Detection of Vibrio parahaemolyticus Based on Magnetic and Upconversion Nanoparticles Combined with Aptamers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Synthesis of Amine-Functionalised Fe3O4 Magnetic Nanoparticles
2.4. UCNP Synthesis and Surface Modifications
2.5. Synthesis of Aptamer-Fe3O4 Magnetic Nanoparticles and Aptamer-UCNPs
2.6. Optimization of Reacted Amount of Aptamer-Fe3O4 Magnetic Nanoparticles and Aptamer-UCNPs
2.7. Detection of V. parahaemolyticus Using the Developed Fluorescent Method
2.8. Detection Specificity
3. Results and Discussion
3.1. Mechanism of V. parahaemolyticus Detection
3.2. Characterisation of Amine-Functionalised Magnetic Fe3O4 Nanoparticle
3.3. UCNP Characterisation
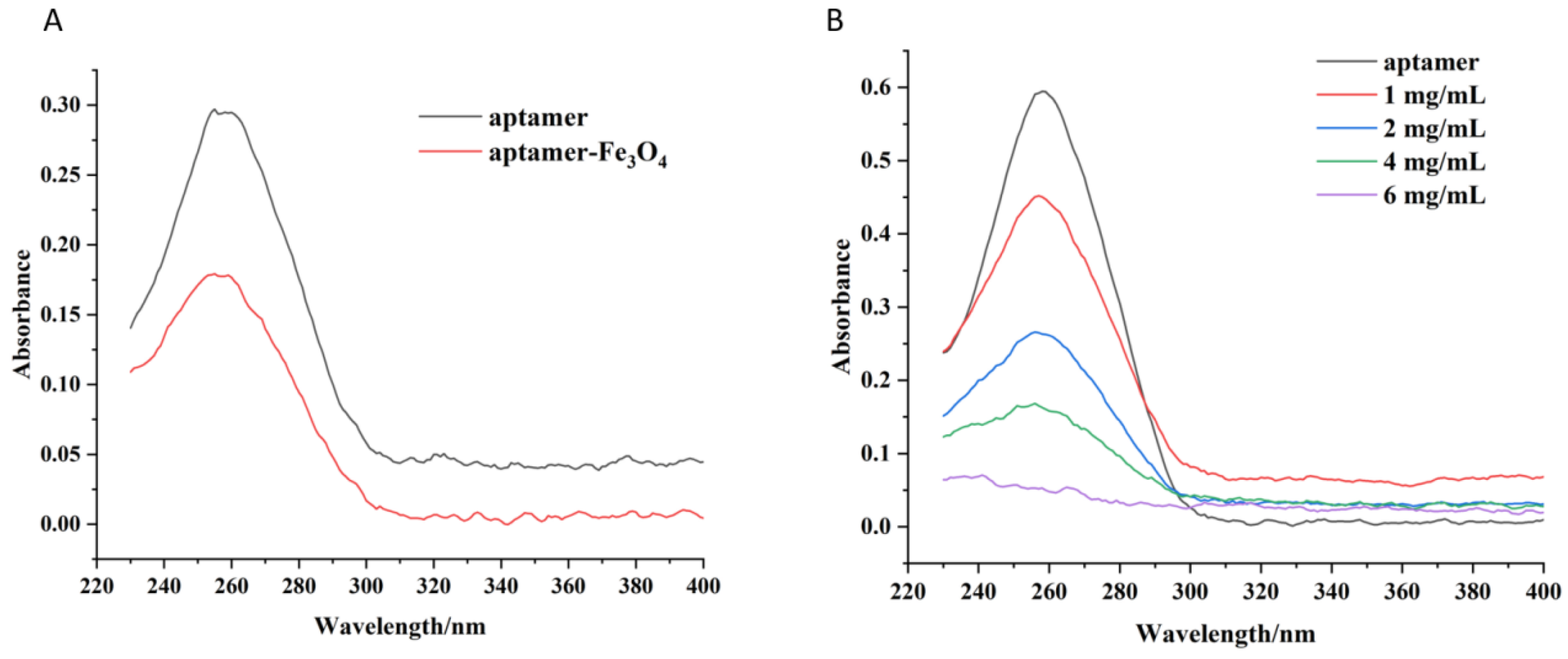
3.4. Aptamer-Fe3O4 Magnetic Nanoparticles and Aptamer-UCNP Characterisation
3.5. Optimal Amount of Aptamer-Fe3O4 Magnetic Nanoparticles and Aptamer-UCNPs
3.6. Detection Method Sensitivity
3.7. Detection Method Specificity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bavisetty, S.C.B.; Vu, H.T.K.; Benjakul, S.; Vongkamjan, K. Rapid pathogen detection tools in seafood safety. Curr. Opin. Food Sci. 2018, 20, 92–99. [Google Scholar] [CrossRef]
- Afreen, M.; Bağdatli, İ. Food-borne pathogens in seafood. EJAR 2021, 5, 44–58. [Google Scholar]
- Cai, G.; Zheng, L.; Liao, M.; Li, Y.; Wang, M.; Liu, N.; Lin, J. A microfluidic immunosensor for visual detection of foodborne bacteria using immunomagnetic separation, enzymatic catalysis and distance indication. Microchim. Acta 2019, 186, 757. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Dhital, R.; Aghvami, S.A.; Mustapha, A.; Zhang, Y.; Lin, M. Separation and detection of E. coli O157:H7 using a SERS-based microfluidic immunosensor. Microchim. Acta 2022, 189, 111. [Google Scholar] [CrossRef]
- Nair, G.B.; Ramamurthy, T.; Bhattacharya, S.K.; Dutta, B.; Takeda, Y.; Sack, D.A. Global dissemination of Vibrio parahaemolyticus Serotype O3:K6 and its serovariants. Clin. Microbiol. Rev. 2007, 20, 39–48. [Google Scholar] [CrossRef]
- Park, K.; Mok, J.S.; Kwon, J.Y.; Ryu, A.R.; Kim, S.H.; Lee, H.J. Food-borne outbreaks, distributions, virulence, and antibiotic resistance profiles of Vibrio parahaemolyticus in Korea from 2003 to 2016: A review. Fish. Aquat. Sci. 2018, 21, 3. [Google Scholar] [CrossRef]
- Chen, L.; Sun, L.; Zhang, R.; Liao, N.; Qi, X.; Chen, J. Surveillance for foodborne disease outbreaks in Zhejiang Province, China, 2015–2020. BMC Public Health 2022, 22, 135. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Malakar, P.K.; Pan, Y.; Zhao, Y.; Zhang, Z. Modeling naturally-occurring Vibrio parahaemolyticus in post-harvest raw shrimps. Food Res. Int. 2023, 173, 113462. [Google Scholar] [CrossRef]
