Dried Herbs as an Easy-to-Use and Cost-Effective Alternative to Essential Oils to Extend the Shelf Life of Sheep Lump Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Packaging of Ewes Cheese Samples
2.2. Samples Cultivation
2.3. Sample Preparation and MALDI-TOF MS Measurement
2.4. Statistical Analysis
3. Results
3.1. Microbiological Quality of Ewe’s Cheese
3.2. Identification of Isolated Microorganisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liegeard, J.; Manning, L. Use of Intelligent Applications to Reduce Household Food Waste. Crit. Rev. Food Sci. Nutr. 2020, 60, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.D.A.; Oliveira, M.S.D. Recent Trends in the Application of Essential Oils: The next Generation of Food Preservation and Food Packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential Application of Essential Oils as Antimicrobial Preservatives in Cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- López-Expósito, I.; Amigo, L.; Recio, I. A Mini-Review on Health and Nutritional Aspects of Cheese with a Focus on Bioactive Peptides. Dairy Sci. Technol. 2012, 92, 419–438. [Google Scholar] [CrossRef]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited Review: Current Production Trends, Farm Structures, and Economics of the Dairy Sheep and Goat Sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef]
- Possas, A.; Bonilla-Luque, O.M.; Valero, A. From Cheese-Making to Consumption: Exploring the Microbial Safety of Cheeses through Predictive Microbiology Models. Foods 2021, 10, 355. [Google Scholar] [CrossRef]
- Kousta, M.; Mataragas, M.; Skandamis, P.; Drosinos, E.H. Prevalence and Sources of Cheese Contamination with Pathogens at Farm and Processing Levels. Food Control 2010, 21, 805–815. [Google Scholar] [CrossRef]
- Oliver, S.P.; Jayarao, B.M.; Almeida, R.A. Foodborne Pathogens in Milk and the Dairy Farm Environment: Food Safety and Public Health Implications. Foodbourne Pathog. Dis. 2005, 2, 115–129. [Google Scholar] [CrossRef]
- Brisabois, A.; Lafarge, V.; Brouillaud, A.; de Buyser, M.L.; Collette, C.; Garin-Bastuji, B.; Thorel, M.F. Pathogenic organisms in milk and milk products: The situation in France and in Europe. Rev. Sci. Tech. 1997, 16, 452–471. [Google Scholar] [CrossRef]
- Sengun, I.; Yaman, D.; Gonul, S. Mycotoxins and Mould Contamination in Cheese: A Review. WMJ 2008, 1, 291–298. [Google Scholar] [CrossRef]
- Nadeem, S.F.; Gohar, U.F.; Tahir, S.F.; Mukhtar, H.; Pornpukdeewattana, S.; Nukthamna, P.; Moula Ali, A.M.; Bavisetty, S.C.B.; Massa, S. Antimicrobial Resistance: More than 70 Years of War between Humans and Bacteria. Crit. Rev. Microb. 2020, 46, 578–599. [Google Scholar] [CrossRef] [PubMed]
- Zantar, S.; Yedri, F.; Mrabet, R.; Laglaoui, A.; Bakkali, M.; Zerrouk, M.H. Effect of Thymus vulgaris and Origanum compactum Essential Oils on the Shelf Life of Fresh Goat Cheese. J. Essent. Oil Res. 2014, 26, 76–84. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microb. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; López De Lacey, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable Gelatin–Chitosan Films Incorporated with Essential Oils as Antimicrobial Agents for Fish Preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Žunić, E. The Bosnian Case: Art, History and Memory. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 2018. [Google Scholar]
- Soliman, K.M.; Badeaa, R.I. Effect of Oil Extracted from Some Medicinal Plants on Different Mycotoxigenic Fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.M.A.; Elbanna, K.; Abulreesh, H.H.; Ramadan, M.F. Bioactive Compounds of Cold-Pressed Thyme (Thymus vulgaris) Oil with Antioxidant and Antimicrobial Properties. J. Oleo Sci. 2016, 65, 629–640. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Royo, M.; Ignacio Maté, J. Antimicrobial Activity of Whey Protein Isolate Edible Films with Essential Oils against Food Spoilers and Foodborne Pathogens. J. Food Sci. 2012, 77, M383–M390. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; Cindio, B.D.; Houghton, P.J.; Menichini, F.; et al. Chemical Analysis, Antioxidant, Antiinflammatory and Anticholinesterase Activities of Origanum ehrenbergii Boiss and Origanum syriacum L. Essential Oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- Moro, A.; Librán, C.M.; Berruga, M.I.; Zalacain, A.; Carmona, M. Mycotoxicogenic Fungal Inhibition by Innovative Cheese Cover with Aromatic Plants: Penicillium verrucosum Inhibition in Cheese by Aromatic Plants. J. Sci. Food Agric. 2013, 93, 1112–1118. [Google Scholar] [CrossRef]
