Comparative Metabolomics Study of Four Kinds of Xihu Longjing Tea Based on Machine Fixing and Manual Fixing Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Xihu Longjing Tea Processing Method
- (1)
- Method 1 involved the fixing of Xihu Longjing tea by machine during both the first and second steps (A1)
- (2)
- Method 2 involved fixing the Xihu Longjing tea fixing by machine in the first step and by hand in the second step (A2)
- (3)
- Method 3 involved fixing the Xihu Longjing tea by hand in the first step and by machine in the second step (A3)
- (4)
- Method 4 involved fixing the Xihu Longjing tea by hand in both the first and second steps (A4)
2.3. Method for Preparing Sample Solution
2.4. Waters Q-EXACTIVE Plus/Dionex U3000 UHPLC
2.5. Data Preprocessing and Statistical Analysis
3. Results and Discussion
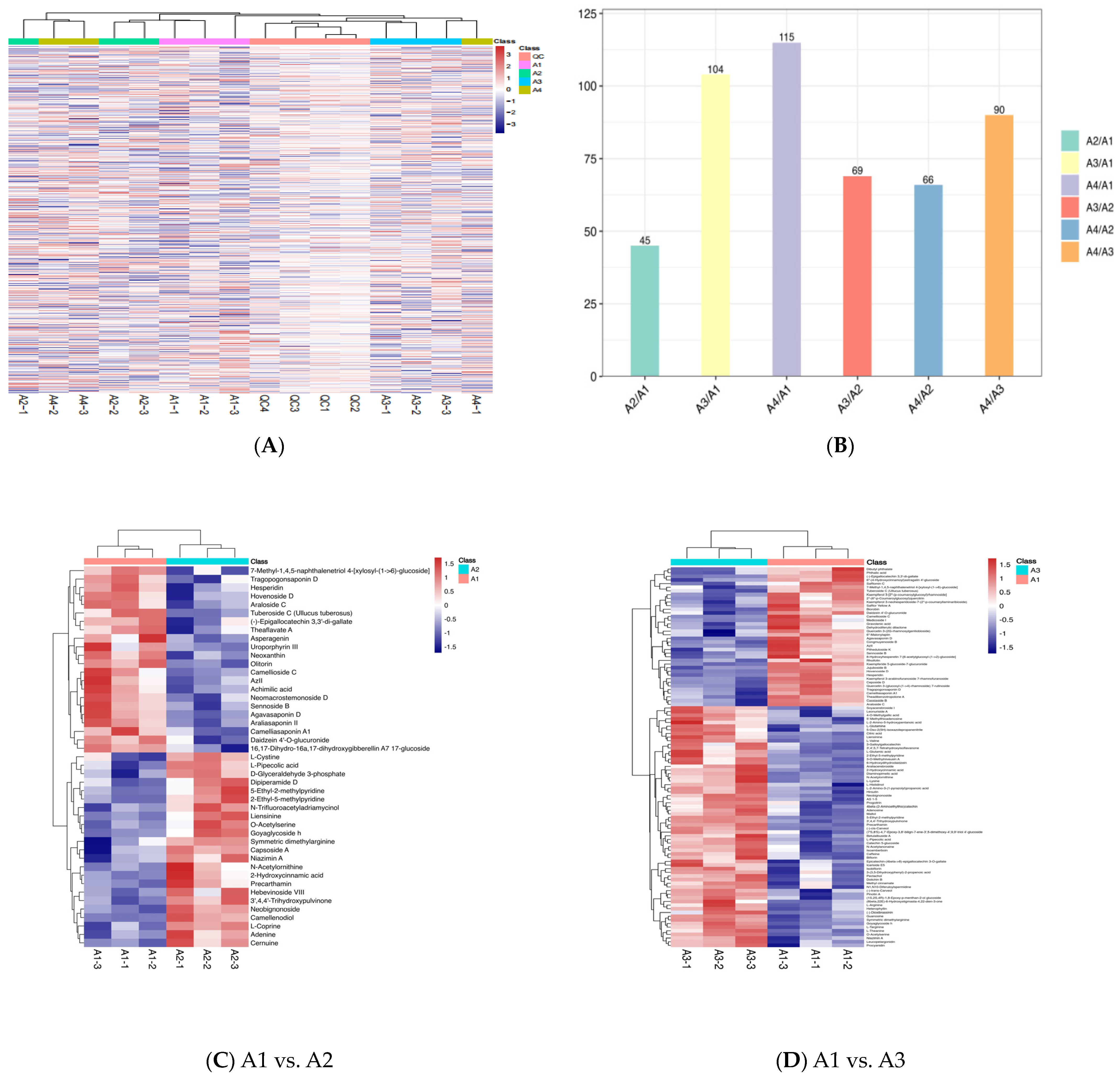
3.1. Metabolite Profiling Analysis
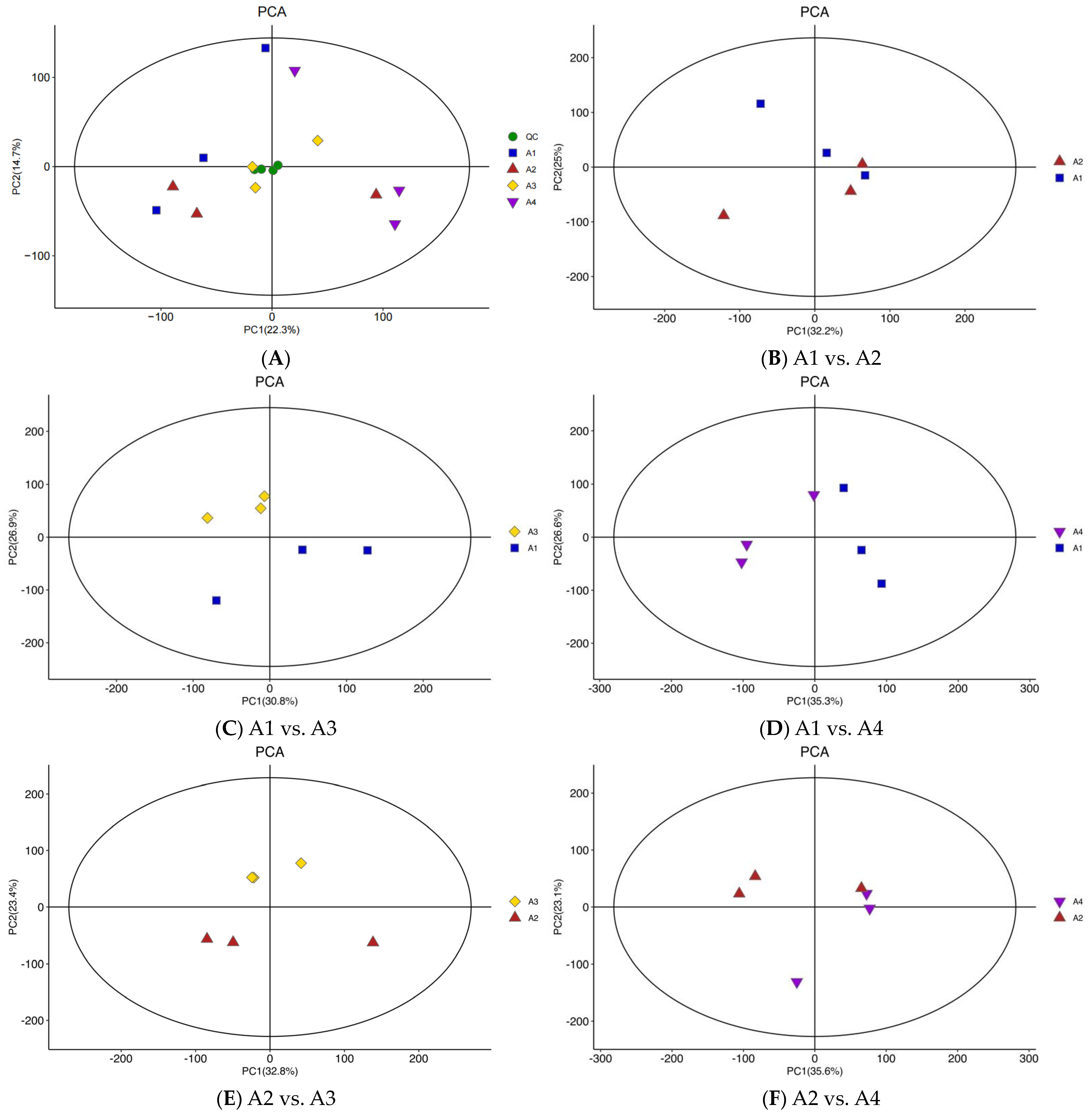
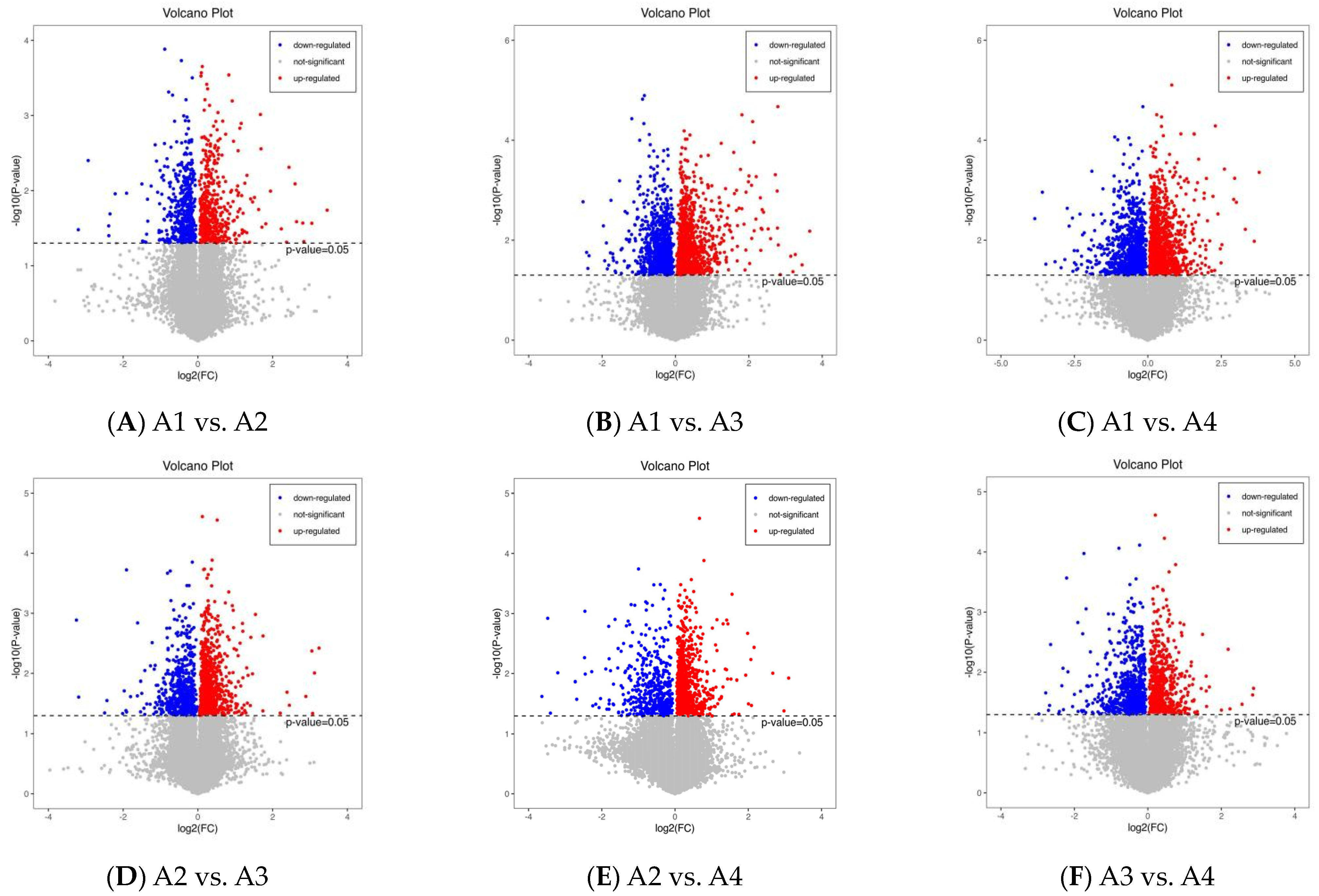
3.2. Differential Metabolite Analysis Based on PCA
