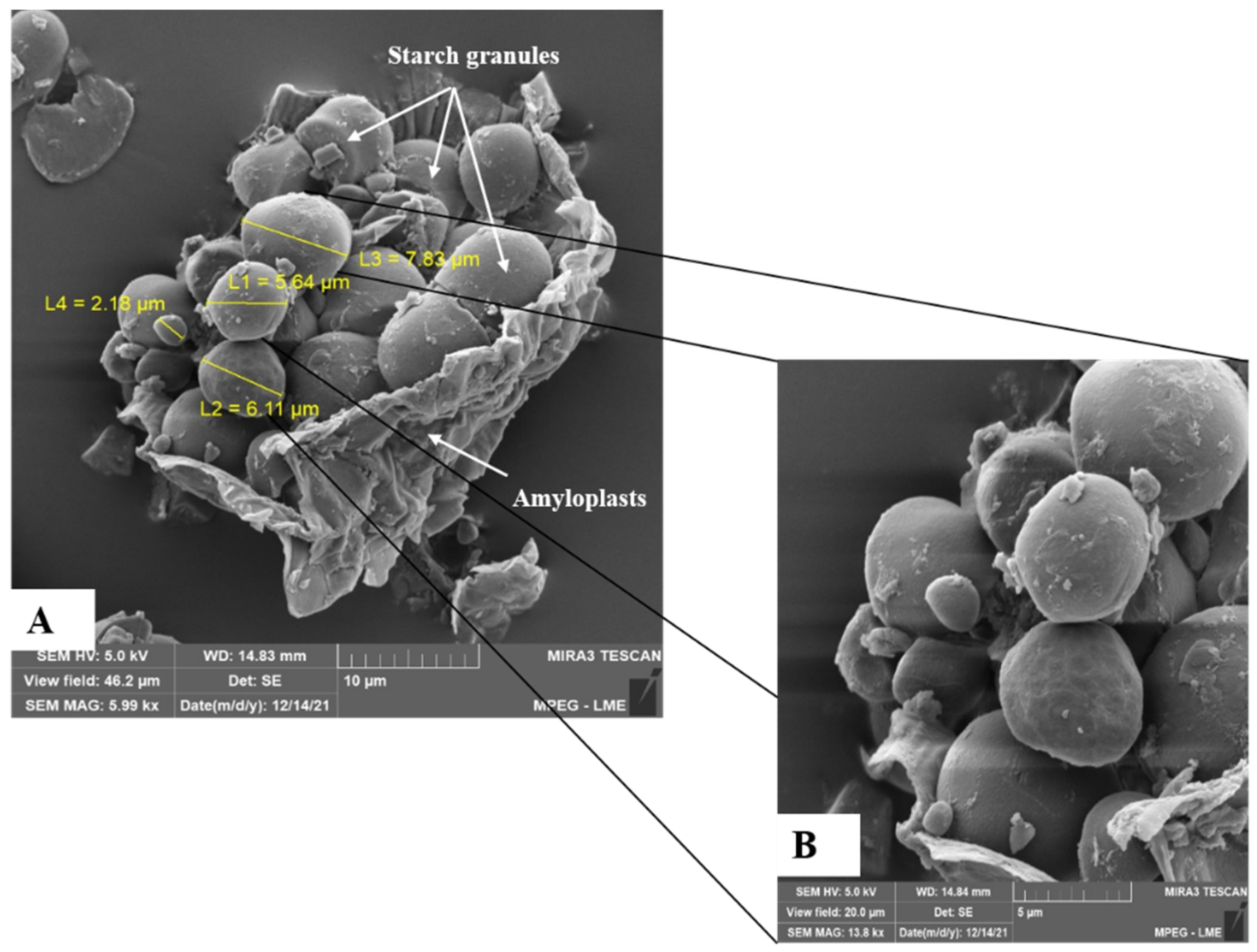
3.7. Morphological Aspects
3.8. Particle size Analysis of Flour
3.9. Technological Functional Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguiar, J.P.L.; Yuyama, K.; Souza, F.C.A. Caracterização dos frutos de pupunheira (Bactris gasipaes Kunth) cultivada na Vila do Equador, RR: O que há de novo? Sci. Amazon. 2019, 8, CA1–CA5. [Google Scholar]
- Chisté, R.C.; Costa, E.L.N.; Monteiro, S.F.; Mercadante, A.Z. Carotenoid and phenolic compound profiles of cooked pulps of orange and yellow peach palm fruits (Bactris gasipaes) from the Brazilian Amazonia. J. Food Compos. Anal. 2021, 99, 103873. [Google Scholar] [CrossRef]
- dos Santos, O.V.; Soares, S.D.; Dias, P.C.S.; do Nascimento, F.D.C.A.; da Conceição, L.R.V.; da Costa, R.S.; da Silva Pena, R. White Peach Palm (Pupunha) a New Bactris gasipaes Kunt variety from the amazon: Nutritional composition, bioactive lipid profile, thermogravimetric and morphological characteristics. J. Food Compos. Anal. 2022, 112, 104684. [Google Scholar] [CrossRef]
- Dos Santos, O.V.; Langley, A.C.D.C.P.; de Lima, A.J.M.; Moraes, V.S.V.; Soares, S.D.; Teixeira-Costa, B.E. Nutraceutical potential of amazonian oilseeds in modulating the immune system against COVID-19—A narrative review. J. Funct. Foods 2022, 94, 105123. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, O.V.; Soares, S.D.; Dias, P.C.S.; de Paula de Almeida Duarte, S.; dos Santos, M.P.L.; das Chagas Alves do Nascimento, F. Chromatographic profile and bioactive compounds found in the composition of pupunha oil (Bactris gasipaes Kunth): Implications for human health. Rev. Nutr. 2020, 33, e190146. [Google Scholar] [CrossRef]
- Muñoz, A.M.; Casimiro-Gonzales, S.; Gómez-Coca, R.B.; Moreda, W.; Best, I.; Cajo-Pinche, M.I.; Loja, J.F.; Ibañez, E.; Cifuentes, A.; Ramos-Escudero, F. Comparison of four oil extraction methods for sinami fruit (Oenocarpus mapora H. Karst): Evaluating quality, polyphenol content and antioxidant activity. Foods 2022, 11, 1518. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Pérez, A.M.; Vaillant, F.; Pineda-Castro, M.L. Physicochemical and antioxidant composition of fresh peach palm (Bactris gasipaes Kunth) fruits in Costa Rica. Braz. J. Food Technol. 2016, 19, e2015097. [Google Scholar] [CrossRef]
- Pinzón-Zárate, L.X.; Hleap-Zapata, J.I.; Ordóñez-Santos, L.E. Análisis de los parámetros de color en salchichas Frankfurt adicionadas con extracto oleoso de residuos de Chontaduro (Bactris gasipaes). Inf. Tecnol. 2015, 26, 45–54. [Google Scholar] [CrossRef]
- Cornelius, J.P.; Weber, J.C.; Sotelo-Montes, C.; Ugarte-Guerra, L.J. Phenotypic correlations and site effects in a peruvian landrace of peach palm (Bactris gasipaes Kunth). Euphytica 2010, 173, 173–183. [Google Scholar] [CrossRef]
- Pinheiro, R.C.; Ballesteros, L.F.; Cerqueira, M.A.; Rodrigues, A.M.C.; Teixeira, J.A.; Silva, L.H.M. Peach palm (Bactris gasipaes Kunth) and mammee apple (Mammea americana L.) seeds: Properties and potential of application in industry. Lwt 2022, 170, 114089. [Google Scholar] [CrossRef]
