Effects of Baking and Frying on the Protein Oxidation of Wheat Dough
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Color Determination
2.4. Secondary Structure of Dough Protein
2.5. Amino Acid Composition Analysis
2.6. Carbonyl Determination
2.7. Free SH Determination
2.8. Free Amino Content Determination
2.9. AGEs Content Determination
2.10. Dityrosine Cozntent Determzination
2.11. Tryptophan Determination
2.12. Determination of Kynurenine and N’-Formylkynurenine
2.13. Determination of Free Radical Scavenging Ability
2.14. In Vitro Protein Digestibility (IVPD)
2.15. Principal Component Analysis (PCA)
2.16. Statistical Analysis
3. Results
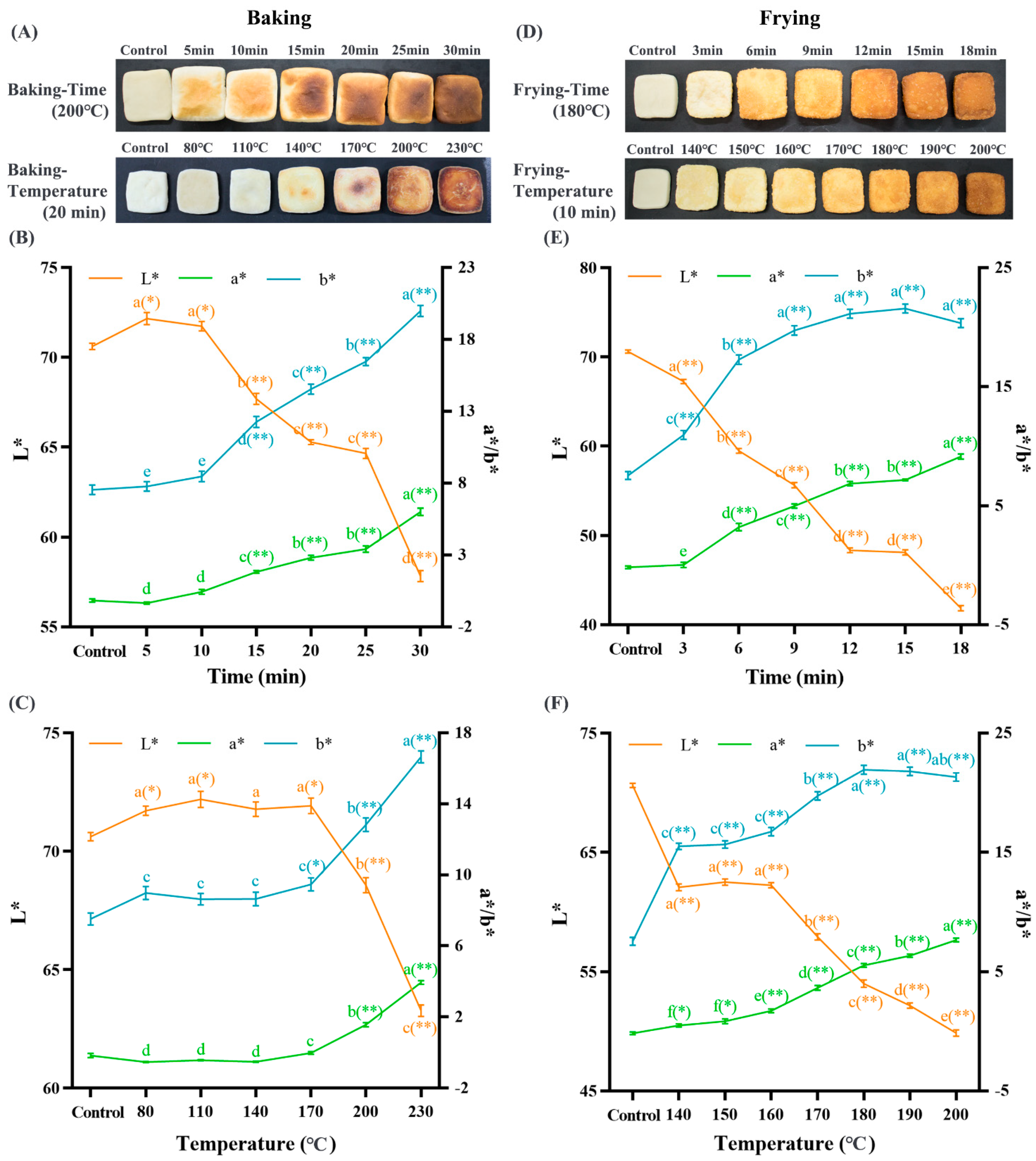
3.1. Baking and Frying Deepen the Dough Color
3.2. Baking and Frying Changed the Secondary Structure of Dough Protein
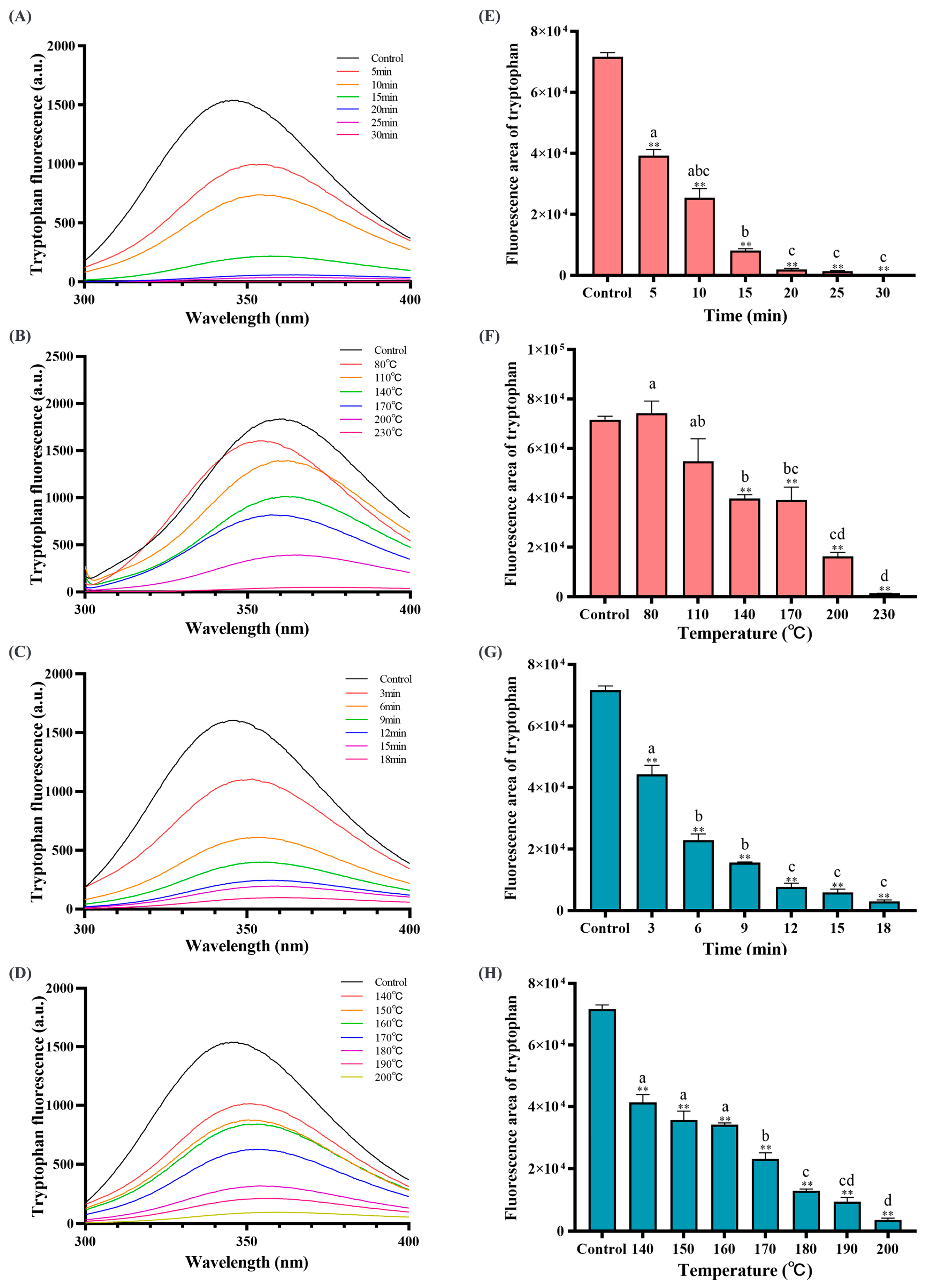
3.3. Baking and Frying Caused Cross-Linking and Oxidation of Dough Proteins
3.4. Baking and Frying Caused Amino Acid Oxidation in the Dough
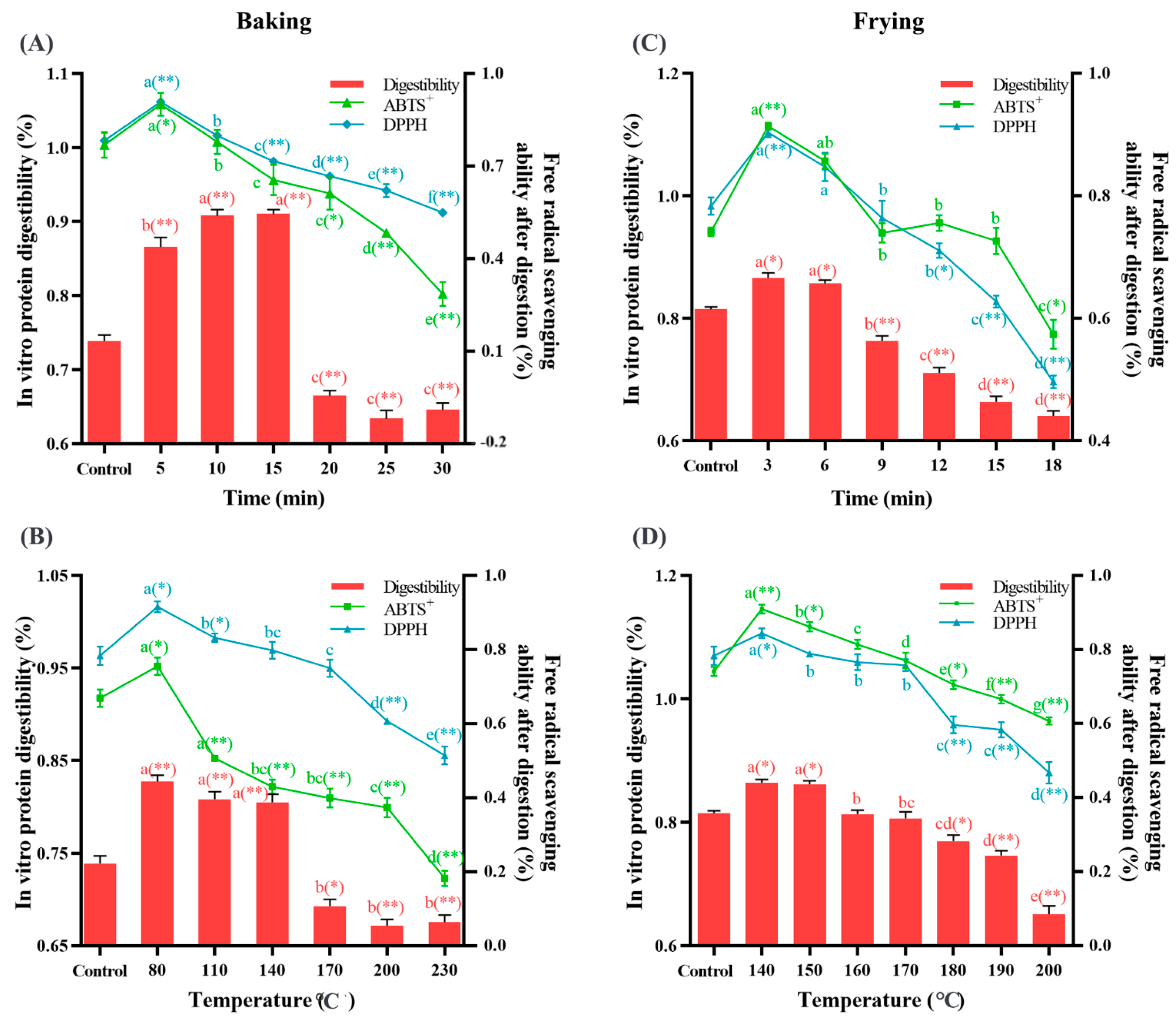
3.5. Baking and Frying Changed the Free Radical Scavenging Rate and Protein Digestibility
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2021, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Wang, H.; Wang, Y.; Ojangba, T.; Nan, H.; Zhang, L.; Yu, Q. Effects of Iron-Catalyzed Oxidation and Methemoglobin Oxidation Systems on Endogenous Enzyme Activity and Myofibrillar Protein Degradation in Yak Meat. Food Chem. 2023, 404, 134647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, Y.; Wang, Q.; Cheng, K.-W.; Chen, F. Application of High Pressure Processing to Improve Digestibility, Reduce Allergenicity, and Avoid Protein Oxidation in Cod (Gadus Morhua). Food Chem. 2019, 298, 125087. [Google Scholar] [CrossRef]
- Qin, R.; Wu, R.; Shi, H.; Jia, C.; Rong, J.; Liu, R. Formation of AGEs in Fish Cakes during Air Frying and Other Traditional Heating Methods. Food Chem. 2022, 391, 133213. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Ding, Y.; Ge, Y.; Xu, Y.; Xie, Y.; Shi, Y.; Le, G. Dityrosine in Food: A Review of Its Occurrence, Health Effects, Detection Methods, and Mitigation Strategies. Compr. Rev. Food Sci. Food Saf. 2023, 22, 355–379. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Yan, B.; Zhang, T.; Gao, Y.; Shi, Y.; Le, G. Health Effects of Dietary Oxidized Tyrosine and Dityrosine Administration in Mice with Nutrimetabolomic Strategies. J. Agric. Food Chem. 2017, 65, 6957–6971. [Google Scholar] [CrossRef]