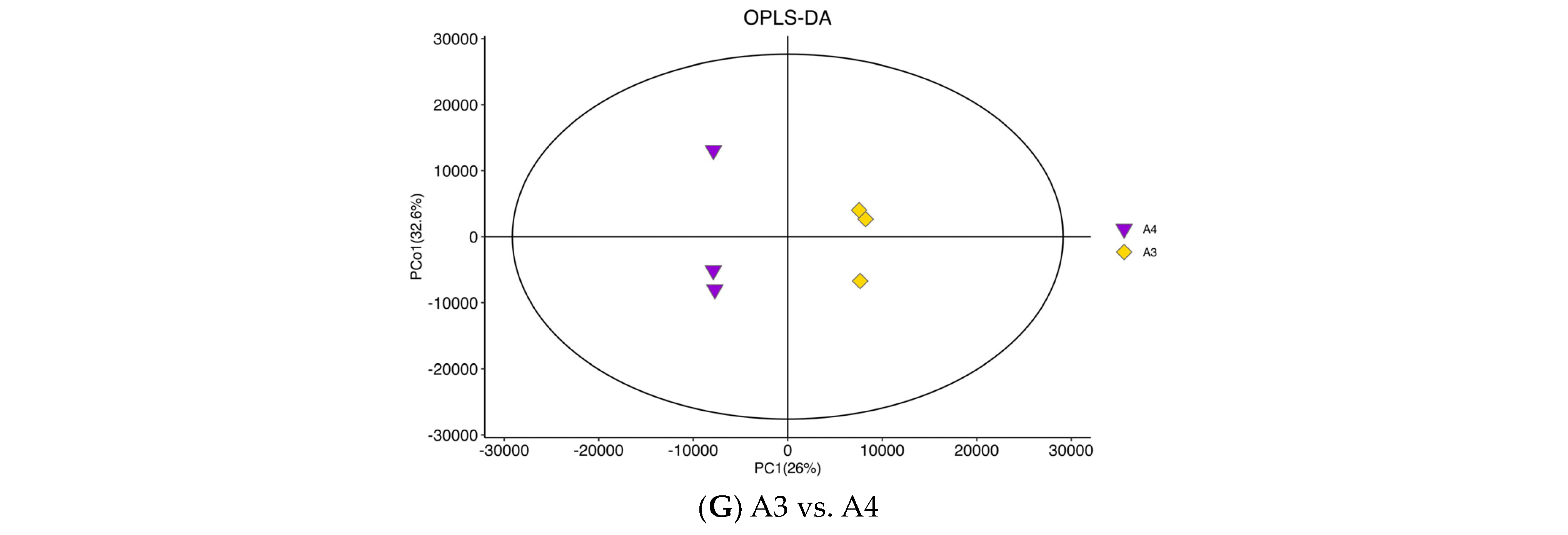
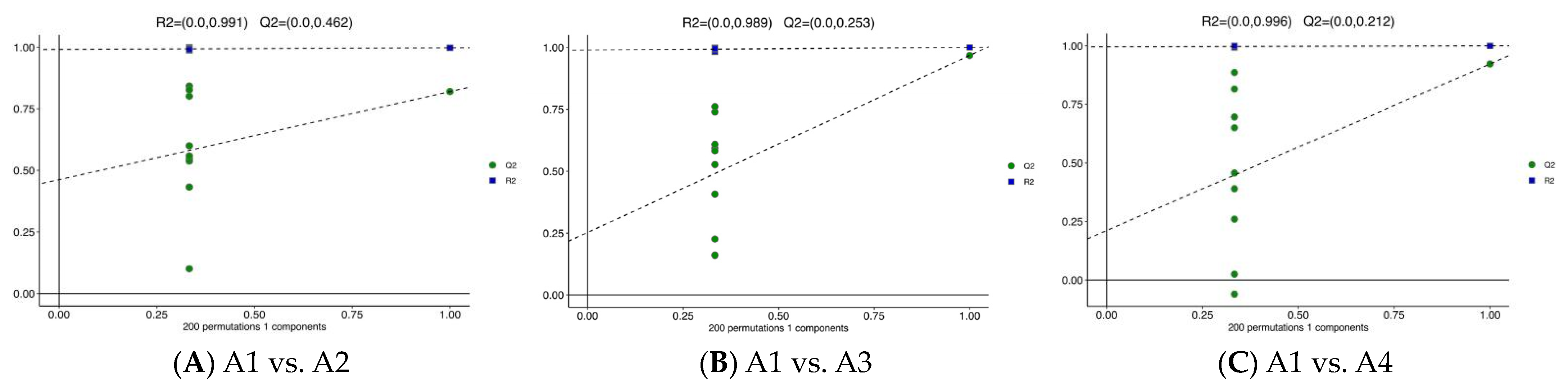
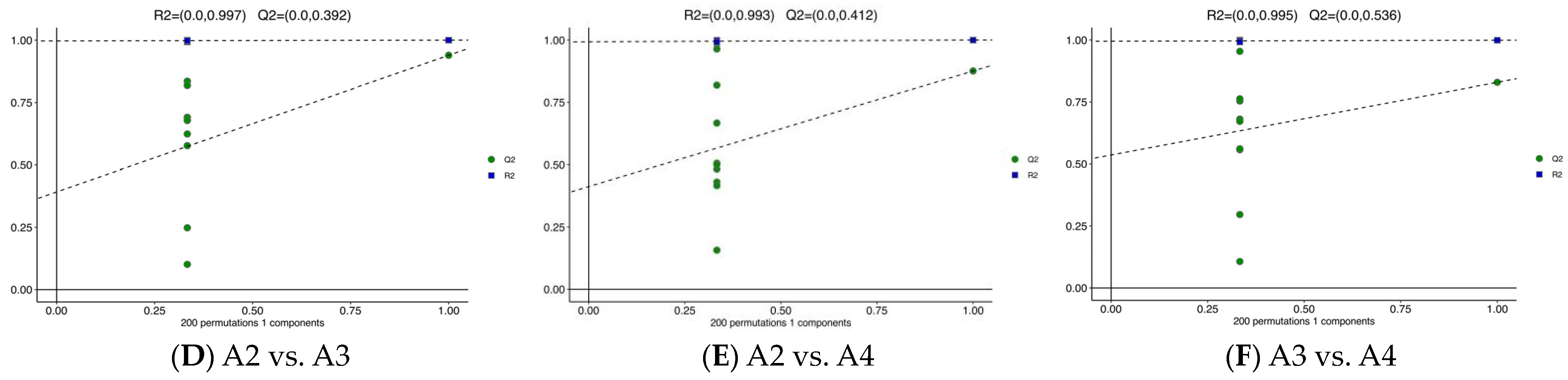
3.3. Differential Metabolite Analysis via OPLS-DA
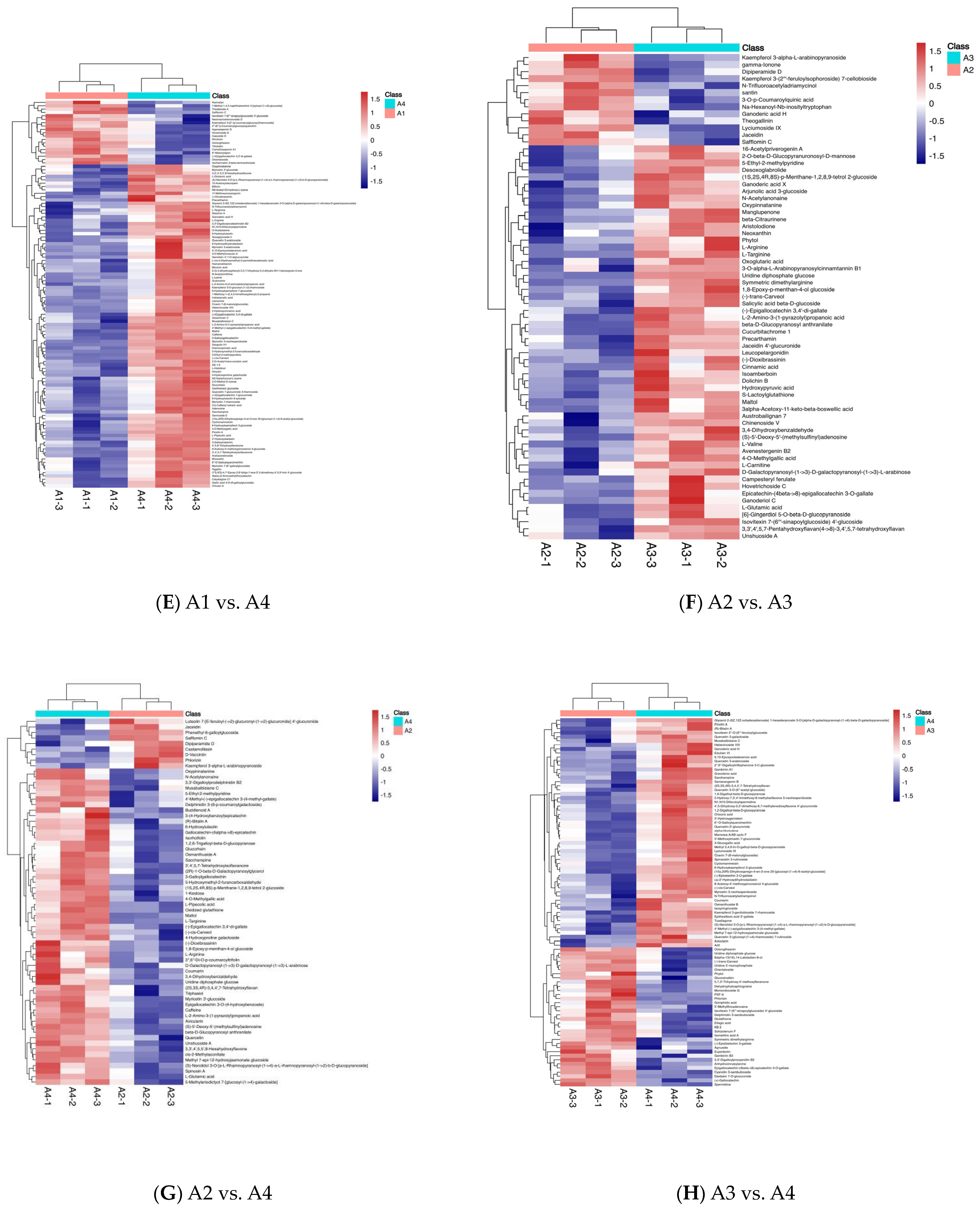
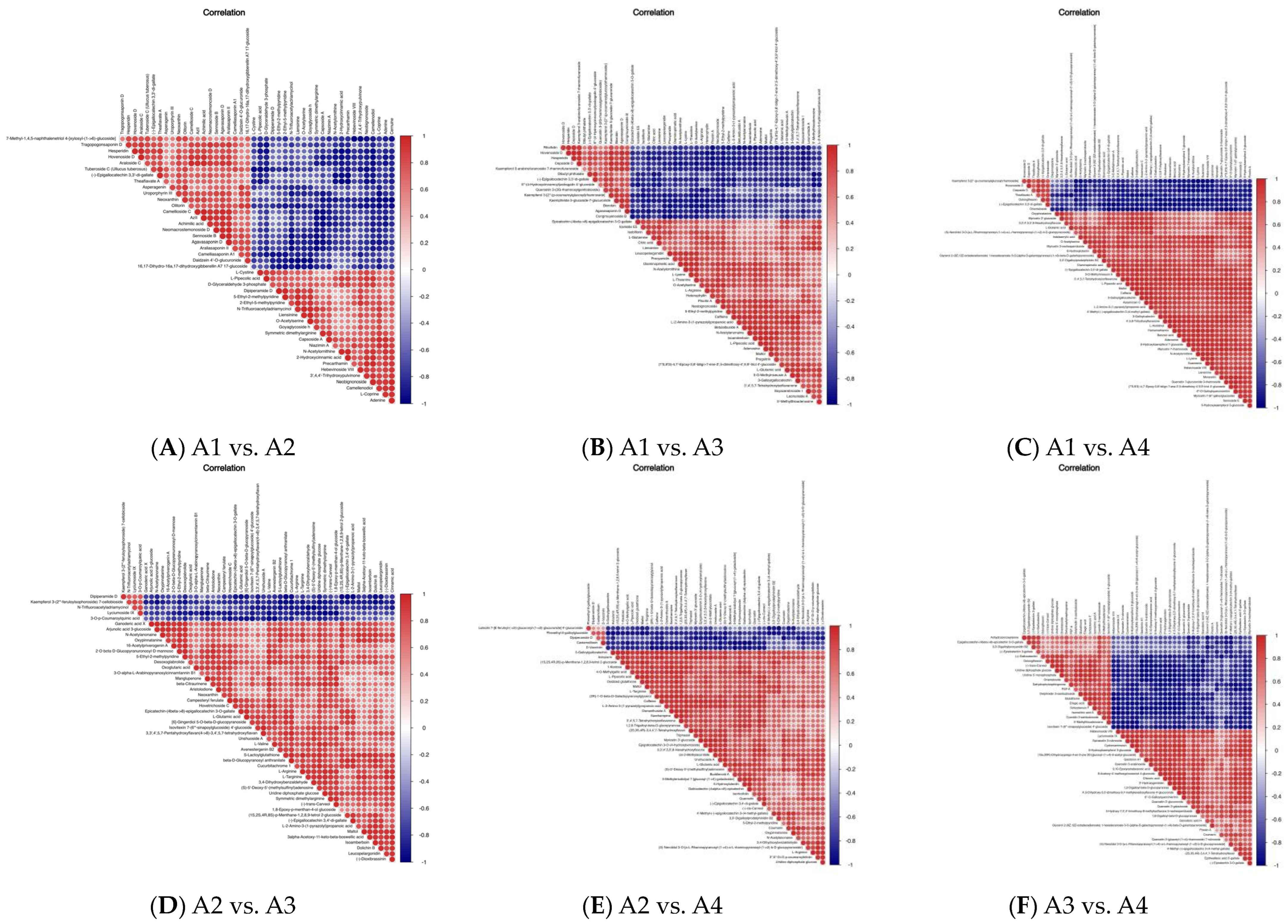
3.4. Differential Metabolite Association Heatmap
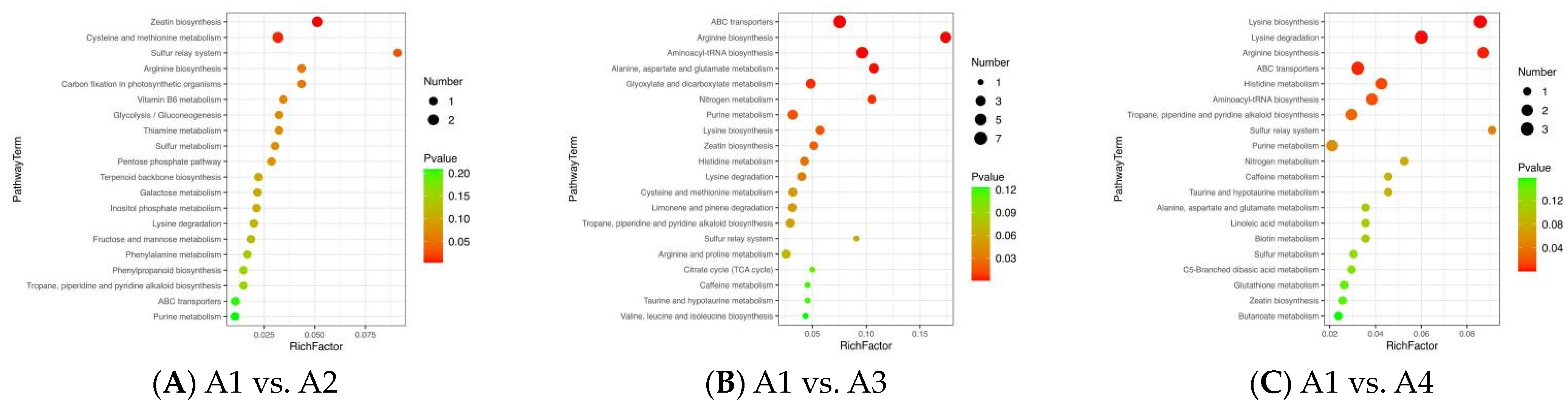
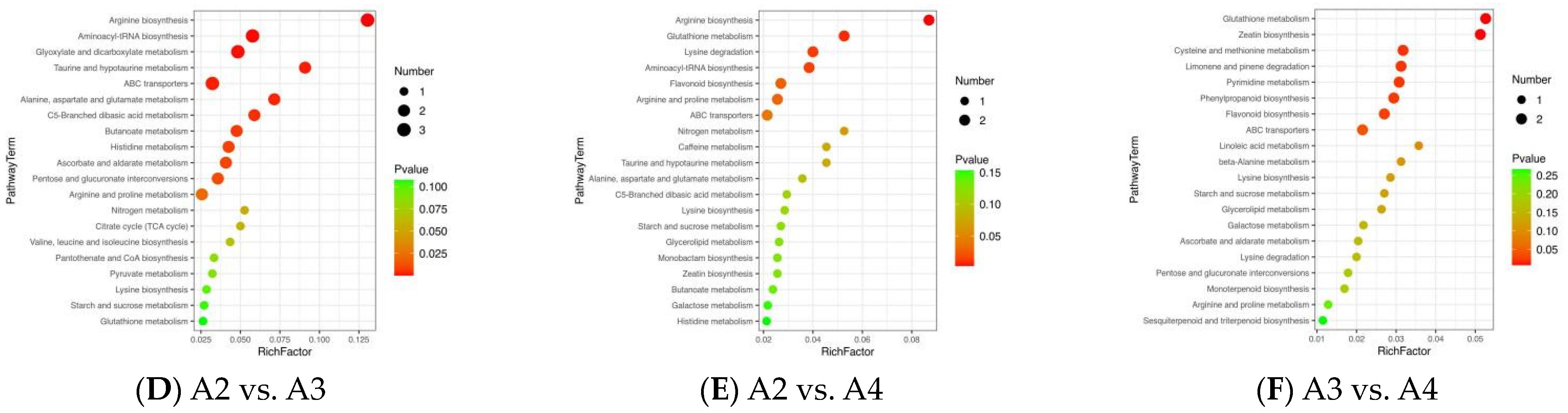
3.5. Differential Metabolic Pathways among the Xihu Longjing Tea Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.Y.; Feng, X.N.; Lyu, C.; Zhou, S.; Liu, Z.X. Effects of different processing methods on the lipid composition of hazelnut oil: A lipidomics analysis. Food Sci. Hum. Wellness 2022, 11, 427–435. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Yang, X.T.; Wang, H.L.; Yang, X.F.; Su, X.Q.; Kong, J.H.; Cheng, Y.L.; Yao, W.R.; Qian, H. Study on the establishment of quality discrimination model of Longjing 43 green tea (Camellia sinensis (L.) Kuntze). J. Appl. Res. Med. Aromat. Plants 2022, 31, 100389–100396. [Google Scholar] [CrossRef]