- Melo, M.C.T.; Costa, L.L.; Pereira, F.C.; Castro, L.M.; Neponuceno, S. Physical and chemical analysis of fruit “in Natura” of pupunha. Ciência E Tecnol. Aliment. 2017, 3, 13–17. [Google Scholar]
- dos Santos, M.A.S.; Protázio, D.C.; da Costa, G.P.; Rebello, F.K.; Martins, C.M.; Bezerra, A.S.; da Silva Nogueira, A. Profile of peach palm fruit consumers in the metropolitan region of Belém, Pará, Brazilian Amazon. Int. J. Innov. Educ. Res. 2021, 9, 550–560. [Google Scholar] [CrossRef]
- Santos, I.L.; Steel, C.J.; Aguiar, J.P.L.; Schmiele, M.; de Sales Ferreira, C.J.; do Amaral Souza, F.D.C. Sensory analysis of extruded corn-based breakfast cereals with whole peach palm fruit (Bactris gasipaes, Kunth) powder. Afr. J. Food Sci. 2017, 11, 310–317. [Google Scholar] [CrossRef]
- Pires, M.B.; Amante, E.R.; Lopes, A.S.; da Cruz Rodrigues, A.M.; da Silva, L.H.M. Peach palm flour (Bactris gasipae Kunth): Potential application in the food industry. Food Sci. Technol. 2019, 39, 613–619. [Google Scholar] [CrossRef]
- Leterme, P.; García, M.F.; Londoño, A.M.; Rojas, M.G.; Buldgen, A.; Souffrant, W.B. Chemical composition and nutritive value of peach palm (Bactris gasipaes Kunth) in rats. J. Sci. Food Agric. 2005, 85, 1505–1512. [Google Scholar] [CrossRef]
- dos Santos, A.B.; Pereira, M.L.A.; de Oliveira Silva, H.G.; de Carvalho, G.G.P.; de Jesus Pereira, T.C.; Ribeiro, L.S.O.; Azevêdo, J.A.G.; das Graças Conceição Parada Costa Silva, M.; Sousa, L.B.; Sousa, L.B.; et al. Intake, digestibility and performance of lambs fed diets containing peach palm meal. Trop. Anim. Health Prod. 2016, 48, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.V.; Valido, I.H.; Paz, W.H.P.; da Silva, F.M.A.; de Souza, A.D.L.; Acho, L.R.D.; Lima, E.S.; Boleti, A.P.A.; Marinho, J.V.N.; Salvador, M.J.; et al. Comparative evaluation of chemical composition and biological activities of tropical fruits consumed in Manaus, Central Amazonia, Brazil. Food Res. Int. 2021, 139, 109836. [Google Scholar] [CrossRef]
- Martínez-Girón, J.; Figueroa-Molano, A.M.; Ordóñez-Santos, L.E. Effect of the addition of peach palm (Bactris gasipaes) peel flour on the color and sensory properties of cakes. Food Sci. Technol. 2017, 37, 418–424. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Neves, B.V.; Pisani, L.P.; de Rosso, V.V. Mayonnaise as a model food for improving the bioaccessibility of carotenoids from Bactris gasipaes fruits. LWT 2020, 122, 109022. [Google Scholar] [CrossRef]
- de Melo Neto, B.A.; Junior, C.C.M.F.; da Silva, E.G.P.; Franco, M.; dos Santos Reis, N.; Ferreira Bonomo, R.C.; de Almeida, P.F.; Pontes, K.V. Biodegradable thermoplastic starch of peach palm (Bactris gasipaes Kunth) fruit: Production and characterisation. Int. J. Food Prop. 2018, 20, S2429–S2440. [Google Scholar] [CrossRef]
- Singo, T.M.; Beswa, D. Effect of roselle extracts on the selected quality characteristics of ice cream. Int. J. Food Prop. 2019, 22, 42–53. [Google Scholar] [CrossRef]