- Zhou, P.; Dong, S.; Zeng, M. Formation of Nε-Carboxymethyl-Lysine and Nε-Carboxyethyl-Lysine in Pacific Oyster (Crassostrea Gigas) Induced by Thermal Processing Methods. Front. Nutr. 2022, 9, 883789. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, S.; Khan, I.A.; Cheng, Y.; Yu, Y.; Zhang, C.; Huang, J.; Huang, M.; Zhou, X. The Effect of Oxidation and Maillard Reaction on Formation of N ε -Carboxymethyllysine and Nε-Carboxyethyllysine in Prepared Chicken Breast. CyTA—J. Food 2019, 17, 685–694. [Google Scholar] [CrossRef]
- Estévez, M.; Luna, C. Dietary Protein Oxidation: A Silent Threat to Human Health? Crit. Rev. Food Sci. Nutr. 2017, 57, 3781–3793. [Google Scholar] [CrossRef]
- Gurer-Orhan, H.; Ercal, N.; Mare, S.; Pennathur, S.; Orhan, H.; Heinecke, J.W. Misincorporation of Free m -Tyrosine into Cellular Proteins: A Potential Cytotoxic Mechanism for Oxidized Amino Acids. Biochem. J. 2006, 395, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Mo, L.; Yang, Y.; Zhang, S.; Xu, J.; Ge, Y.; Xu, Y.; Shi, Y.; Le, G. Processing Milk Causes the Formation of Protein Oxidation Products Which Impair Spatial Learning and Memory in Rats. RSC Adv. 2019, 9, 22161–22175. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Lin, S.; Li, B.; Yang, Y.; Tang, X.; Shi, Y.; Sun, J.; Le, G. Oxidized Pork Induces Oxidative Stress and Inflammation by Altering Gut Microbiota in Mice. Mol. Nutr. Food Res. 2020, 64, 1901012. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Wang, F.; Zhao, Y.-S.; Pan, H.-Z. Evaluation of Oxidative Stress in Colorectal Cancer Patients. Biomed. Environ. Sci. 2008, 21, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Faure, P.; Lafond, J.