- De Carvalho, R.J.; De Souza, G.T.; Honório, V.G.; De Sousa, J.P.; Da Conceição, M.L.; Maganani, M.; De Souza, E.L. Comparative Inhibitory Effects of Thymus Vulgaris L. Essential Oil against Staphylococcus aureus, Listeria monocytogenes and Mesophilic Starter Co-Culture in Cheese-Mimicking Models. Food Microb. 2015, 52, 59–65. [Google Scholar] [CrossRef]
- De Souza, G.T.; De Carvalho, R.J.; De Sousa, J.P.; Tavares, J.F.; Schaffner, D.; De Souza, E.L.; Magnani, M. Effects of the Essential Oil from Origanum vulgare L. on Survival of Pathogenic Bacteria and Starter Lactic Acid Bacteria in Semihard Cheese Broth and Slurry. J. Food Prot. 2016, 79, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Marcial, G.E.; Gerez, C.L.; De Kairuz, M.N.; Araoz, V.C.; Schuff, C.; De Valdez, G.F. Influence of Oregano Essential Oil on Traditional Argentinean Cheese Elaboration: Effect on Lactic Starter Cultures. Rev. Argent. Microbiol. 2016, 48, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Licon, C.C.; Moro, A.; Librán, C.M.; Molina, A.M.; Zalacain, A.; Berruga, M.I.; Carmona, M. Volatile Transference and Antimicrobial Activity of Cheeses Made with Ewes’ Milk Fortified with Essential Oils. Foods 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of Essential Oils: A Review on Their Interaction with Food Components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Graciá, C.; González-Bermúdez, C.A.; Cabellero-Valcárcel, A.M.; Santaella-Pascual, M.; Frontela-Saseta, C. Use of Herbs and Spices for Food Preservation: Advantages and Limitations. Curr. Opin. Food Sci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.; Brooks, J.D.; Corke, H. Antibacterial and Antioxidant Effects of Five Spice and Herb Extracts as Natural Preservatives of Raw Pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Mendonca, A.; Jackson-Davis, A.; Moutiq, R.; Thomas-Popo, E. Use of Natural Antimicrobials of Plant Origin to Improve the Microbiological Safety of Foods. In Food and Feed Safety Systems and Analysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 249–272. ISBN 978-0-12-811835-1. [Google Scholar]
- Corbo, M.R.; Bevilacqua, A.; Campaniello, D.; D’Amato, D.; Speranza, B.; Sinigaglia, M. Prolonging Microbial Shelf Life of Foods through the Use of Natural Compounds and Non-thermal Approaches—A Review. Int. J. Food Sci. Technol. 2009, 44, 223–241. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential Oils Extracted from Different Species of the Lamiaceae Plant Family as Prospective Bioagents against Several Detrimental Pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- Stankovic, M. Lamiaceae Species; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant Activity and Polyphenol Content of Turkish Thyme (Thymus vulgaris) Monitored by Liquid Chromatography and Tandem Mass Spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- Schmitz, S.; Weidenbörner, M.; Kunz, B. Herbs and Spices as Selective Inhibitors of Mould Growth. Chem. Mikrobiol. Technol. Lebensm. 1993, 15, 175–177. [Google Scholar]
- Vázquez, B.I.; Fente, C.; Franco, C.M.; Vázquez, M.J.; Cepeda, A. Inhibitory Effects of Eugenol and Thymol on Penicillium citrinum Strains in Culture Media and Cheese. Int. J. Food Microbiol. 2001, 67, 157–163. [Google Scholar] [CrossRef]
- ISO 4833-2:2013; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms: Colony Count at 30 °C by the Pour Plate Technique. BSI British Standards: London, UK, 2013.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Coliforms. Colony-Count Technique. BSI British Standards: London, UK, 2006.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria. Colony-Count Technique at 30 °C. BSI British Standards: London, UK, 1998.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. BSI British Standards: London, UK, 2008.