- Salazar, D.; Arancibia, M.; Raza, K.; López-Caballero, M.E.; Montero, M.P. Influence of underutilized unripe banana (Cavendish) flour in the formulation of healthier chorizo. Foods 2021, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.H.; Lindemann, I.D.S.; Ferreira, C.D.; Pohndorf, R.S.; Vanier, N.L.; de Oliveira, M. Influence of drying temperature on the structural and cooking quality properties of black rice. Cereal Chem. 2018, 95, 564–574. [Google Scholar] [CrossRef]
- Lang, G.H.; Lindemann, I.D.S.; Ferreira, C.D.; Pohndorf, R.S.; Vanier, N.L.; de Oliveira, M. Fluidized-bed drying of black rice grains: Impact on cooking properties, in vitro starch digestibility, and bioaccessibility of phenolic compounds. J. Food Sci. 2020, 85, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, W.C.; da Silva Araújo, C.; Macedo, L.L.; Maradini Filho, A.M.; Saraiva, S.H.; Teixeira, L.J.Q. Influence of drying temperature on drying kinetics, energy consumption, bioactive compounds and cooking quality of pasta enriched with spinach. J. Food Process Eng. 2020, 43, e13571. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International; AOAC: Washington, DC, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- FAO/WHO. Food Energy: Methods of Analysis and Conversion Factors; FAO: Rome, Italy, 2002. [Google Scholar]
- Mclellan, M.R.; Lind, L.R.; Kime, R.W. Hue angle determinations and statistical analysis for multiquadrant hunter L,a,b data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- Palavecino, P.M.; Penci, M.C.; Calderón-Domínguez, G.; Ribotta, P.D. Chemical composition and physical properties of sorghum flour prepared from different sorghum hybrids grown in Argentina. Starch-Stärke 2016, 68, 1055–1064. [Google Scholar] [CrossRef]
- Alves, B.S.F.; Pereira Junior, J.B.; Carvalho, F.I.M.; Dantas Filho, H.A.; Fernandes Dantas, K.G. Mineral composition of Amazonian fruits by flame atomic absorption spectrometry using multivariate analysis. Biol. Trace Elem. Res. 2019, 189, 259–266. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybidic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Godoy, H.T.; Rodriguez-Amaya, D.B. Occurrence of cis-isomers of provitamin A in Brazilian fruits. J. Agric. Food Chem. 1994, 42, 1306–1313. [Google Scholar] [CrossRef]
- Zanotto, D.L.; Bellaver, C. Método de Determinação da Granulometria de Ingredientes Para Uso em Rações de Suínos e Aves. Embrapa/CNPSA. 1996. Available online: https://www.infoteca.cnptia.embrapa.br/handle/doc/433741 (accessed on 20 October 2022).
- Anderson, R.A.; Conway, H.F.; Pfeifer, V.; Griffin, E.L. Gelatinization of corn grits by roll and extrusion cooking. Cereal Sci. Today 1969, 14, 4–12. [Google Scholar]
- Coffmann, C.N.; Garcia, V. V Functional properties and amino acid content of a protein isolate from mung bean flour. Int. J. Food Sci. Technol. 1977, 12, 473–484. [Google Scholar] [CrossRef]