-L. Measurement of Plasma Sulfhydryl and Carbonyl Groups as a Possible Indicator of Protein Oxidation. In Analysis of Free Radicals in Biological Systems; Favier, A.E., Cadet, J., Kalyanaraman, B., Fontecave, M., Pierre, J.L., Eds.; Birkhäuser: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Wasim, R.; Mahmood, T.; Siddiqui, M.H.; Ahsan, F.; Shamim, A.; Singh, A.; Shariq, M.; Parveen, S. Aftermath of AGE-RAGE Cascade in the Pathophysiology of Cardiovascular Ailments. Life Sci. 2022, 307, 120860. [Google Scholar] [CrossRef] [PubMed]
- Sahi, A.K.; Verma, P.; Varshney, N.; Gundu, S.; Mahto, S.K. Revisiting Methodologies for In Vitro Preparations of Advanced Glycation End Products. Appl. Biochem. Biotechnol. 2022, 194, 2831–2855. [Google Scholar] [CrossRef] [PubMed]
- Schröter, D.; Höhn, A. Role of Advanced Glycation End Products in Carcinogenesis and Their Therapeutic Implications. Curr. Pharm. Des. 2019, 24, 5245–5251. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, R.; Wang, H.; Hua, C.; Song, S.; Zhou, G.; Zhang, W. Effects of Oxidation In Vitro on Structures and Functions of Myofibrillar Protein from Beef Muscles. J. Agric Food. Chem. 2019, 67, 5866–5873. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, L.; Pereira, J.; Zhang, J.; Zhang, W. Insights into Digestibility and Peptide Profiling of Beef Muscle Proteins with Different Cooking Methods. J. Agric Food. Chem. 2020, 68, 14243–14251. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Liang, Y.; Wu, W. Potential Implications of Oxidative Modification on Dietary Protein Nutritional Value: A Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 714–751. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comparative Peptidomic Profile and Bioactivities of Cooked Beef, Pork, Chicken and Turkey Meat after in Vitro Gastro-Intestinal Digestion. J. Proteom. 2019, 208, 103500. [Google Scholar] [CrossRef] [PubMed]
- Van Steertegem, B.; Pareyt, B.; Slade, L.; Levine, H.; Brijs, K.; Delcour, J.A. Impact of Heat Treatment on Wheat Flour Solvent Retention Capacity (SRC) Profiles. Cereal Chem. 2013, 90, 608–610. [Google Scholar] [CrossRef]
