Opuntia Ficus-Indica Peel By-Product as a Natural Antioxidant Food Additive and Natural Anticoccidial Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical Reagents
2.3. Microwave-Assisted Extraction
2.4. Determination of Phenolic Compound Contents and Antioxidant Activities
2.4.1. Determination of Total Phenolic Content (TPC)
2.4.2. Determination of Antioxidant Activity (DPPH Radical Scavenging Assay)
2.4.3. Ferric Reducing Power (FRAP)
2.5. Phenolic Compound Profile
2.6. Margarine Preparation and Oxidative Stability
2.7. Determination of the Anticoccidial Activity of OFI Peel Optimum Extract
2.8. Experimental Design and Statistical Analyses
3. Results and Discussion
3.1. Phenolic Compounds
3.2. Margarine Oxidative Stability
3.3. Anticoccidial Activities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loiseau, E.; Saikku, L.; Antikainen, R.; Droste, N.; Hansjürgens, B.; Pitkänen, K.; Leskinen, P.; Kuikman, P.; Thomsen, M. Green economy and related concepts: An overview. J. Clean. Prod. 2016, 139, 361–371. [Google Scholar] [CrossRef]
- Du, K.; Cheng, Y.; Yao, X. Environmental regulation, green technology innovation, and industrial structure upgrading: The road to the green transformation of Chinese cities. Energy Econ. 2021, 98, 105247. [Google Scholar] [CrossRef]
- Oloruntobi, O.; Mokhtar, K.; Rozar, N.M.; Gohari, A.; Asif, S.; Chuah, L.F. Effective technologies and practices for reducing pollution in warehouses-a review. Clean. Eng. Technol. 2023, 13, 100622. [Google Scholar] [CrossRef]
- Amrane-Abider, M.; Nerín, C.; Tamendjari, A.; Serralheiro, M.L.M. Phenolic composition, antioxidant and antiacetylcholinesterase activities of Opuntia ficus-indica peel and flower teas after in vitro gastrointestinal digestion. J. Sci. Food Agric. 2022, 102, 4401–4409. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Jiménez-Martínez, C.; Sandoval, G. Revalorization of cactus pear (Opuntia spp.) wastes as a source of antioxidants. Food Sci. Technol. 2011, 31, 782–788. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Avellone, G.; Carnaroglio, D.; Danzì, C.; Timpanaro, G.; Meneguzzo, F.; Ilharco, L.M.; Pagliaro, M. Economic and technical feasibility of betanin and pectin extraction from Opuntia ficus-indica peel via microwave-assisted hydrodiffusion. ACS Omega 2019, 4, 12121–12124. [Google Scholar] [CrossRef]
- Forni, E.; Penci, M.; Polesello, A. A preliminary characterization of some pectins from quince fruit (Cydonia oblonga Mill.) and prickly pear (Opuntia ficus indica) peel. Carbohydr. Polym. 1994, 23, 231–234. [Google Scholar] [CrossRef]
- Gopi, D.; Kanimozhi, K.; Kavitha, L. Opuntia ficus indica peel derived pectin mediated hydroxyapatite nanoparticles: Synthesis, spectral characterization, biological and antimicrobial activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 135–143. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response surface methodology as a tool to optimize the extraction of bioactive compounds from plant sources. J. Sci. Food Agric. 2023, 103, 26–36. [Google Scholar] [CrossRef]
- Amrane-Abider, M.; Nerin, C.; Canellas, E.; Benkerrou, F.; Louaileche, H. Modeling and optimization of phenolic compounds extraction from prickly pear (Opuntia ficus-indica) seeds via ultrasound-assisted technique. Ann. Univ. Dunarea De Jos Galati. Fascicle VI-Food Technol. 2018, 42, 109–121. [Google Scholar]
- Benkerrou, F.; Bachir bey, M.; Amrane, M.; Louaileche, H. Ultrasonic-assisted extraction of total phenolic contents from Phoenix dactylifera and evaluation of antioxidant activity: Statistical optimization of extraction process parameters. J. Food Meas. Charact. 2018, 12, 1910–1916. [Google Scholar] [CrossRef]