- Silva, R.B.; Silva-Júnior, E.V.; Rodrigues, L.C.; Andrade, L.H.C.; da Silva, S.I.; Harand, W.; Oliveira, A.F.M. A comparative study of nutritional composition and potential use of some underutilized tropical fruits of Arecaceae. An. Acad. Bras. Cienc. 2015, 87, 1701–1709. [Google Scholar] [CrossRef]
- Martínez-Girón, J.; Rodríguez-Rodríguez, X.; Pinzón-Zárate, L.X.; Ordóñez-Santos, L.E. Caracterización fisicoquímica de harina de residuos del fruto de chontaduro (Bactris gasipaes Kunth, Arecaceae) obtenida por secado convectivo. Corpoica Cienc. Y Tecnol. Agropecu. 2017, 18, 599–613. [Google Scholar] [CrossRef]
- Giombelli, C.; Raspe, D.; Donadone, D.; da Silva, C.; Barros, B. Chemical composition and functional properties of dietary fiber concentrates obtained from peach palm by-product. J. Braz. Chem. Soc. 2023, 34, 927–936. [Google Scholar] [CrossRef]
- FAO/WHO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; FAO: Rome, Italy, 2013. [Google Scholar]
- Basto, G.J.; Carvalho, C.W.P.; Soares, A.G.; Costa, H.T.G.B.; Chávez, D.W.H.; de Oliveira Godoy, R.L.; Pacheco, S. Physicochemical properties and carotenoid content of extruded and non-extruded corn and peach palm (Bactris gasipaes, Kunth). LWT-Food Sci. Technol. 2016, 69, 312–318. [Google Scholar] [CrossRef]
- Torres-Vargas, O.L.; Luzardo-Ocampo, I.; Hernandez-Becerra, E.; Rodríguez-García, M.E. Physicochemical characterization of unripe and ripe chontaduro (Bactris gasipaes Kunth) fruit flours and starches. Starch-Stärke 2021, 73, 2000242. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, L.; Gutin, B.; Zhu, H. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur. J. Clin. Nutr. 2019, 73, 1172–1178. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.M.; Greenwood, D.C.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Norat, T. Dietary fiber and breast cancer risk: A systematic review and meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1394–1402. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 2010, 58, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Negu, A.; Zegeye, A.; Astatkie, T. Development and quality evaluation of wheat based cookies enriched with fenugreek and oat flours. J. Food Sci. Technol. 2020, 57, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Giombelli, C.; Iwassa, I.J.; da Silva, C.; Bolanho Barros, B.C. Valorization of peach palm by-product through subcritical water extraction of soluble sugars and phenolic compounds. J. Supercrit. Fluids 2020, 165, 104985. [Google Scholar] [CrossRef]
- Suzauddula, M.; Hossain, M.B.; Farzana, T.; Orchy, T.N.; Islam, M.N.; Hasan, M.M. Incorporation of oat flour into wheat flour noodle and evaluation of its physical, chemical and sensory attributes. Braz. J. Food Technol. 2021, 24, e2020252. [Google Scholar] [CrossRef]
- USDA (United State Department of Agriculture). Nutrient Database for Standard—Reference—Release 15: Nutrient; USDA: Washington, DC, USA, 2002.