- Tilley, K.A.; Benjamin, R.E.; Bagorogoza, K.E.; Okot-Kotber, B.M.; Prakash, O.; Kwena, H. Tyrosine Cross-Links: Molecular Basis of Gluten Structure and Function. J Agric. Food Chem. 2001, 49, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.-L.; Cha, S.-H.; Park, S.-B.; Jiang, S.; Hyun, T.K.; Jang, K.-I. Quality Characteristics of Noodles Produced Using Steam-Treated Dough Prepared with Psyllium Husk and Soaked-and-Dried Soybean. J. Food Process. Preserv. 2023, 2023, 1–14. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, B.; Wu, Y.; Zhu, H.; Lian, H.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Effects of Sourdough Addition on the Textural and Physiochemical Attributes of Microwaved Steamed-Cake. LWT 2021, 146, 11396. [Google Scholar] [CrossRef]
- Kukurová, K.; Morales, F.J.; Bednáriková, A.; Ciesarová, Z. Effect of L-Asparaginase on Acrylamide Mitigation in a Fried-Dough Pastry Model. Mol. Nutr. Food Res. 2009, 53, 1532–1539. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Effect of Heat Treatment on Protein Oxidation in Pig Meat. Meat Sci. 2012, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Shen, S.; Yu, W.; Bu, Q.; Ding, Z.; Fu, J. Insights into Peptide Profiling of Sturgeon Myofibrillar Proteins with Low Temperature Vacuum Heating. J. Sci. Food Agric. 2023, 103, 2858–2866. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Cui, C.; Sun, W. Influence of Linoleic Acid-Induced Oxidative Modifications on Physicochemical Changes and in Vitro Digestibility of Porcine Myofibrillar Proteins. LWT 2015, 61, 414–421. [Google Scholar] [CrossRef]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-Based Method for Determination of Protein Carbonylation in Muscle and Meat. Food Chem. 2016, 197, 670–675. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, X.; Yang, Y.; Chen, Y.; Tang, X.; Wei, S.; Li, S. Effects of High-Speed Shear Homogenization on the Emulsifying and Structural Properties of Myofibrillar Protein under Low-Fat Conditions. J. Sci. Food Agric. 2019, 99, 6500–6508. [Google Scholar] [CrossRef] [PubMed]