- Amrane-Abider, M.; Nerin, C.; Cannelas, E.; Zeroual, B.; Hadjal, S.; Louaileche, H. Prickly pear (Opuntia ficus-indica) seeds as a source of phenolic compounds: Microwave-assisted extraction optimization and effect on food lipid oxidations. Ann. Univ. Dunarea De Jos Galati. Fascicle VI-Food Technol. 2018, 42, 23–35. [Google Scholar]
- Bouchez, A.; Vauchel, P.; Périno, S.; Dimitrov, K. Multi-Criteria Optimization including Environmental Impacts of a Microwave-Assisted Extraction of Polyphenols and Comparison with an Ultrasound-Assisted Extraction Process. Foods 2023, 12, 1750. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Larrea-Wachtendorff, D.; Donsi, F.; Ferrari, G. Functionalization of pasta through the incorporation of bioactive compounds from agri-food by-products: Fundamentals, opportunities, and drawbacks. Trends Food Sci. Technol. 2022, 122, 49–65. [Google Scholar] [CrossRef]
- Gorji, S.G.; Smyth, H.E.; Sharma, M.; Fitzgerald, M. Lipid oxidation in mayonnaise and the role of natural antioxidants: A review. Trends Food Sci. Technol. 2016, 56, 88–102. [Google Scholar] [CrossRef]
- Ouahrani, S.; Casal, S.; Bachir-bey, M.; Zaidi, F. Impact of Moringa oleífera leaves extract in the stabilization of margarine under accelerated storage. J. Food Meas. Charact. 2023, 17, 1455–1466. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Abd El-Rahman, G.I.; Behairy, A.; Beheiry, R.R.; Hendam, B.M.; Alsubaie, F.M.; Khalil, S.R. Influence of feeding quinoa (Chenopodium quinoa) seeds and prickly pear fruit (Opuntia ficus indica) peel on the immune response and resistance to Aeromonas sobria infection in Nile tilapia (Oreochromis niloticus). Animals 2020, 10, 2266. [Google Scholar] [CrossRef]
- Mahrose, K.M. Prickly Pear (Opuntia spp.) in Animal and Poultry Feed. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 827–839. [Google Scholar]
- Badr, S.E.; Fattah, M.S.A.; Elsaid, A.S. Productive performance and meat quality of commercial Cobb chicken fed diets containing different levels of prickly pear fruits (Opuntia ficus indica) peel. Bull. Natl. Res. Cent. 2019, 43, 195. [Google Scholar] [CrossRef]
- Debbou-Iouknane, N.; Nerín, C.; Amrane-Abider, M.; Ayad, A. In vitro anticoccidial effects of Olive Leaf (Olea europaea L. var. Chemlal) extract against broiler chickens Eimeria oocysts. Vet. Ir Zootech. 2021, 79, 1–8. [Google Scholar]
- Herrero-Encinas, J.; Huerta, A.; Blanch, M.; Pastor, J.J.; Morais, S.; Menoyo, D. Impact of Dietary Supplementation of Spice Extracts on Growth Performance, Nutrient Digestibility and Antioxidant Response in Broiler Chickens. Animals 2023, 13, 250. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Armson, A.; Lymbery, A.J.; Zahedi, A.; Ash, A. Medicinal plants as a source of antiparasitics: An overview of experimental studies. Pathog. Glob. Health 2023, 117, 535–553. [Google Scholar] [CrossRef]
- Abebe, E.; Gugsa, G. A review on poultry coccidiosis. Abyssinia J. Sci. Technol. 2018, 3, 1–12. [Google Scholar]
- Saeed, Z.; Alkheraije, K.A. Botanicals: A promising approach for controlling cecal coccidiosis in poultry. Front. Vet. Sci. 2023, 10, 1157633. [Google Scholar] [CrossRef]
- Amrane-Abider, M.; Imre, M.; Herman, V.; Debbou-Iouknane, N.; Zemouri-Alioui, S.; Khaled, S.; Bouiche, C.; Nerín, C.; Acaroz, U.; Ayad, A. Bioactive Compounds and In Vitro Antioxidant and Anticoccidial Activities of Opuntia ficus-indica Flower Extracts. Biomedicines 2023, 11, 2173. [Google Scholar] [CrossRef]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol 2004, 26, 211–219. [Google Scholar]
- Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- ISO 6886:2016; Animal and Vegetable Fats and Oils. Determination of the Oxidation Stability (Accelerated Oxidation Test) (2nd ed.). ISO International Standard: Geneva, Switzerland, 2006; pp. 1–14.