- Gong, S.Y.; Mao, Z.F.; Lu, D.B.; Zhang, Y.B.; Qian, X.D.; Sun, L.Y.; Fang, J.H. Effect of processing technology on quality of spring (high-grade) longjing tea. China Tea 2011, 12, 8–11. [Google Scholar]
- Shen, H. Effects of different processing methods on the quality of high-grade West Lake Longjing tea-comparison from sensory evaluation results. Tea Proc. China 2010, 3, 33–35. [Google Scholar]
- Wang, H.; Gong, S.Y.; Wei, M.X.; Zhu, Z.C.; Lu, W.Y.; Xia, J.F.; Nie, S.Z.; Chen, M.H. Effects of tea varieties and processing technology on the quality of changxing bamboo shoot tea. Chin. Tea 2014, 3, 18–21. [Google Scholar]
- Zhang, C.; Suen, C.L.-C.; Yang, C.; Quek, S.Y. Antioxidant capacity and major polyphenol composition of teas as affected by geographical location, plantation elevation and leaf grade. Food Chem. 2018, 244, 109–119. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wei, K.; Cheng, H.; Li, X.; Gong, W. Geographical tracing of Xihu Longjing tea using high performance liquid chromatography. Food Chem. 2014, 146, 98–103. [Google Scholar] [CrossRef]