- Kačániová, M.; Terentjeva, M.; Kunová, S.; Nagyová, Ľ.; Horská, E.; Haščík, P.; Kluz, M.; Puchalski, C. Microorganisms Isolated from the Slovak Traditional Cheese “Parenica” and Their Identification with Mass Spectrometry. Sci. Pap. Anim. Sci. Biotechnol. 2018, 51, 76–82. [Google Scholar]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef]
- Sadeghi, E.; Mohammadi, A.; Jamilpanah, M.; Bashiry, M.; Bohlouli, S. Antimicrobial Effects of Mentha pulegium Essential Oil on Listeria monocytogenes in Iranian White Cheese. J. Food Qual. Hazards Control. 2016, 3, 20–24. [Google Scholar]
- Govaris, A.; Botsoglou, E.; Sergelidis, D.; Chatzopoulou, P.S. Antibacterial Activity of Oregano and Thyme Essential Oils against Listeria monocytogenes and Escherichia coli O157:H7 in Feta Cheese Packaged under Modified Atmosphere. LWT—Food Sci. Technol. 2011, 44, 1240–1244. [Google Scholar] [CrossRef]
- Kavas, G.; Kavas, N.; Saygili, D. The Effects of Thyme and Clove Essential Oil Fortified Edible Films on the Physical, Chemical and Microbiological Characteristics of Kashar Cheese. J. Food Qual. 2015, 38, 405–412. [Google Scholar] [CrossRef]
- Zandona, E.; Perković, I.; Aladić, K.; Blažić, M. Quality And Shelf Life Of Skuta Whey Cheese Packed Under Vacuum And Modified Atmosphere In Presence Or Absence Of The Hemp Seed Powder. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 483–495. [Google Scholar]