- Carvalho, F.S.; Wenceslau, A.A.; Teixeira, M.; Carneiro, J.A.M.; Melo, A.D.B.; Albuquerque, G.R. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet. Parasitol. 2011, 176, 95–100. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Ahmad, J.; Langrish, T. Optimisation of total phenolic acids extraction from mandarin peels using microwave energy: The importance of the Maillard reaction. J. Food Eng. 2012, 109, 162–174. [Google Scholar] [CrossRef]
- Bansod, S.P.; Parikh, J.K.; Sarangi, P.K. Pineapple peel waste valorization for extraction of bio-active compounds and protein: Microwave assisted method and Box Behnken design optimization. Environ. Res. 2023, 221, 115237. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Abou-Elella, F.M.; Ali, R.F.M. Antioxidant and anticancer activities of different constituents extracted from Egyptian prickly pear Cactus (Opuntia ficus-indica) Peel. Biochem. Anal. Biochem. 2014, 3, 158. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Chougui, N.; Louaileche, H.; Mohedeb, S.; Mouloudj, Y.; Hammoui, Y.; Tamendjari, A. Physico-chemical characterisation and antioxidant activity of some Opuntia ficus-indica varieties grown in North Algeria. Afr. J. Biotechnol. 2013, 12, 299–307. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. 12—Methods for the assessment of antioxidant activity in foods11This chapter is reproduced to a large extent from an article in press by the authors in the Journal of Functional Foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 287–333. [Google Scholar]
- Gómez-Maqueo, A.; Escobedo-Avellaneda, Z.; Welti-Chanes, J. Phenolic compounds in mesoamerican fruits—Characterization, health potential and processing with innovative technologies. Int. J. Mol. Sci. 2020, 21, 8357. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Yang, B.; Du, J.; Chen, L.; Li, Y.; Guo, F. Structures, Sources, Identification/Quantification Methods, Health Benefits, Bioaccessibility, and Products of Isorhamnetin Glycosides as Phytonutrients. Nutrients 2023, 15, 1947. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Ammar, I.; Salem, M.B.; Harrabi, B.; Mzid, M.; Bardaa, S.; Sahnoun, Z.; Attia, H.; Ennouri, M. Anti-inflammatory activity and phenolic composition of prickly pear (Opuntia ficus-indica) flowers. Ind. Crops Prod. 2018, 112, 313–319. [Google Scholar] [CrossRef]
- Brahmi, F.; Blando, F.; Sellami, R.; Mehdi, S.; De Bellis, L.; Negro, C.; Haddadi-Guemghar, H.; Madani, K.; Makhlouf-Boulekbache, L. Optimization of the conditions for ultrasound-assisted extraction of phenolic compounds from Opuntia ficus-indica [L.] Mill. flowers and comparison with conventional procedures. Ind. Crops Prod. 2022, 184, 114977. [Google Scholar] [CrossRef]