- Gujral, H.S.; Rosell, C.M. Functionality of Rice Flour Modified with a Microbial Transglutaminase. J. Cereal Sci. 2004, 39, 225–230. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Zheng, X.; Yang, Y.; Xiao, D.; Zhang, H.; Ai, B.; Sheng, Z. κ-Carrageenan Inhibits the Formation of Advanced Glycation End Products in Cakes: Inhibition Mechanism, Cake Characteristics, and Sensory Evaluation. Food Chem. 2023, 429, 136583. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L.; Park, D.; Ooizumi, T. Variation in the Cross-Linking Pattern of Porcine Myofibrillar Protein Exposed to Three Oxidative Environments. J. Agric. Food Chem. 2009, 57, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Fluorescence Spectroscopy as a Novel Approach for the Assessment of Myofibrillar Protein Oxidation in Oil-in-Water Emulsions. Meat Sci. 2008, 80, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the Antioxidant Properties of Quinoa Flour through Fermentation with Selected Autochthonous Lactic Acid Bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef]
- Liu, T.X.; Zhao, M. Physical and Chemical Modification of SPI as a Potential Means to Enhance Small Peptide Contents and Antioxidant Activity Found in Hydrolysates. Innov. Food Sci. Emerg. Technol. 2010, 11, 677–683. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, J.; Li, B.; Lu, M.; Le, G.; Xie, Y. Metabolomics Based On1 H-Nmr Reveal the Regulatory Mechanisms of Dietary Methionine Restriction on Splenic Metabolic Dysfunction in Obese Mice. Foods 2021, 10, 2439. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Faubion, J.M. Effect of an Enzyme Preparation on Wheat Flour and Dough Color, Mixing, and Test Baking. LWT—Food Sci. Technol. 2009, 42, 1461–1467. [Google Scholar] [CrossRef]
- Žilić, S.; Kocadağlı, T.; Vančetović, J.; Gökmen, V. Effects of Baking Conditions and Dough Formulations on Phenolic Compound Stability, Antioxidant Capacity and Color of Cookies Made from Anthocyanin-Rich Corn Flour. LWT—Food Sci. Technol. 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Mendoza, F.; Dejmek, P.; Aguilera, J.M. Calibrated Color Measurements of Agricultural Foods Using Image Analysis. Postharvest Biol. Technol. 2006, 41, 285–295. [Google Scholar] [CrossRef]
- Vieira, E.C.S.; Mársico, E.T.; Conte-Junior, C.A.; Damiani, C.; da Cruz Silva Canto, A.C.V.; Monteiro, M.L.G.; da Silva, F.A. Effects of Different Frying Techniques on the Color, Fatty Acid Profile, and Lipid Oxidation of Arapaima Gigas. J. Food Process. Preserv. 2018, 42, e13820. [Google Scholar] [CrossRef]