- Mamiro, P.S.; Mwanri, A.W.; Mongi, R.J.; Chivaghula, T.J.; Nyagaya, M.; Ntwenya, J. Effect of cooking on tannin and phytate content in different bean (Phaseolus vulgaris) varieties grown in Tanzania. Afr. J. Biotechnol. 2017, 16, 1186–1191. [Google Scholar] [CrossRef]
- Akin, M.; Eyduran, S.P.; Eyduran, E.; Reed, B.M. Analysis of macro nutrient related growth responses using multivariate adaptive regression splines. Plant Cell Tissue Organ Cult. 2020, 140, 661–670. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Kostov, K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: Focusing on the processes of insulin secretion and signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A focus on calcium-rich mineral waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef]
- Bhattarai, H.K.; Shrestha, S.; Rokka, K.; Shakya, R. Vitamin D, calcium, parathyroid hormone, and sex steroids in bone health and effects of aging. J. Osteoporos. 2020, 2020, 9324505. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: A systematic review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e3. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations. Available online: http://faostat.fao.org/ (accessed on 10 October 2022).
- Rojas-Garbanzo, C.; Pérez, A.M.; Pineda Castro, M.L.; Vaillant, F. Major physicochemical and antioxidant changes during peach-palm (Bactris gasipaes H.B.K.) flour processing. Fruits 2012, 67, 415–427. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Lang, G.H.; da Silva Lindemann, I.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; de Oliveira, M. Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- de Prado, J.M.; de Passos, G.R.; dos Santos, T.B.; da Silva, C.N.S.; Lemes, A.C.; de Aleluia Batista, K.; de Souza Sora, G.T.; Polesi, L.F.; de Paula, L.C. Production and characterization of a carotenoid-rich peach palm flour. J. Eng. Exact Sci. 2022, 8, 14866-01i. [Google Scholar] [CrossRef]
- de Castro, N.T.; de Alencar, E.R.; Zandonadi, R.P.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Botelho, R.B.A. Influence of cooking method on the nutritional quality of organic and conventional brazilian vegetables: A study on sodium, potassium, and carotenoids. Foods 2021, 10, 1782. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. Lwt 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, J.; Zhang, S.; Guan, C.; Wang, G. Effects of three cooking methods on content changes and absorption efficiencies of carotenoids in maize. Food Funct. 2020, 11, 944–954. [Google Scholar] [CrossRef]
- Britton, G.; Khachik, F. Carotenoids: Nutrition and Health. In Carotenoids in Food; Birkhäuser Basel: Basel, Switzerland, 2009; pp. 45–66. [Google Scholar]
- Farina, V.; Cinquanta, L.; Vella, F.; Niro, S.; Panfili, G.; Metallo, A.; Cuccurullo, G.; Corona, O. Evolution of carotenoids, sensory profiles and volatile compounds in microwave-dried fruits of three different loquat cultivars (Eriobotrya japonica Lindl.). Plant Foods Hum. Nutr. 2020, 75, 200–207. [Google Scholar] [CrossRef]