- De Leo, M.; De Abreu, M.B.; Pawlowska, A.; Cioni, P.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Bockstaele, F.V.; Penagos, I.A.; Rondou, K.; Dewettinck, K. Margarine and Fat Spreads. In Fat Mimetics for Food Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 366–391. [Google Scholar]
- Prior, E. Usage des corps gras alimentaires dans les différents secteurs de la technologie alimentaire. In Lipides et Corps Gras Alimentaires; Lavoisier Tec et Doc: Paris, France, 2003; pp. 87–147. [Google Scholar]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.; Ferreira, O.; Ferreira, I.C. Optimization and comparison of maceration and microwave extraction systems for the production of phenolic compounds from Juglans regia L. for the valorization of walnut leaves. Ind. Crops Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef]
- Kutlu, N.; Isci, A.; Sakiyan, O.; Yilmaz, A.E. Extraction of Phenolic Compounds from Cornelian Cherry (Cornus mas L.) Using Microwave and Ohmic Heating Assisted Microwave Methods. Food Bioprocess Technol. 2021, 14, 650–664. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.; Alami, M.; Khomeiri, M. Microwave-assisted extraction of phenolic compounds from olive leaves; a comparison with maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Serra, J.J.; Mura, J.; Fagoaga, C.; Castellano, G. Oxidative Stability of Margarine is Improved by Adding Natural Antioxidants from Herbs and Spices. 2023; preprint. [Google Scholar] [CrossRef]
- Martínez-Girón, J. Ultrasound-assisted production of sweet pepper (Capsicum annuum) oleoresin and tree tomato (Solanum betaceum Cav.) juice: A potential source of bioactive compounds in margarine. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Nahed, A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F. Phytochemical control of poultry coccidiosis: A review. Poult. Sci. 2022, 101, 101542. [Google Scholar]
- Debbou-Iouknane, N.; Nerín, C.; Amrane, M.; Ghemghar, M.; Madani, K.; Ayad, A. In vitro anticoccidial activity of olive pulp (Olea europaea L. var. chemlal) extract against Eimeria oocysts in broiler chickens. Acta Parasitol. 2019, 64, 887–897. [Google Scholar] [CrossRef]
- Murshed, M.; Aljawdah, H.M.; Mares, M.; Al-Quraishy, S. In Vitro: The Effects of the Anticoccidial Activities of Calotropis procera Leaf Extracts on Eimeria stiedae Oocysts Isolated from Rabbits. Molecules 2023, 28, 3352. [Google Scholar] [CrossRef]
- Kerboeuf, D.; Riou, M.; Guégnard, F. Flavonoids and related compounds in parasitic disease control. Mini Rev. Med. Chem. 2008, 8, 116–128. [Google Scholar] [CrossRef]