- Jin, J.; Shao, Z.Q.; Chang, L.M. Discussion on the processing technology of Longjing tea automatic continuous production line with different defoliation methods. Tea 2017, 43, 157–160. [Google Scholar]
- Xia, J.R.; Xu, L.Y.; Yang, Q.; Miu, Q.; Xia, J.M. Analysis of Longjing tea mechanism technology. Tea Proc. China 2016, 1, 20–23. [Google Scholar]
- Jiang, B.F. Research and application of new semi-continuous processing technology of Longjing tea. Agri. Sci. Tech. Shanghai 2016, 2, 21–22. [Google Scholar]
- Shi, D.L.; Lu, D.B.; Jin, J. Small-scale processing mode and technology integration of Longjing tea. Chin. Tea 2015, 5, 21–22. [Google Scholar]
- Chen, F.; Zou, X.W. A brief analysis of the reasons for the formation of the quality of West Lake Longjing tea. Tea Proc. China 2011, 3, 27–30. [Google Scholar]
- Wang, D.B.; Xu, J.; Ru, L.J.; Wang, M.M.; Huang, H.X. Reasons for quality defects of Longjing tea and improvement methods. Chin. Tea 2019, 3, 46–48. [Google Scholar]
- Shi, D.L.; Yu, J.Z.; Liu, X.H.; Guo, M.M.; Zhang, W. Study on the effect of different technology and different pot method on the quality of Longjing tea. J. Southwest Norm. Univ. 2010, 35, 173–177. [Google Scholar]
- Kang, M.L.; Xue, X.C.; Ling, J.G. Optimization of processing parameters of Longjing tea with summer and autumn tea. Sci. Tech. Food Ind. 2007, 28, 145–147. [Google Scholar]
- Ge, Y.T.; Sheng, L.F.; Gao, L.H.; Zhu, Y.W.; Tang, D.S.; Liang, H.L.; Huang, H.T. Identification of key control points in Longjing tea mechanism process based on leaf temperature monitoring. Zhejiang Agri. Sci. 2019, 60, 425–436. [Google Scholar]
- Han, Y.C.; Chen, H.J.; Gao, H.Y.; Fang, X.J. Effect of soaking conditions on antioxidant properties of West Lake Longjing and correlation analysis. Chin. J. Food 2018, 18, 128–136. [Google Scholar]
- Yin, J.F.; Xu, Y.Q.; Chen, G.S.; Wang, F.; Chen, J.X. Effects of different types of drinking water on flavor and main quality components of West Lake Longjing tea. Chin. Tea 2018, 5, 21–26. [Google Scholar]