- Ferrari Felisberto, M.H.; Souza Costa, M.; Villas Boas, F.; Lopes Leivas, C.; Maria Landi Franco, C.; Michielon de Souza, S.; Pedrosa Silva Clerici, M.T.; Mach Côrtes Cordeiro, L. Characterization and technological properties of peach palm (Bactris gasipaes Var. Gasipaes) fruit starch. Food Res. Int. 2020, 136, 109569. [Google Scholar] [CrossRef] [PubMed]
- de Melo Neto, B.A.; Fernandes, B.S.; Junior, C.C.M.F.; Franco, M.; Bonomo, R.C.F.; de Almeida, P.F.; Pontes, K.V. Thermal-morphological characterisation of starch from peach-palm (Bactris gasipaes Kunth) fruit (Pejibaye). Int. J. Food Prop. 2017, 20, 1007–1015. [Google Scholar] [CrossRef]
- de Melo Neto, B.A.; Bonomo, R.C.F.; Franco, M.; de Almeida, P.F.; Pontes, K.V. Starch extraction from the peach palm (Bactris gasepaes Kunth.) fruit: A model approach for yield increase. Eng. Agric. 2017, 37, 148–159. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: A review. Starch-Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of adulterated neem and flaxseed oil compositions by FTIR spectroscopy and multivariate regression analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Rai, A.; Mohanty, B.; Bhargava, R. Supercritical extraction of sunflower oil: A central composite design for extraction variables. Food Chem. 2016, 192, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Jiang, F.-C.; Cai, J.; Hu, S.; Zhou, R.; Liu, G.; Wang, Y.-H.; Wang, H.-B.; He, J.-R.; Xiong, X.-G. Facile microencapsulation of olive oil in porous starch granules: Fabrication, characterization, and oxidative stability. Int. J. Biol. Macromol. 2018, 111, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Valencia, G.A.; Moraes, I.C.F.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.D.A. Physicochemical, morphological, and functional properties of flour and starch from peach palm (Bactris gasipaes K.) fruit. Starch-Stärke 2015, 67, 163–173. [Google Scholar] [CrossRef]
- Richardson, S.; Gorton, L. Characterisation of the Substituent distribution in starch and cellulose derivatives. Anal. Chim. Acta 2003, 497, 27–65. [Google Scholar] [CrossRef]
- Koksel, H.; Masatcioglu, T.; Kahraman, K.; Ozturk, S.; Basman, A. Improving effect of lyophilization on functional properties of resistant starch preparations formed by acid hydrolysis and heat treatment. J. Cereal Sci. 2008, 47, 275–282. [Google Scholar] [CrossRef]
- Jefferson, A.; Adolphus, K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: A systematic review. Front. Nutr. 2019, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.M.; Bordenave, N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutr. Rev. 2021, 78, 13–20. [Google Scholar] [CrossRef] [PubMed]
- SILVA, L.H. Desenvolvimento de pão de fôrma com a adição de farinha de “Okara”. Braz. J. Food Technol. 2010, 12, 315–322. [Google Scholar] [CrossRef]
- Zhou, S.; Reddy, C.K.; Du, B.; Xu, B. Pasting, thermal, and functional properties of wheat flour and rice flour formulated with chestnut flour. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100290. [Google Scholar] [CrossRef]