- Alnassan, A.A.; Thabet, A.; Daugschies, A.; Bangoura, B. In vitro efficacy of allicin on chicken Eimeria tenella sporozoites. Parasitol. Res. 2015, 114, 3913–3915. [Google Scholar] [CrossRef]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Massi, P.; Tosi, G.; Fiorentini, L.; Piva, A.; Grilli, E. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult. Sci. 2020, 99, 5350–5355. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.Z.; Abbas, A.; Raza, M.A.; Khan, M.K.; Saleemi, M.K.; Saeed, Z. In vitro anticoccidial activity of Trachyspermum ammi (Ajwain) extract on oocysts of Eimeria species of Chicken. Adv. Life Sci. 2019, 7, 44–47. [Google Scholar]
- Ali, M.; Chand, N.; Khan, R.U.; Naz, S.; Gul, S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar] [CrossRef]
- Tanweer, A.J.; Chand, N.; Saddique, U.; Bailey, C.; Khan, R. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol. Res. 2014, 113, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
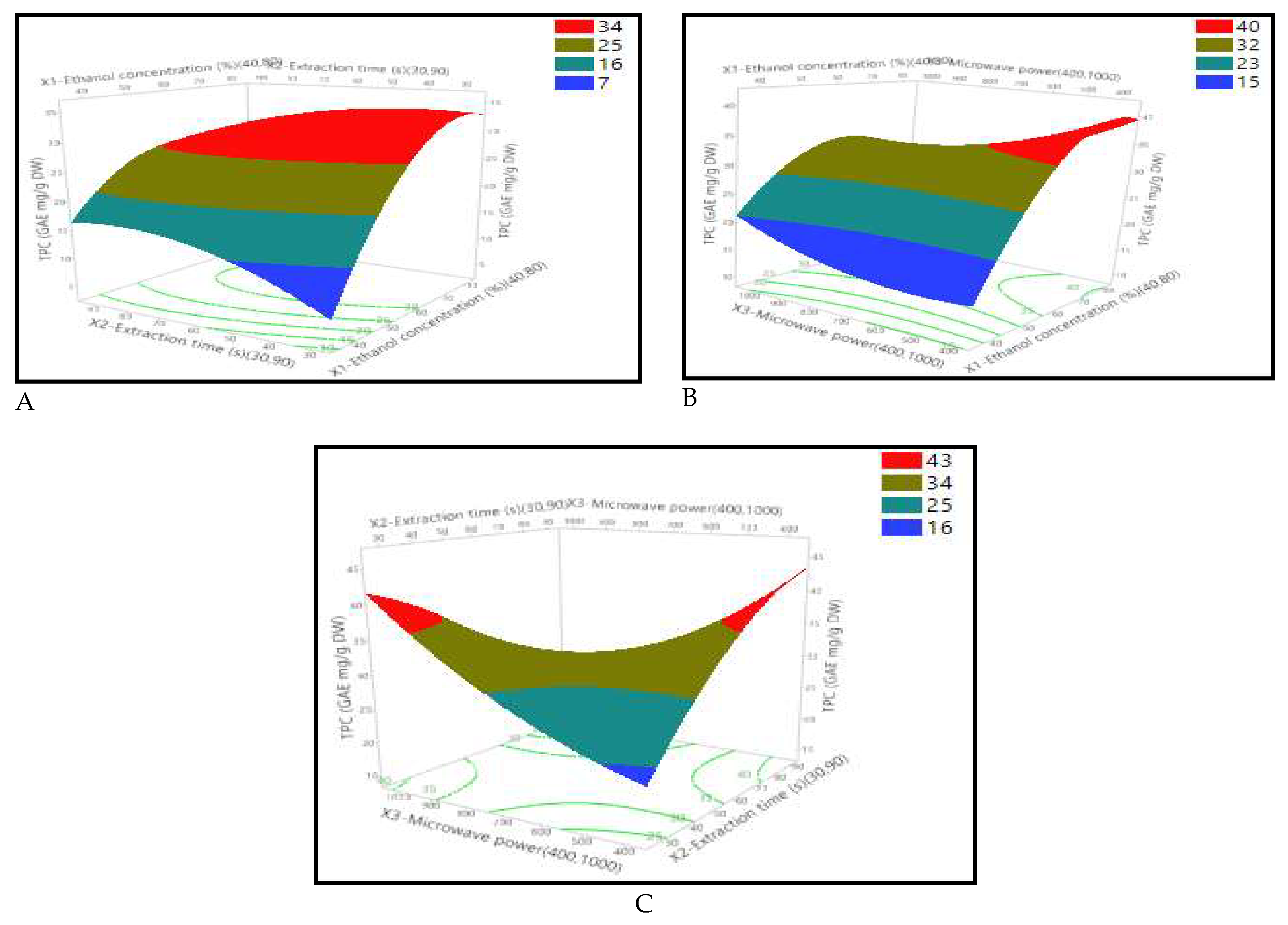




| Run | Variable Levels | TPC (GAE mg/g DW) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Observed | Predicted | |
| 1 | 40 | 30 | 700 | 12.59 | 14.47 |
| 2 | 60 | 60 | 700 | 32.32 | 31.47 |
| 3 | 60 | 90 | 1000 | 19.59 | 20.12 |
| 4 | 80 | 90 | 700 | 26.97 | 25.08 |
| 5 | 80 | 60 | 400 | 37.24 | 38.78 |
| 6 | 80 | 30 | 700 | 34.67 | 33.70 |
| 7 | 60 | 60 | 700 | 33.82 | 31.47 |
| 8 | 60 | 90 | 400 | 40.11 | 40.50 |
| 9 | 60 | 60 | 700 | 29.27 | 31.47 |
| 10 | 40 | 60 | 400 | 21.66 | 20.30 |
| 11 | 40 | 60 | 1000 | 25.36 | 23.86 |
| 12 | 60 | 30 | 1000 | 39.28 | 38.89 |
| 13 | 80 | 60 | 1000 | 27.94 | 29.30 |
| 14 | 60 | 30 | 400 | 24.90 | 24.37 |
| 15 | 40 | 90 | 700 | 19.47 | 20.44 |
| Source | DF | Sum of Squares | F Ratio | Prob > F |
|---|---|---|---|---|
| TPC | ||||
| X1 | 1 | 245.0340 | 42.5626 | 0.0013 * |
| X2 | 1 | 214.0661 | 37.1834 | 0.0017 * |
| X3 | 1 | 30.7549 | 5.3422 | 0.0688 |
| X1X2 | 1 | 53.1776 | 9.2370 | 0.0288 * |
| X1X3 | 1 | 42.2435 | 7.3377 | 0.0423 * |
| X2X3 | 1 | 304.5200 | 52.8954 | 0.0008 * |
| X1X1 | 1 | 117.9654 | 20.4907 | 0.0062 * |
| X2X2 | 1 | 27.5454 | 4.7847 | 0.0804 |
| X3X3 | 1 | 13.2628 | 2.3038 | 0.18952 |
| Model | 9 | 886.31 | 16.71 | 0.0032 * |
| Lack of fit | 3 | 18.0061 | 1.1137 | 0.5053 |
| Error | 5 | 28.7851 | ||
| Total model | 14 | 895.0955 | ||
| R2 = 0.97 | ||||
| Adj. R2 = 0.90 |
| Variables | p-Value | |
|---|---|---|
| X2-Extraction time (s) × X3-Microwave power (W) |  | 0.00077 |
| X1-Ethanol concentration (%) |  | 0.00089 |
| X1-Ethanol concentration (%) × X1-Ethanol concentration (%) |  | 0.00625 |
| X1-Ethanol concentration (%) × X2-Extraction time (s) |  | 0.02877 |
| X1-Ethanol concentration (%) × X3-Microwave power (W) |  | 0.04228 |
| X2-Extraction time (s) × X2-Extraction time (s) |  | 0.08046 |