- Ouyang, K.; Xie, H.; Wang, Y.; Ning, F.; Xiong, H.; Zhao, Q. Color Formation during the Fibrillization of Whey Protein Isolate: Maillard Reaction and Protein Oxidation. Food Hydrocoll. 2023, 142, 108819. [Google Scholar] [CrossRef]
- Li, B.B.; Lv, Y.Y.; Wei, S.; Zhang, S.B.; Zhao, Y.Y.; Hu, Y. Sen Effects of Protein Oxidation on the Rheological Behaviour of Different Wheat Flour. Int. J. Food Sci. Technol. 2022, 57, 4602–4612. [Google Scholar] [CrossRef]
- Mejri, M.; Roge, B.; Bensouissi, A.; Michels, F.; Mathlouthi, M. Effects of Some Additives on Wheat Gluten Solubility: A Structural Approach. Food Chem. 2005, 92, 7–15. [Google Scholar] [CrossRef]
- Yin, Y.; Xing, L.; Zhang, W. Moderate Protein Oxidation Improves Bovine Myofibril Digestibility by Releasing Peptides in the S2 Region of Myosin: A Peptidomics Perspective. J. Agric. Food Chem. 2023, 71, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Jood, S.; Schofield, J.D.; Tsiami, A.A.; Bollecker, S. Effect of Composition of Glutenin Subfractions on Rheological Properties of Wheat. J. Food Biochem. 2000, 24, 275–298. [Google Scholar] [CrossRef]
- Hopp, T.P. Protein Surface Analysis Methods for Identifying Antigenic Determinants and Other Interaction Sites. J. Immunol. Methods 1986, 88, 1–18. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Wang, Z.; Emara, A.M.; Hu, Y.; He, Z. Effects of In Vitro Oxidation on Myofibrillar Protein Charge, Aggregation, and Structural Characteristics. Food Chem. 2020, 332, 127396. [Google Scholar] [CrossRef]
- Headlam, H.A.; Davies, M.J. Markers of Protein Oxidation: Different Oxidants Give Rise to Variable Yields of Bound and Released Carbonyl Products. Free Radic. Biol. Med. 2004, 36, 1175–1184. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative Modification of Soy Protein by Peroxyl Radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of Pitanga Leaf Extracts on Lipid and Protein Oxidation of Pork Burger during Shelf-Life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pischetsrieder, M. Are Dietary