- Ndraha, N.; Hsiao, H. A climate-driven model for predicting the level of Vibrio parahaemolyticus in oysters harvested from Taiwanese farms using elastic net regularized regression. Microb. Risk Anal. 2022, 21, 100201. [Google Scholar] [CrossRef]
- Su, Y.C.; Duan, J.; Wu, W.-H. Selectivity and specificity of a chromogenic medium for detecting Vibrio parahaemolyticus. J. Food Prot. 2005, 68, 1454–1456. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhao, C.; Li, L.; Xu, K.; Wang, J.; Song, X.; Li, H. Production of phage display-derived peptide and the application for detecting Vibrio parahaemolyticus by combined PCR technology. Food Anal. Methods 2020, 13, 1906–1917. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Bai, H.; Li, X.; Lv, P.; Guo, A. Specific Detection of Vibrio Parahaemolyticus by Fluorescence Quenching Immunoassay Based on Quantum Dots. Appl. Biochem. Biotechnol. 2014, 173, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Kong, L.; Lu, Z.; Qiao, J.; Lv, F.; Meng, F.; Bie, X. Research on nanogold-assisted HRM-qPCR technology for highly sensitive and accurate detection of Vibrio parahaemolyticus. LWT Food Sci. Technol. 2022, 162, 113488. [Google Scholar] [CrossRef]
- Feng, Z.-S.; Li, J.-Y.; Zhang, J.-Y.; Li, F.-Y.; Guan, H.-X.; Zhang, R.-Q.; Liu, H.; Guo, Q.; Shen, X.-X.; Kan, B.; et al. Development and evaluation of a sensitive recombinase aided amplification assay for rapid detection of Vibrio parahaemolyticus. J. Microbiol. Methods 2022, 193, 106404. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Chen, S.; Jiang, L.; Ren, J.; Ling, N.; Su, J.; Zhao, Y.; Jiang, Y.; Xue, F.; Tang, F.; et al. A polymerase chain reaction based lateral flow test strip with propidium monoazide for detection of viable Vibrio parahaemolyticus in codfish. Microchem. J. 2020, 159, 105418. [Google Scholar] [CrossRef]
- Yang, G.; Wei, C.; Tang, Y.; Zhang, J.; Zhang, X.; Chi, H.; Tao, L.; Kong, C. A novel fluorescence immunoassay based on inner filter effect and gold nanoclusters for Vibrio parahaemolyticus determination. Results Chem. 2021, 3, 100208. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, M.; Kim, B. Phage biocontrol of zoonotic food-borne pathogen Vibrio parahaemolyticus for seafood safety. Food Control 2023, 144, 109334. [Google Scholar] [CrossRef]
- Ndraha, N.; Huang, L.; Wu, V.C.H.; Hsiao, H.-I. Vibrio parahaemolyticus in seafood: Recent progress in understanding influential factors at harvest and food-safety intervention approaches. Curr. Opin. Food Sci. 2023, 48, 100927. [Google Scholar] [CrossRef]