- Muñoz-Tébar, N.; De La Vara, J.A.; Ortiz De Elguea-Culebras, G.; Cano, E.L.; Molina, A.; Carmona, M.; Berruga, M.I. Enrichment of Sheep Cheese with Chia (Salvia hispanica L.) Oil as a Source of Omega-3. LWT 2019, 108, 407–415. [Google Scholar] [CrossRef]
- Šaková, N.; Sádecká, J.; Lejková, J.; Puškárová, A.; Koreňová, J.; Kolek, E.; Valík, Ľ.; Kuchta, T. Characterization of May Bryndza Cheese from Various Regions in Slovakia Based on Microbiological, Molecular and Principal Volatile Odorants Examination. J. Food Nutr. Res. 2015, 54, 239–251. [Google Scholar]
- Lauková, A.; Micenková, L.; Pogány Simonová, M.; Focková, V.; Ščerbová, J.; Tomáška, M.; Dvorožňáková, E.; Kološta, M. Microbiome Associated with Slovak Traditional Ewe’s Milk Lump Cheese. Processes 2021, 9, 1603. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Kunová, S.; Haščík, P.; Kowalczewski, P.Ł.; Štefániková, J. Diversity of Microbiota in Slovak Summer Ewes’ Cheese “Bryndza”. Open Life Sci. 2021, 16, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of Edible Films and Coatings in Cheese Preservation: Opportunities and Challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sangoyomi, T.E.; Owoseni, A.; Okerokun, O. Prevalence of Entero-Pathogenic and Lactic Acid Bacteria Species in Wara: A Local Cheese from Nigeria. Afr. J. Microbiol. Res. 2010, 4, 1624–1630. [Google Scholar]