AGEs/ALEs a Risk to Human Health and, If so, What Is the Mechanism of Action? Mol. Nutr. Food Res. 2007, 51, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein Oxidation in Emulsified Cooked Burger Patties with Added Fruit Extracts: Influence on Colour and Texture Deterioration during Chill Storage. Meat Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, Y.; Han, J.; Chen, Q.; Kong, B. Structure-Modification by Moderate Oxidation in Hydroxyl Radical-Generating Systems Promote the Emulsifying Properties of Soy Protein Isolate. Food Struct. 2015, 6, 21–28. [Google Scholar] [CrossRef]
- Li, B.; Xu, Y.; Li, J.; Niu, S.; Wang, C.; Zhang, N.; Yang, M.; Zhou, K.; Chen, S.; He, L.; et al. Effect of Oxidized Lipids Stored under Different Temperatures on Muscle Protein Oxidation in Sichuan-Style Sausages during Ripening. Meat Sci. 2019, 147, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Niu, S.; Wang, X.; Chen, F. Effect of Oxidation Induced by Hydroxyl Radical-Mediated Model on Molecular Structural and Physical Character of Egg White Powder. Int. J. Food Sci. Technol. 2018, 53, 2282–2289. [Google Scholar] [CrossRef]
- Quansah, J.K.; Udenigwe, C.C.; Saalia, F.K.; Yada, R.Y. The Effect of Thermal and Ultrasonic Treatment on Amino Acid Composition, Radical Scavenging and Reducing Potential of Hydrolysates Obtained from Simulated Gastrointestinal Digestion of Cowpea Proteins. Plant Foods Hum. Nutr. 2013, 68, 31–38. [Google Scholar] [CrossRef]
- Dorta, E.; Ávila, F.; Fuentes-Lemus, E.; Fuentealba, D.; López-Alarcón, C. Oxidation of Myofibrillar Proteins Induced by Peroxyl Radicals: Role of Oxidizable Amino Acids. Food Res. Int. 2019, 126, 108580. [Google Scholar] [CrossRef]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef]
- Salminen, H.; Jaakkola, H.; Heinonen, M. Modifications of Tryptophan Oxidation by Phenolic-Rich Plant Materials. J. Agric. Food Chem. 2008, 56, 11178–11186. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Li, H.; Wang, Z.; Emara, A.M.; Zhang, D.; He, Z. Does Protein Oxidation Affect Proteolysis in Low Sodium Chinese Traditional Bacon Processing? Meat Sci. 2019, 150, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Davies, K.J.A. Dityrosine: A Marker for Oxidatively Modified Proteins and Selective Proteolysis. Methods Enzymol. 1994, 233, 363–371. [Google Scholar] [PubMed]
