Fu Brick Tea as a Staple Food Supplement Attenuates High Fat Diet Induced Obesity in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Different Tea Noodles
2.2. Determination of the Nutritional Components of Noodles
2.3. Animals and Experimental Design
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Histological and Immunohistochemical Examination
2.6. Biochemical Analyses of the Serum and Liver Samples
2.7. RNA Extraction and Real-Time Quantitative PCR
2.8. Analysis of the Gut Microbiota by 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Supplement of FBT Changed the Nutritional Composition of Noodles
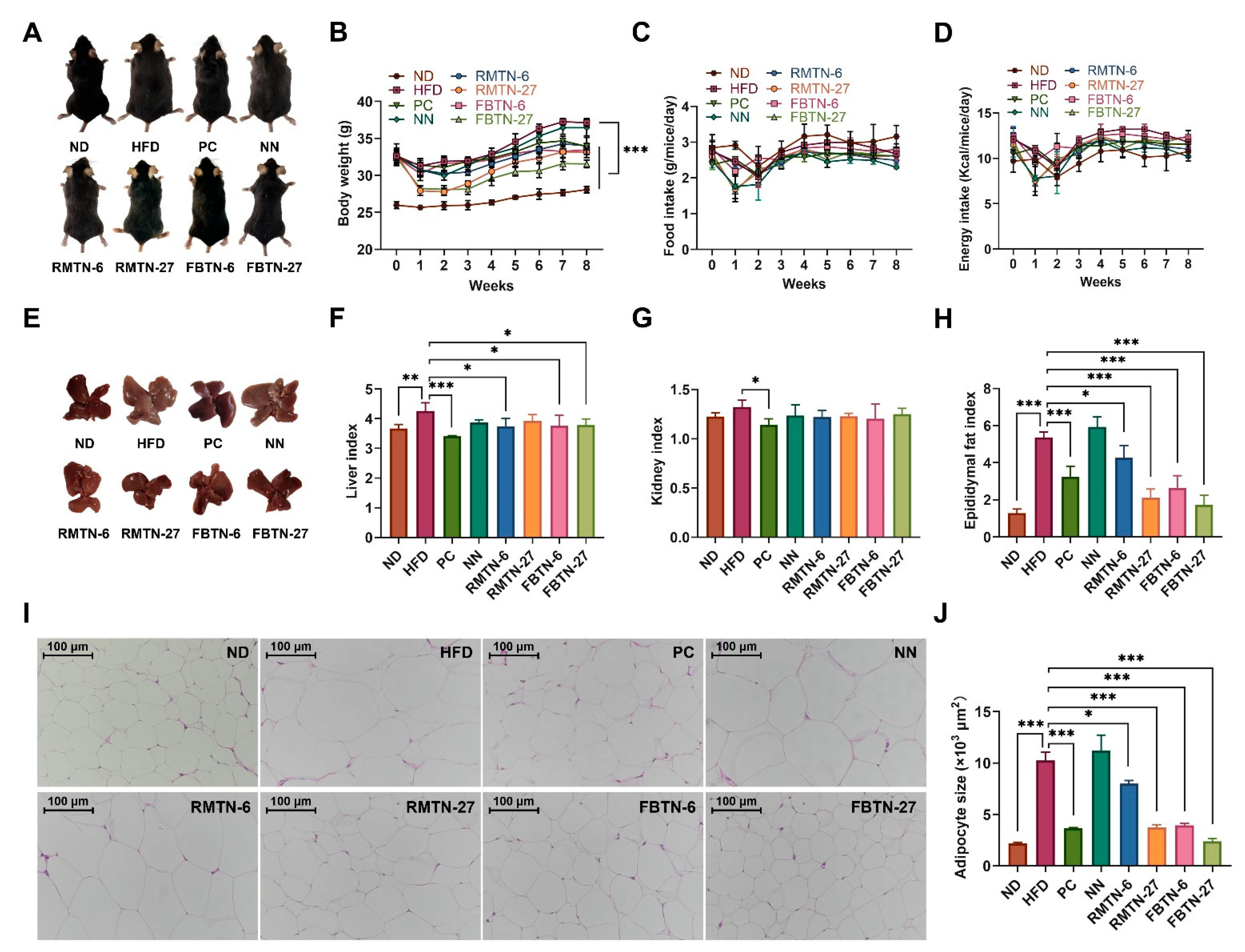
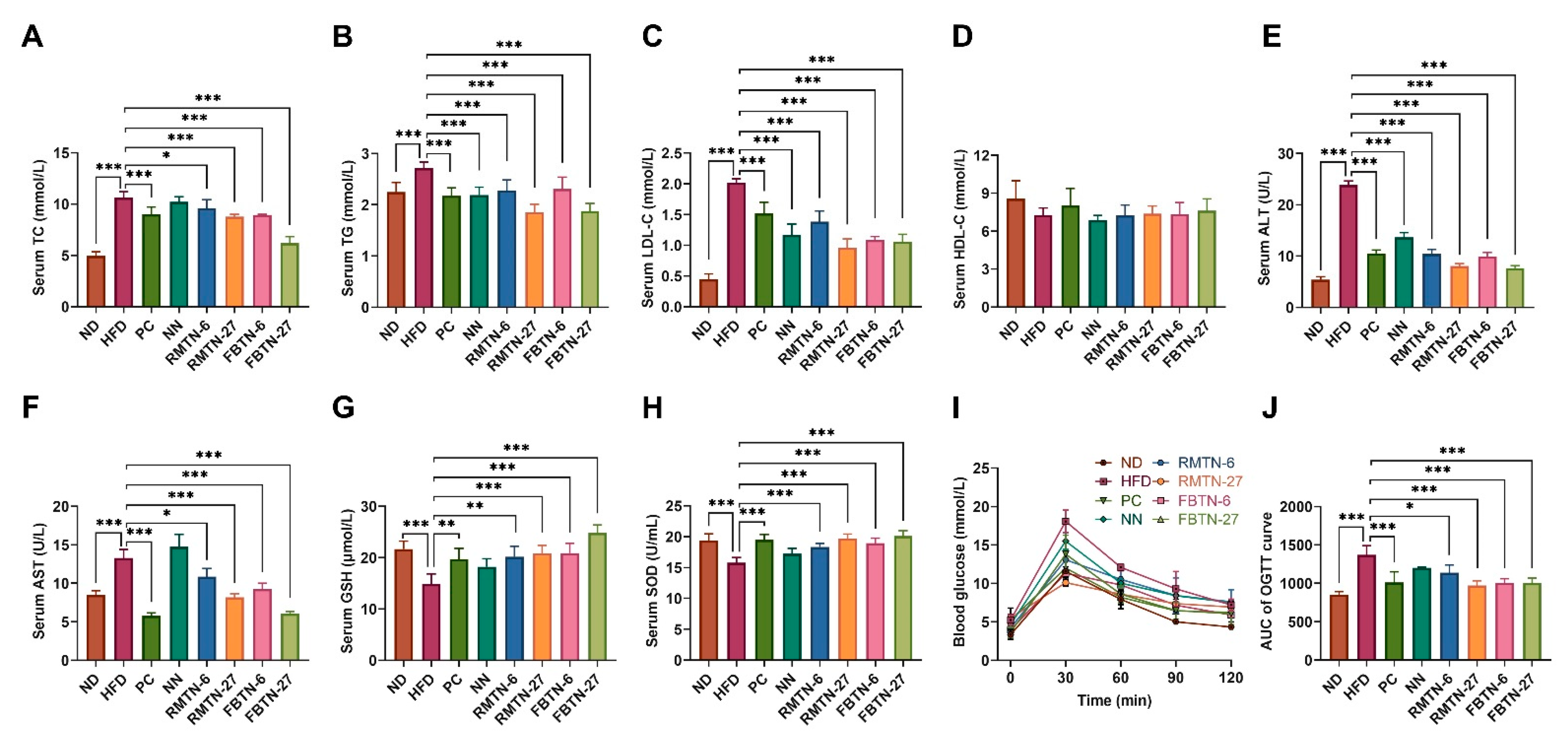
3.2. Impact of FBTN on Fat Accumulation, Serum Biochemical Indices, and OGTT in HFD-Induced Mice