- Kazeminia, M.; Mahmoudi, R.; Ghajarbygi, P.; Moosavi, S. The Effect of Seasonal Variation on the Chemical and Microbial Quality of Raw Milk Samples Used in Qazvin, Iran. J. Chem. Health Risks 2019, 9, 157–165. [Google Scholar] [CrossRef]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of Antilisterial Action of Cilantro Oil on Vacuum Packed Ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
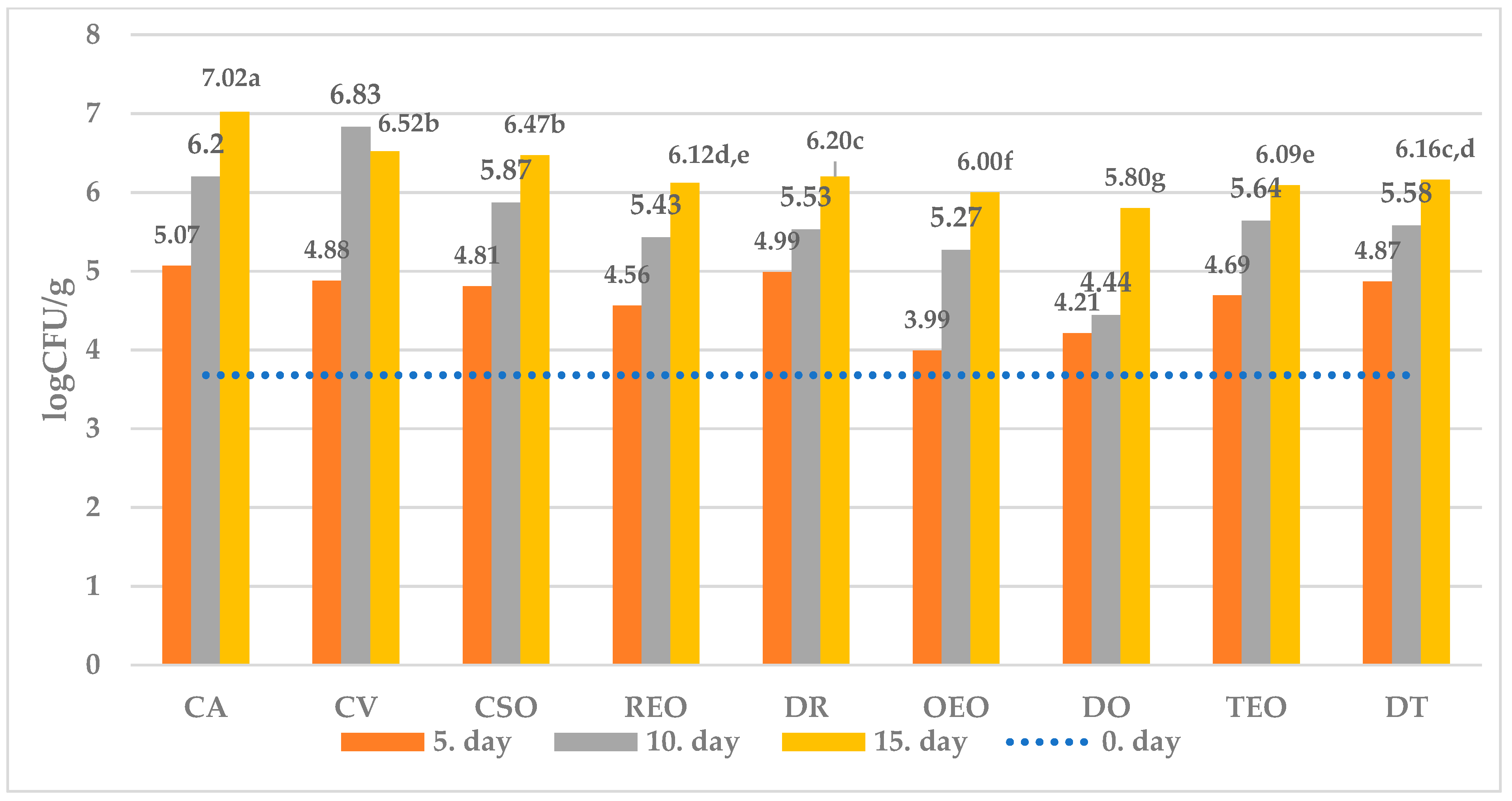

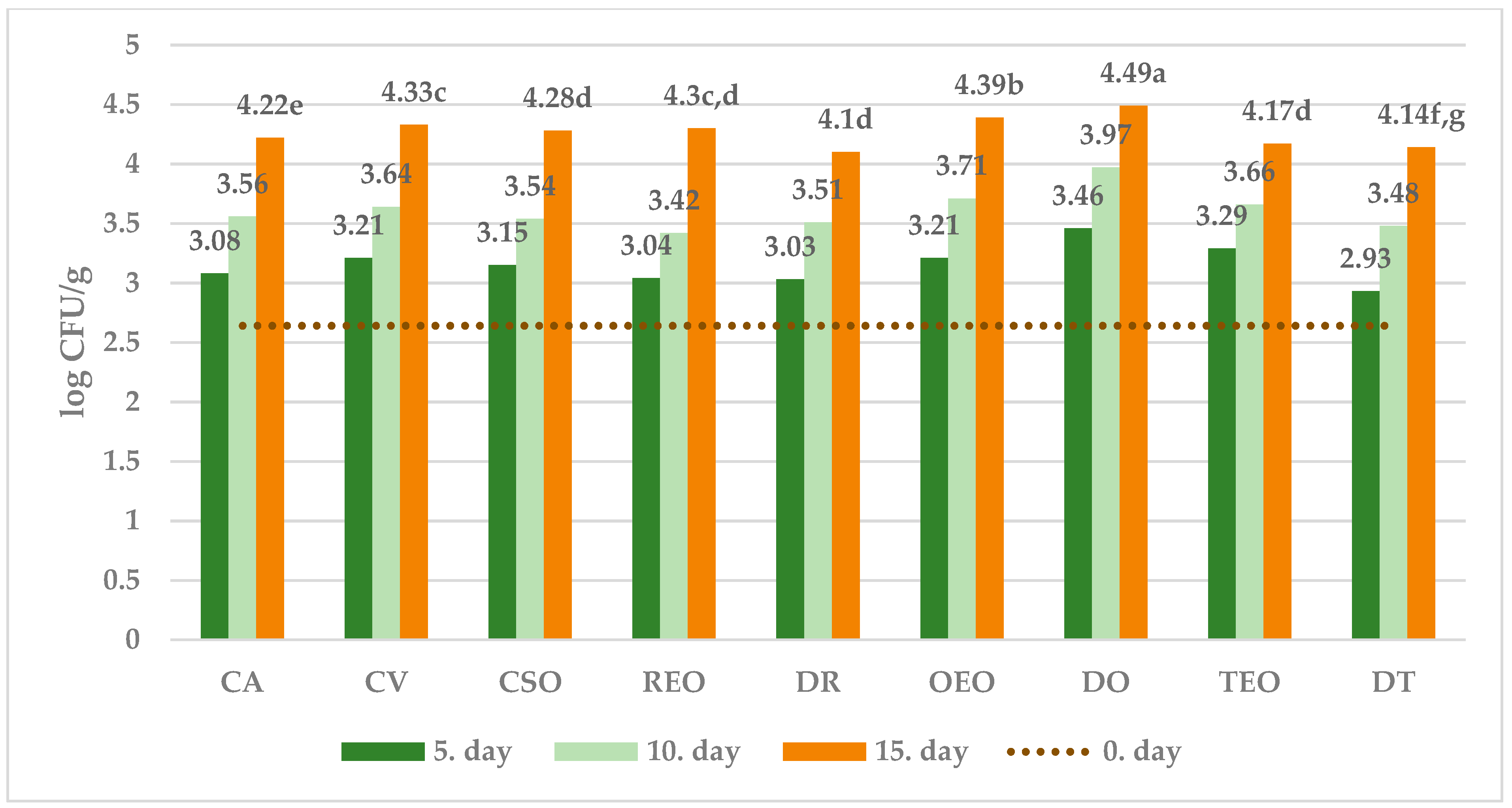

| Control group | CA | samples of ewe’s cheese packaged under aerobic conditions and stored at temperature 4 °C |
| Control group with vacuum packaging | CV | samples of ewe’s cheese, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Control group with sunflower oil | CSO | samples of ewe’s cheese treated with sunflower oil, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Samples with 1% rosemary essential oil | REO | samples of ewe’s cheese treated with 1% REO, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Samples with 1% thyme essential oil | TEO | samples of ewe’s cheese treated with 1% TEO, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Samples with 1% oregano