- Schaumburg, F.; Carrell, C.S.; Henry, C.S. Rapid bacteria detection at low concentrations using sequential immunomagnetic separation and paper-based isotachophoresis. Anal. Chem. 2019, 91, 9623–9630. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, L.; Hao, H.; Zhang, Z.; Ding, C.; Zhang, G.; Bi, J.; Yan, S.; Liu, G.; Hou, H. A novel photoelectrochemical aptamer sensor based on rare-earth doped Bi2WO6 and Ag2S for the rapid detection of Vibrio parahaemolyticus. Microchem. J. 2021, 165, 106132. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wu, S.; Chen, X.; Huang, Y.; Wang, Z. Selection and Identification of a DNA Aptamer Targeted to Vibrio parahemolyticus. J. Agric. Food Chem. 2012, 60, 4034–4038. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Hassan, M.; Ouyang, Q.; Chen, Q. Lanthanide ion (Ln3+)-based upconversion sensor for quantification of food contaminants: A review. Compr. Rev. Food Sci. 2021, 20, 3531–3578. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bao, J.; Wang, L.; Zhang, F.; Li, Y. One-pot synthesis and bioapplication of amine-functionalized magnetite nanoparticles and hollow nanospheres. Chem. Eur. J. 2006, 12, 6341–6347. [Google Scholar] [CrossRef]
- Nodehi, R.; Shayesteh, H.; Rahbar-Kelishami, A. Fe3O4@NiO core–shell magnetic nanoparticle for highly efficient removal of Alizarin red S anionic dye. Int. J. Environ. Sci. Technol. 2022, 19, 2899–2912. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4:Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 2008, 19, 345606. [Google Scholar] [CrossRef]
- Li, H.; Ahmad, W.; Rong, Y.; Chen, Q.; Zuo, M.; Ouyang, Q.; Guo, Z. Designing an aptamer based magnetic and upconversion nanoparticles conjugated fluorescence sensor for screening Escherichia coli in food. Food Control 2020, 107, 106761. [Google Scholar] [CrossRef]
- Sheng, W.; Shi, Y.; Ma, J.; Wang, L.; Zhang, B.; Chang, Q.; Duan, W.; Wang, S. Highly sensitive atrazine fluorescence immunoassay by using magnetic separation and upconversion nanoparticles as labels. Microchim. Acta 2019, 186, 564. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, H.; Shi, Z.; Duan, N.; Fang, C.C.; Dai, S.; Wang, Z. Aptamer-based fluorescence biosensor for chloramphenicol determination using upconversion nanoparticles. Food Control 2015, 50, 597–604. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Ali, S.; Haruna, S.A.; He, P.; Li, H.; Ouyang, Q.; Chen, Q. Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Control 2021, 122, 107808. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Ying, N.; Wang, Y.; Song, X.; Yang, L.; Qin, B.; Wu, Y.; Fang, W. Lateral flow colorimetric biosensor for detection of Vibrio parahaemolyticus based on hybridization chain reaction and aptamer. Microchim. Acta 2021, 188, 381. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Song, Y.; Han, L.; Zhou, D.; Wang, Y.; Pan, L.; Tu, K. An Ultrasensitive Upconversion Fluorescence Aptasensor Based on Graphene Oxide Release and Magnetic Separation for Staphylococcus aureus Detection. Food Anal. Methods 2022, 15, 2791–2800. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, S.; Yu, Z.; Hui, Y.; Li, T.; Wu, D.; Bi, W.; Gan, N.; Jia, Z. On-site and dual-mode detection of live Vibrio parahaemolyticus in waters: A universal pathogen sensing platform based on a smart hydrogel aptasensor imbedded with gold nanoclusters. Sens. Actuators B Chem. 2022, 366, 131947. [Google Scholar] [CrossRef]
- Zhai, Y.; Meng, X.; Li, L.; Liu, Y.; Xu, K.; Zhao, C.; Wang, J.; Song, X.; Li, J.; Jin, M. Rapid detection of Vibrio parahaemolyticus using magnetic nanobead-based immunoseparation and quantum dot-based immunofluorescence. RSC Adv. 2021, 11, 38638–38647. [Google Scholar] [CrossRef]
- Ren, Y.; Cao, L.; Zhang, X.; Jiao, R.; Ou, D.; Wang, Y.; Zhang, D.; Shen, Y.; Ling, N.; Ye, Y. A novel fluorescence resonance energy transfer (FRET)-based paper sensor with smartphone for quantitative detection of Vibrio parahaemolyticus. Food Control 2023, 145, 109412. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wu, J.; Ying, D.; Duan, N.; Wang, Z.; Wu, S. Multifunctional magnetic composite nanomaterial for colorimetric-SERS dual-Mode detection and photothermal sterilization of Vibrio parahaemolyticus. Chem. Eng. J. 2023, 477, 147113. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Ozbay, G. Utilizing HRPzyme, a cost-effective Vibrio parahaemolyticus detection method. LWT 2023, 189, 115461. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Li, W.; Wu, L.; Lv, T.; Zhang, Y.; Sun, J.; Shentu, X.; Yu, X.; Wu, Y. Detection of Vibrio parahaemolyticus Based on Magnetic and Upconversion Nanoparticles Combined with Aptamers. Foods 2023, 12, 4433. https://doi.org/10.3390/foods12244433
Song X, Li W, Wu L, Lv T, Zhang Y, Sun J, Shentu X, Yu X, Wu Y. Detection of Vibrio parahaemolyticus Based on Magnetic and Upconversion Nanoparticles Combined with Aptamers. Foods. 2023; 12(24):4433. https://doi.org/10.3390/foods12244433
Chicago/Turabian StyleSong, Xinjie, Wei Li, Li Wu, Tianfeng Lv, Yao Zhang, Juan Sun, Xuping Shentu, Xiaoping Yu, and Yuanfeng Wu. 2023. "Detection of Vibrio parahaemolyticus Based on Magnetic and Upconversion Nanoparticles Combined with Aptamers" Foods 12, no. 24: 4433. https://doi.org/10.3390/foods12244433
APA StyleSong, X., Li, W., Wu, L., Lv, T., Zhang, Y., Sun, J., Shentu, X., Yu, X., & Wu, Y. (2023). Detection of Vibrio parahaemolyticus Based on Magnetic and Upconversion Nanoparticles Combined with Aptamers. Foods, 12(24), 4433. https://doi.org/10.3390/foods12244433