- Chen, Z.; Leinisch, F.; Greco, I.; Zhang, W.; Shu, N.; Chuang, C.Y.; Lund, M.N.; Davies, M.J. Characterisation and Quantification of Protein Oxidative Modifications and Amino Acid Racemisation in Powdered Infant Milk Formula. Free Radic. Res. 2019, 53, 68–81. [Google Scholar] [CrossRef]
- Chen, Y.; Han, P.; Ma, B.; Wang, X.; Ma, M.; Qiu, N.; Fu, X. Effect of Thermal Treatment on the Antioxidant Activity of Egg White Hydrolysate and the Preparation of Novel Antioxidant Peptides. Int. J. Food Sci. Technol. 2022, 57, 2590–2599. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a Radical Scavenging Peptide from Fermented Mussel Sauce and Its Antioxidant Properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Sun, W. Effect of Protein Oxidation on the in Vitro Digestibility of Soy Protein Isolate. Food Chem. 2013, 141, 3224–3229. [Google Scholar] [CrossRef]
- Lin, L.; Jiao, M.; Zhao, M.; Sun, W. In Vitro Gastrointestinal Digest of Catechin-Modified β-Conglycinin Oxidized by Lipoxygenase-Catalyzed Linoleic Acid Peroxidation. Food Chem. 2019, 280, 154–163. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Li, Q.; Zhang, L.; Wang, Z.; Han, L.; Yu, Q. Oxidation of Myofibrillar Protein and Crosslinking Behavior during Processing of Traditional Air-Dried Yak (Bos Grunniens) Meat in Relation to Digestibility. LWT 2021, 142, 110984. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Yang, Y.; Cui, X.; Mwabulili, F.; Xie, Y. Effects of Baking and Frying on the Protein Oxidation of Wheat Dough. Foods 2023, 12, 4479. https://doi.org/10.3390/foods12244479
Liu R, Yang Y, Cui X, Mwabulili F, Xie Y. Effects of Baking and Frying on the Protein Oxidation of Wheat Dough. Foods. 2023; 12(24):4479. https://doi.org/10.3390/foods12244479
Chicago/Turabian StyleLiu, Ru, Yuhui Yang, Xiaojie Cui, Fred Mwabulili, and Yanli Xie. 2023. "Effects of Baking and Frying on the Protein Oxidation of Wheat Dough" Foods 12, no. 24: 4479. https://doi.org/10.3390/foods12244479