essential oil | OEO | samples of ewe’s cheese treated with 1% OEO, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Samples with dried rosemary | DR | samples of ewe’s cheese treated with 1% DR, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Samples with dried thyme | DT | samples of ewe’s cheese treated with 1% DT, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Samples with dried oregano | DO | samples of ewe’s cheese treated with 1% DO, were vacuum-sealed in polyethylene bags and stored at a temperature of 4 °C |
| Sample | TVC (k ± c.i.) × 10−1 | R2 | CB (k ± c.i.) × 10−1 [day−1] | R2 | LAB (k ± c.i.) × 10−1 [day−1] | R2 | MFF (k ± c.i.) × 10−1 [day−1] | R2 |
|---|---|---|---|---|---|---|---|---|
| [day−1] | ||||||||
| C | 2.35 ± 0.01 | 0.987 | 1.87 ± 0.01 | 0.999 | 1.00 ± 0.03 | 0.990 | 1.09 ± 0.01 | 0.923 |
| CV | 2.00 ± 0.01 | 0.986 | 1.71 ± 0.02 | 0.997 | 1.09 ± 0.02 | 0.992 | 1.22 ± 0.17 | 0.780 |
| CSO | 1.96 ± 0.02 | 0.988 | 1.69 ± 0.02 | 0.999 | 1.03 ± 0.03 | 0.982 | 1.02 ± 0.02 | 0.974 |
| REO | 1.67 ± 0.01 | 0.997 | 1.15 ± 0.01 | 0.973 | 0.99 ± 0.02 | 0.952 | 0.60 ± 0.02 | 0.899 |
| DR | 1.80 ± 0.02 | 0.958 | 1.17 ± 0.02 | 0.952 | 0.93 ± 0.03 | 0.991 | 0.71 ± 0.02 | 0.957 |
| TEO | 1.74 ± 0.01 | 0.974 | 1.24 ± 0.02 | 0.966 | 1.04 ± 0.02 | 0.988 | 0.82 ± 0.01 | 0.939 |