- Hatamian, M.; Noshad, M.; Abdanan-Mehdizadeh, S.; Barzegar, H. Effect of roasting treatment on functional and antioxidant properties of chia seed flours. NFS J. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.; An, F.; He, H.; Geng, F.; Song, H.; Huang, Q. Structural and rheological characterization of pectin from passion fruit (Passiflora edulis f. flavicarpa) peel extracted by high-speed shearing. Food Hydrocoll. 2021, 114, 106555. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Igwe, V.S.; Echeta, C.K. The functional properties of foods and flours. Int. J. Adv. Acad. Res. Sci. 2019, 5, 2488–9849. [Google Scholar]
- Das, P.C.; Khan, M.J.; Rahman, M.S.; Majumder, S.; Islam, M.N. Comparison of the physico-chemical and functional properties of mango kernel flour with wheat flour and development of mango kernel flour based composite cakes. NFS J. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Ofori, J.; Tortoe, C.; Agbenorhevi, J.K. Physicochemical and functional properties of dried okra (Abelmoschus esculentus L.) seed flour. Food Sci. Nutr. 2020, 8, 4291–4296. [Google Scholar] [CrossRef]
- Kanetro, B.; Riyanto, M.; Pujimulyani, D.; Huda, N. Improvement of functional properties of jack bean (Canavalia ensiformis) flour by germination and its relation to amino acids profile. Curr. Res. Nutr. Food Sci. 2021, 9, 812–822. [Google Scholar] [CrossRef]
- Zielinska, E. Evaluating the Functional characteristics of certain insect flours (non-defatted/defatted flour) and their protein preparations. Molecules 2022, 27, 6339. [Google Scholar] [CrossRef]





| Components | Pulp | Flour | ||
|---|---|---|---|---|
| Raw | Cooked | Raw | Cooked | |
| Moisture (g/100 g wet basis—wb) | 61.00 ± < 0.01 b | 70.50 ± 0.50 a | 9.67 ± 0.29 c | 5.83 ± 0.29 d |
| Ash (g/100 g dry matter—dm) | 9.68 ± 1.30 b | 13.93 ± 0.65 a | 2.21 ± < 0.01 c | 2.12 ± < 0.01 c |
| Proteins (g/100 g dm) | 7.90 ± < 0.01 c | 7.59 ± 0.23 c | 16.96 ± < 0.01 b | 19.99 ± 0.45 a |
| Lipids (g/100 g dm) | 10.21 ± 1.12 a | 8.63 ± 0.84 a | 10.81 ± 0.31 a | 7.86 ± 0.52 a |
| Total carbohydrates (g/100 g dm) | 72.08 ± 2.94 a | 70.58 ± 0.14 a | 69.92 ± 0.69 a | 69.59 ± 0.53 a |
| Total dietary fiber (g/100 g dm) | 13.64 ± 2.27 ab | 12.81 ± 2.18 ab | 16.89 ± 0.84 a | 8.19 ± 0.19 b |
| Insoluble dietary fiber (g/100 g dm) | 9.30 ± 0.72 b | 7.32 ± 0.01 b | 14.72 ± 0.70 a | 6.03 ± 1.38 b |
| Soluble dietary fiber (g/100 g dm) | 5.41 ± 1.85 ab | 6.74 ± 0.18 a | 2.66 ± 0.11 b | 2.16 ± 0.19 b |
| Total sugars (g/100 g dm) | 59.18 ± 1.11 a | 49.10 ± 0.57 b | 24.74 ± 0.45 c | 12.46 ± 0.12 d |
| Reducing sugars (g/100 g dm) | 12.58 ± 0.21 a | 3.86 ± < 0.01 c | 12.92 ± < 0.01 a | 6.98 ± 0.29 b |
| Non-reducing sugars (g/100 g dm) | 46.60 ± 1.02 a | 45.24 ± 0.61 b | 11.82 ± 0.45 c | 5.48 ± 0.26 d |
| Total energetic value (kcal/100 g wb) | 160.58 ± 0.50 b | 115.19 ± 1.20 c | 401.85 ± 0.10 a | 400.00 ± 1.70 a |
| aw | 0.980 ± < 0.01 a | 0.981 ± < 0.01 a | 0.206 ± < 0.01 c | 0.246 ± < 0.01 b |