- Chen, Q.; Zhao, J.; Guo, Z.; Wang, X. Determination of caffeine content and main catechins contents in green tea (Camellia sinensis L.) using taste sensor tech nique and multivariate calibration. J. Food Compos. Anal. 2010, 23, 353–358. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Vittayapadung, S. Identification of the green tea grade level using electronic tongue and pattern recognition. Food Res. Int. 2008, 41, 500–504. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kan, Z.P.; Henry, J.T.; Ling, T.J.; Ho, C.T.; Li, D.Y.; Wan, X.C. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, W.; Lu, M.; Xie, D.; Tan, J.; Yang, C.; Zhu, Y.; Lv, H.; Peng, Q.; Zhang, Y.; et al. Metabolomic analysis reveals the composition differences in 13 Chinese tea cultivars of different manufacturing suitabilities. J. Sci. Food Agric. 2018, 98, 1153–1161. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.H.; Zhang, J.J.; Huang, Y.Q.; Yang, W.; Lu, K.H.; Zhu, J.Y. Toxic cyanobacteria induce coupled changes in gut microbiota and co-metabolite of freshwater gastropods. Environ. Pollut. 2023, 338, 122651–122660. [Google Scholar] [CrossRef] [PubMed]
- Davosir, D.; Sola, I. Membrane permeabilizers enhance biofortification of Brassica microgreens by interspecific transfer of metabolites from tea (Camellia sinensis). Food Chem. 2023, 420, 136186. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Zhang, Y.; Ren, D.; Qin, X.; Wang, N.; Yang, X. Fu Brick Tea Alleviates Constipation via Regulating the Aquaporins-Mediated Water Transport System in Association with Gut Microbiota. J. Agric. Food Chem. 2023, 71, 3862–3865. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Z. The classification and prediction of green teas by elector chemical response data extraction and fusion approaches based on the combination of e-nose and e-tongue. RSC Adv. 2015, 5, 106959–106970. [Google Scholar] [CrossRef]