| DT | 1.78 ± 0.01 | 0.971 | 1.40 ± 0.03 | 0.986 | 0.93 ± 0.02 | 0.972 | 0.92 ± 0.01 | 0.957 |
| OEO | 1.49 ± 0.02 | 0.955 | 1.41 ± 0.02 | 0.949 | 1.16 ± 0.03 | 0.996 | 0.50 ± 0.02 | 0.915 |
| DO | 1.20 ± 0.02 | 0.886 | 1.34 ± 0.01 | 0.955 | 1.29 ± 0.01 | 0.985 | 0.60 ± 0.02 | 0.966 |
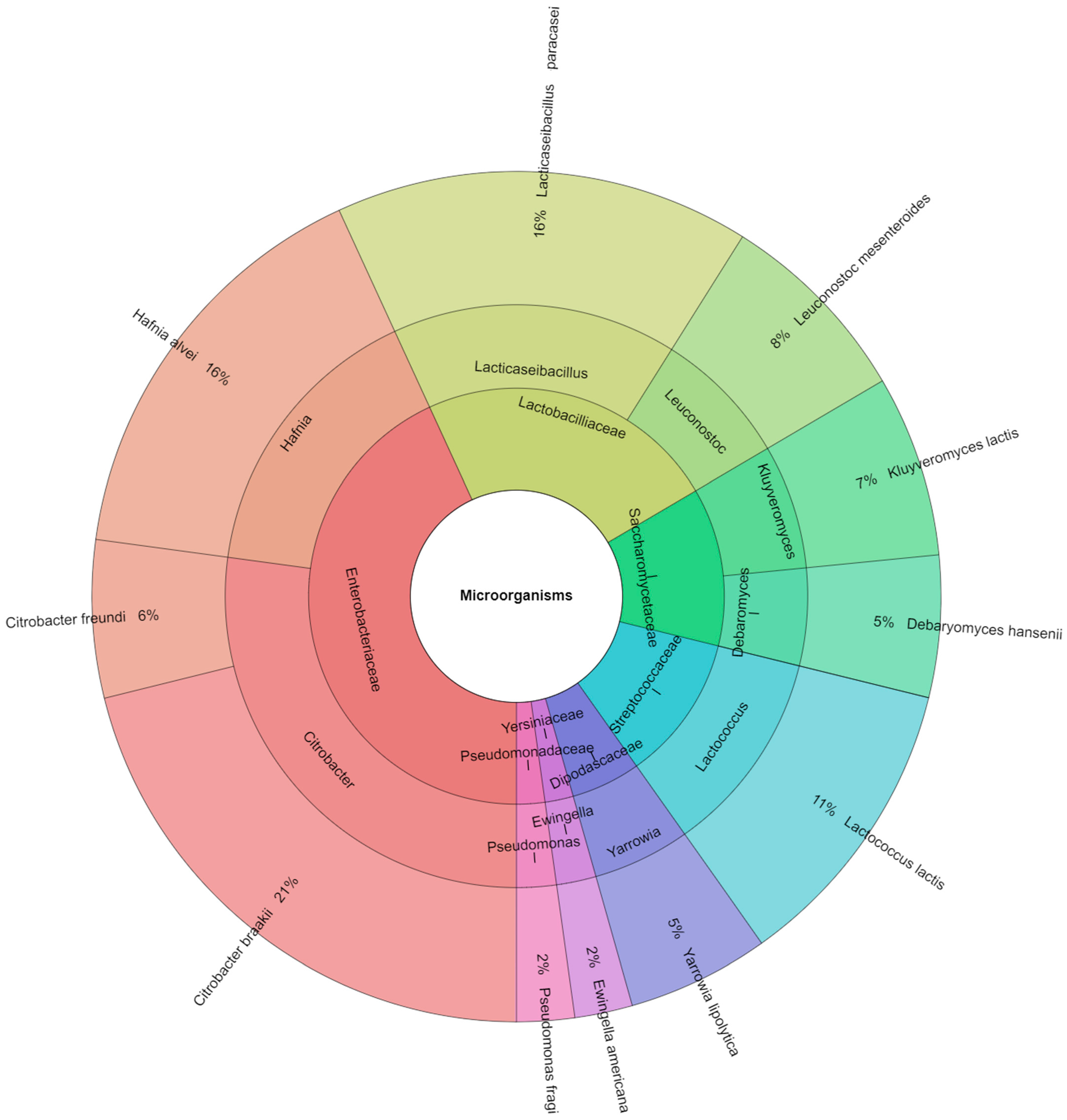
| Microorganisms | Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CA | CV | CSO | REO | TEO | OEO | DR | DT | DO | Total | |
| Citrobacter braakii | 19 | 15 | 14 | 4 | 5 | 3 | 3 | 2 | 2 | 67 |
| Citrobacter freundi | 9 | 6 | 3 | - | - | 1 | - | - | - | 19 |
| Debaryomyces hansenii | 5 | 5 | 3 | 1 | 2 | 1 | - | - | 17 | |
| Hafnia alvei | 17 | 12 | 11 | 3 | 2 | 1 | 2 | 2 | 1 | 51 |
| Ewingella americana | 4 | 2 | 1 | - | - | - | - | - | - | 7 |
| Kluyveromyces lactis | 3 | 5 | 3 | 1 | 1 | 3 | 2 | 3 | 1 | 22 |
| Pseudomonas fragi | 4 | 2 | 1 | - | - | - | - | - | - | 7 |
| Lacticaseibacillus paracasei | 2 | 8 | 5 | 4 | 5 | 8 | 4 | 5 | 9 | 50 |
| Lactococcus lactis | 5 | 3 | 7 | 5 | 3 | 5 | 4 | 2 | 2 | 36 |
| Leuconostoc mesenteroides | 1 | 5 | 4 | 2 | 1 | 1 | 3 | 2 | 5 | 24 |
| Yarrowia lipolytica | 2 | 3 | 2 | 3 | - | 3 | 2 | 1 | 1 | 17 |