| pH | 6.44 ± 0.08 a | 6.18 ± 0.03 a | 6.15 ± 0.05 b | 6.25 ± 0.13 a |
| Total titratable acidity (g citric acid/100 g wb) | 8.96 ± < 0.01 c | 4.48 ± < 0.01 d | 21.32 ± 0.30 b | 23.45 ± 0.60 a |
| Parameter | Pulp | Flour | ||
|---|---|---|---|---|
| Raw | Cooked | Raw | Cooked | |
| L* | 76.52 ± 4.78 a | 70.37 ± 1.26 b | 80.23 ± 2.2 a | 72.03 ± 1.61 b |
| a* | −0.43 ± 0.21 a | −1.59 ± 0.37 b | −1.39 ± 0.04 b | −0.47 ± 0.12 a |
| b* | +18.56 ± 0.86 b | +25.88 ± 0.67 a | +16.0 ± 0.72 b | +24.93 ± 1.66 a |
| C* | 18.57 ± 0.86 b | 25.93 ± 0.69 a | 15.17 ± 0.1 c | 24.93 ± 1.64 a |
| h° | 91.35 ± 0.75 b | 93.50 ± 0.73 a | 94.92 ± 0.02 a | 90.94 ± 0.27 b |
| WI | 70.00 ± 3.46 a | 60.60 ± 0.43 b | 74.58 ± 0.61 a | 62.48 ± 0.23 b |
| Components | Pulp | Flour | ||
|---|---|---|---|---|
| Raw | Cooked | Raw | Cooked | |
| TPC (mg GAE/100 g dry matter—dm) | 63.10 ± 3.10 a | 51.39 ± 5.47 b | 48.47 ± 0.98 b | 12.16 ± 2.27 c |
| Total flavonoids (mg quercetin/100 g dm) | 19.31 ± 0.13 a | 10.17 ± 0.42 b | 2.37 ± 0.18 c | 2.16 ± < 0.01 c |
| Ascorbic acid (mg/100 g dm) | 88.15 ± < 0.1 a | 70.63 ± 6.13 b | 7.90 ± < 0.1 c | 6.06 ± < 0.1 c |
| Total carotenoids (μg β-carotene/100 g dm) | 4.92 ± 0.20 ab | 4.62 ± 0.65 ab | 4.39 ± 0.02 b | 5.53 ± 0.21 a |
| Property | Flour | |
|---|---|---|
| Raw Pulp | Cooked Pulp | |
| WSI (%) | 26.57 ± < 0.01 a | 18.05 ± 1.84 b |
| WAI (g/g dry matter) | 1.46 ± 0.04 b | 2.07 ± 0.08 a |
| OAC (g/g dry matter) | 1.04 ± 0.04 a | 0.89 ± 0.03 b |
| FC (%) | 11.11 ± 2.24 | nr |
| FS | nr | nr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, S.D.; dos Santos, O.V.; da Conceição, L.R.V.; Costi, H.T.; Silva Júnior, J.O.C.; Nascimento, F.d.C.A.d.; Pena, R.d.S. Nutritional and Technological Properties of Albino Peach Palm (Bactris gasipaes) from the Amazon: Influence of Cooking and Drying. Foods 2023, 12, 4344. https://doi.org/10.3390/foods12234344
Soares SD, dos Santos OV, da Conceição LRV, Costi HT, Silva Júnior JOC, Nascimento FdCAd, Pena RdS. Nutritional and Technological Properties of Albino Peach Palm (Bactris gasipaes) from the Amazon: Influence of Cooking and Drying. Foods. 2023; 12(23):4344. https://doi.org/10.3390/foods12234344
Chicago/Turabian StyleSoares, Stephanie Dias, Orquídea Vasconcelos dos Santos, Leyvison Rafael Vieira da Conceição, Hilton Túlio Costi, José Otávio Carrera Silva Júnior, Francisco das Chagas Alves do Nascimento, and Rosinelson da Silva Pena. 2023. "Nutritional and Technological Properties of Albino Peach Palm (Bactris gasipaes) from the Amazon: Influence of Cooking and Drying" Foods 12, no. 23: 4344. https://doi.org/10.3390/foods12234344
APA StyleSoares, S. D., dos Santos, O. V., da Conceição, L. R. V., Costi, H. T., Silva Júnior, J. O. C., Nascimento, F. d. C. A. d., & Pena, R. d. S. (2023). Nutritional and Technological Properties of Albino Peach Palm (Bactris gasipaes) from the Amazon: Influence of Cooking and Drying. Foods, 12(23), 4344. https://doi.org/10.3390/foods12234344