- Xu, L.; Yan, S.-M.; Ye, Z.-H.; Fu, X.-S.; Yu, X.-P. Combining Electronic tongue array and chemometrics for discriminating the specific geographical origins of green tea. J. Anal. Methods Chem. 2013, 2013, 350801. [Google Scholar] [CrossRef]
- Yu, P.; Yeo, A.S.-L.; Low, M.-Y.; Zhou, W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Mao, Y.; Zhao, Y.; Huang, H.; Yin, J.; Yu, J.; Zhang, J. Comparative Metabolomics Study of Four Kinds of Xihu Longjing Tea Based on Machine Fixing and Manual Fixing Methods. Foods 2023, 12, 4486. https://doi.org/10.3390/foods12244486
Cui H, Mao Y, Zhao Y, Huang H, Yin J, Yu J, Zhang J. Comparative Metabolomics Study of Four Kinds of Xihu Longjing Tea Based on Machine Fixing and Manual Fixing Methods. Foods. 2023; 12(24):4486. https://doi.org/10.3390/foods12244486
Chicago/Turabian StyleCui, Hongchun, Yuxiao Mao, Yun Zhao, Haitao Huang, Junfeng Yin, Jizhong Yu, and Jianyong Zhang. 2023. "Comparative Metabolomics Study of Four Kinds of Xihu Longjing Tea Based on Machine Fixing and Manual Fixing Methods" Foods 12, no. 24: 4486. https://doi.org/10.3390/foods12244486