| X3-Microwave power (W) |  | 0.14409 |
| X3-Microwave power (W) × X3-Microwave power (W) |  | 0.18927 |
| X2-Extraction time (s) |  | 0.47001 |
| Antioxidant Activities IC50 (mg/mL) | |
| DPPH radical | 12.99 ± 0.36 |
| Ferric reducing power | 6.57 ± 0.05 |
| Rancimat (hours) | |
| Margarine control | 12.82 ± 0.51 a |
| Margarine vitamin E | 14.33 ± 0.22 b |
| Margarine enriched (OFI) 50 ppm | 15.86 ± 0.17 c |
| Margarine enriched (OFI) 100 ppm | 16.02 ± 0.41 d |
| Compounds | [M-H] | Retention Time (min) | Concentration (µg/g) | Molecular Formula | Fragment Ions (m/z) | |
|---|---|---|---|---|---|---|
| 1 | Vanilic acid | 167 | 0.65 | 60.09 ± 0.13 | C8H8O4 | 167 (100), 153 (5), 137 (3), 123 (1) |
| 2 | Coumaric acid | 163 | 0.82 | 93.12 ± 0.25 | C9H8O3 | 119 (100),163 (22), 91 (2) |
| 3 | Protocatechuic acid | 153 | 3.08 | 81.80 ± 0.19 | C7H6O4 | 153 (39), 109 (100), 111 (29) |
| 4 | Isorhamnetin | 315 | 6.88 | 155.37 ± 0.51 | C16H12O7 | 315 (100), 301 (7), 297 (6), 285 (2) |
| 5 | Dihydrokaempferol | 287 | 7.15 | 206.16 ± 0. 30 | C15H12O6 | 289 (100), 273 (2), 271 (49), 153 (11) |
| 6 | Kaempferol-3-O-rutinoside | 593 | 7.46 | 112.81 ± 0.15 | C27H30O15 | 287 (100), 146 (7) |
| 7 | Isorhamnetin 3-O-rutinoside | 623 | 7.78 | 108.79 ± 0.21 | C28H32O16 | 315 (42), 314 (100) |
| 8 | Isorhamnetin 3-O-glucoside | 477 | 8.10 | 95.80 ± 0.12 | C22H22O12 | 315 (100), 300 (80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrane-Abider, M.; Imre, M.; Herman, V.; Debbou-Iouknane, N.; Saci, F.; Boudries, H.; Madani, K.; Merzouk, H.; Ayad, A. Opuntia Ficus-Indica Peel By-Product as a Natural Antioxidant Food Additive and Natural Anticoccidial Drug. Foods 2023, 12, 4403. https://doi.org/10.3390/foods12244403
Amrane-Abider M, Imre M, Herman V, Debbou-Iouknane N, Saci F, Boudries H, Madani K, Merzouk H, Ayad A. Opuntia Ficus-Indica Peel By-Product as a Natural Antioxidant Food Additive and Natural Anticoccidial Drug. Foods. 2023; 12(24):4403. https://doi.org/10.3390/foods12244403
Chicago/Turabian StyleAmrane-Abider, Meriem, Mirela Imre, Viorel Herman, Nedjima Debbou-Iouknane, Fairouz Saci, Hafid Boudries, Khodir Madani, Hafida Merzouk, and Abdelhanine Ayad. 2023. "Opuntia Ficus-Indica Peel By-Product as a Natural Antioxidant Food Additive and Natural Anticoccidial Drug" Foods 12, no. 24: 4403. https://doi.org/10.3390/foods12244403
APA StyleAmrane-Abider, M., Imre, M., Herman, V., Debbou-Iouknane, N., Saci, F., Boudries, H., Madani, K., Merzouk, H., & Ayad, A. (2023). Opuntia Ficus-Indica Peel By-Product as a Natural Antioxidant Food Additive and Natural Anticoccidial Drug. Foods, 12(24), 4403. https://doi.org/10.3390/foods12244403