| Total | 71 | 66 | 54 | 22 | 18 | 27 | 21 | 17 | 21 | 317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunová, S.; Taglieri, I.; Haščík, P.; Ben Hsouna, A.; Mnif, W.; Venturi, F.; Sanmartin, C.; Čmiková, N.; Kluz, M.I.; Kačániová, M. Dried Herbs as an Easy-to-Use and Cost-Effective Alternative to Essential Oils to Extend the Shelf Life of Sheep Lump Cheese. Foods 2023, 12, 4487. https://doi.org/10.3390/foods12244487
Kunová S, Taglieri I, Haščík P, Ben Hsouna A, Mnif W, Venturi F, Sanmartin C, Čmiková N, Kluz MI, Kačániová M. Dried Herbs as an Easy-to-Use and Cost-Effective Alternative to Essential Oils to Extend the Shelf Life of Sheep Lump Cheese. Foods. 2023; 12(24):4487. https://doi.org/10.3390/foods12244487
Chicago/Turabian StyleKunová, Simona, Isabella Taglieri, Peter Haščík, Anis Ben Hsouna, Wissem Mnif, Francesca Venturi, Chiara Sanmartin, Natália Čmiková, Maciej Ireneusz Kluz, and Miroslava Kačániová. 2023. "Dried Herbs as an Easy-to-Use and Cost-Effective Alternative to Essential Oils to Extend the Shelf Life of Sheep Lump Cheese" Foods 12, no. 24: 4487. https://doi.org/10.3390/foods12244487
APA StyleKunová, S., Taglieri, I., Haščík, P., Ben Hsouna, A., Mnif, W., Venturi, F., Sanmartin, C., Čmiková, N., Kluz, M. I., & Kačániová, M. (2023). Dried Herbs as an Easy-to-Use and Cost-Effective Alternative to Essential Oils to Extend the Shelf Life of Sheep Lump Cheese. Foods, 12(24), 4487. https://doi.org/10.3390/foods12244487