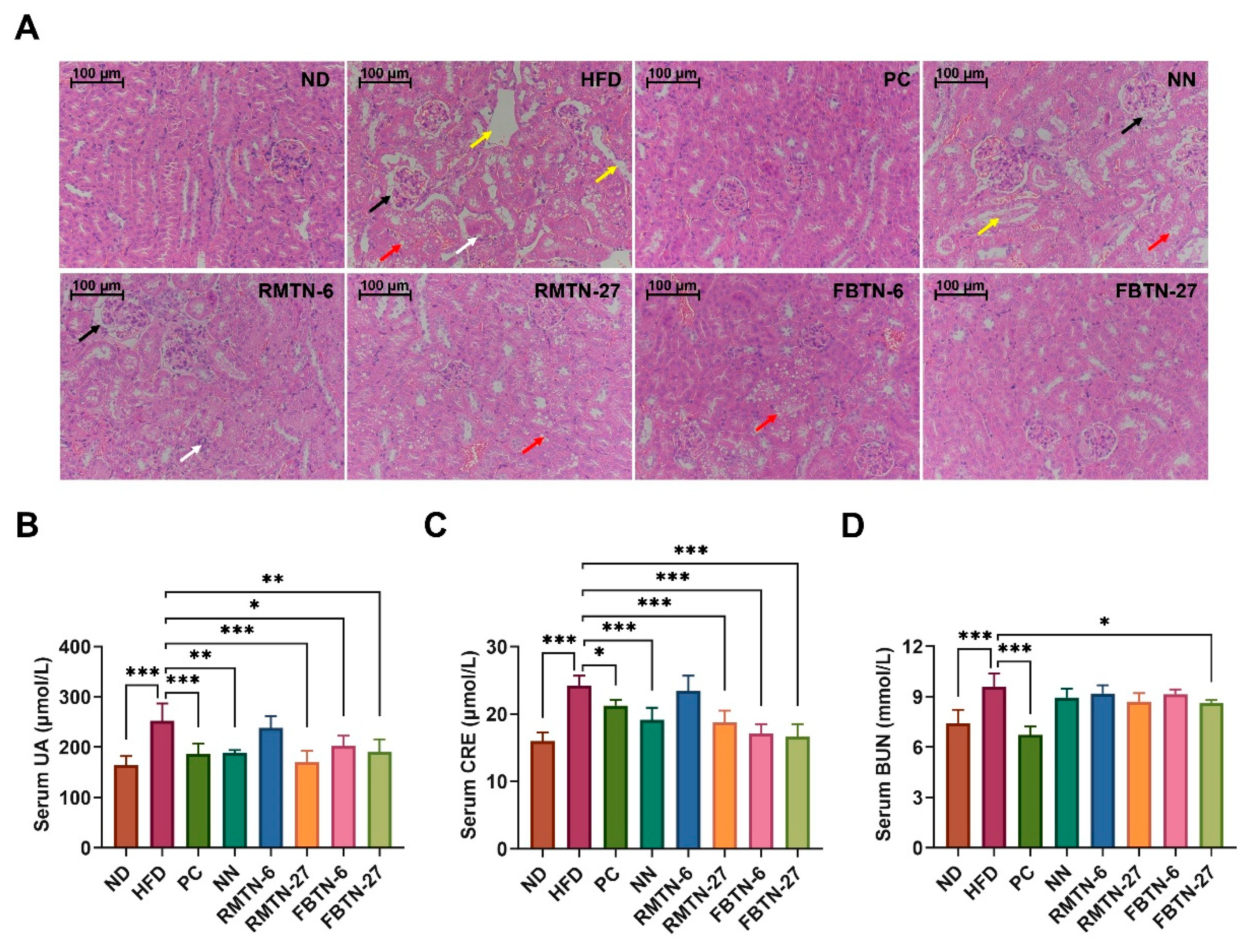
3.3. Effect of FBTN on HFD-Induced Organ Damage in Mice
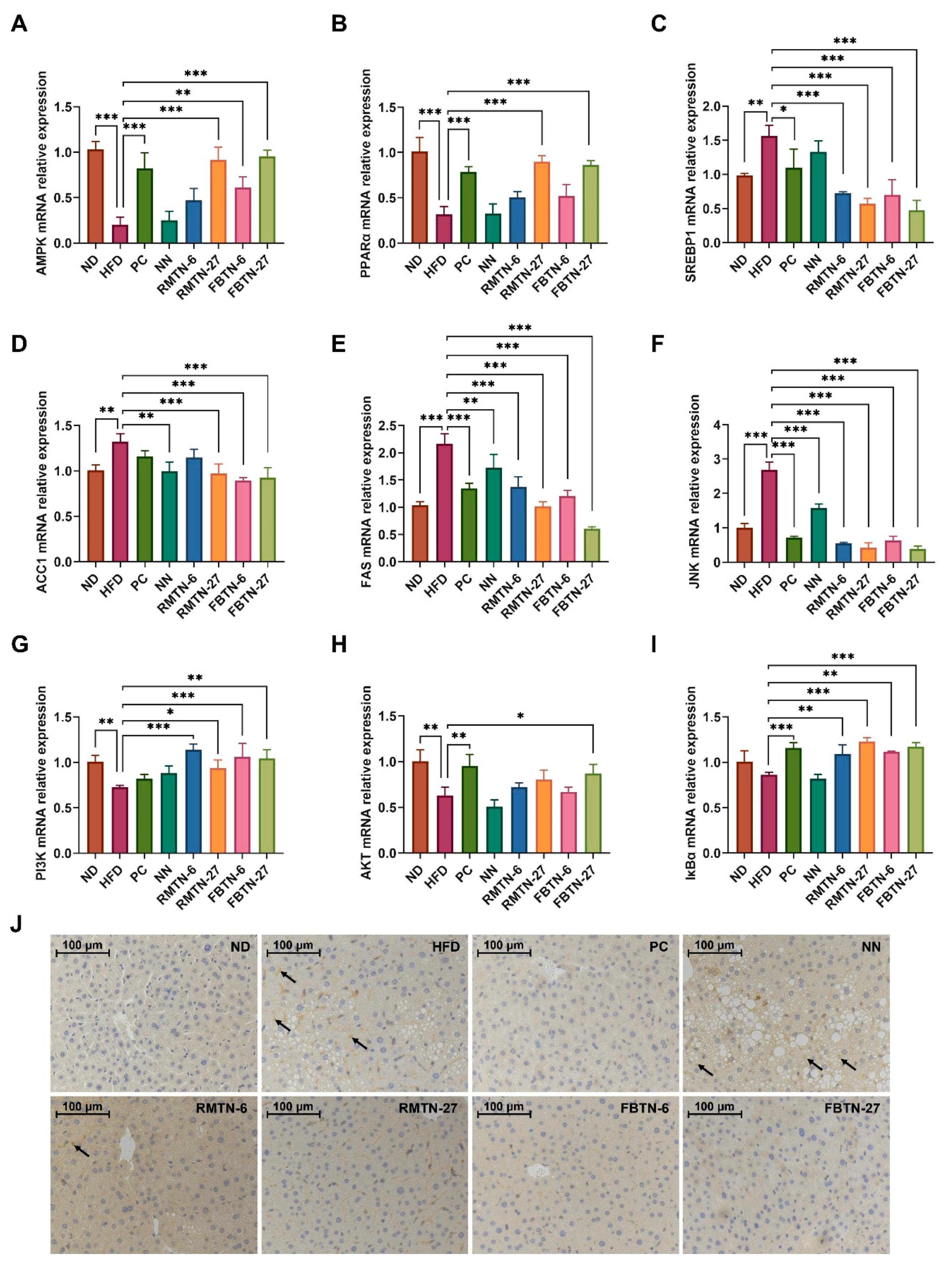
3.4. Effect of FBTN on the Expression of Genes Related to Lipid Metabolism and Inflammatory Responses in the Liver of HFD-Induced Mice
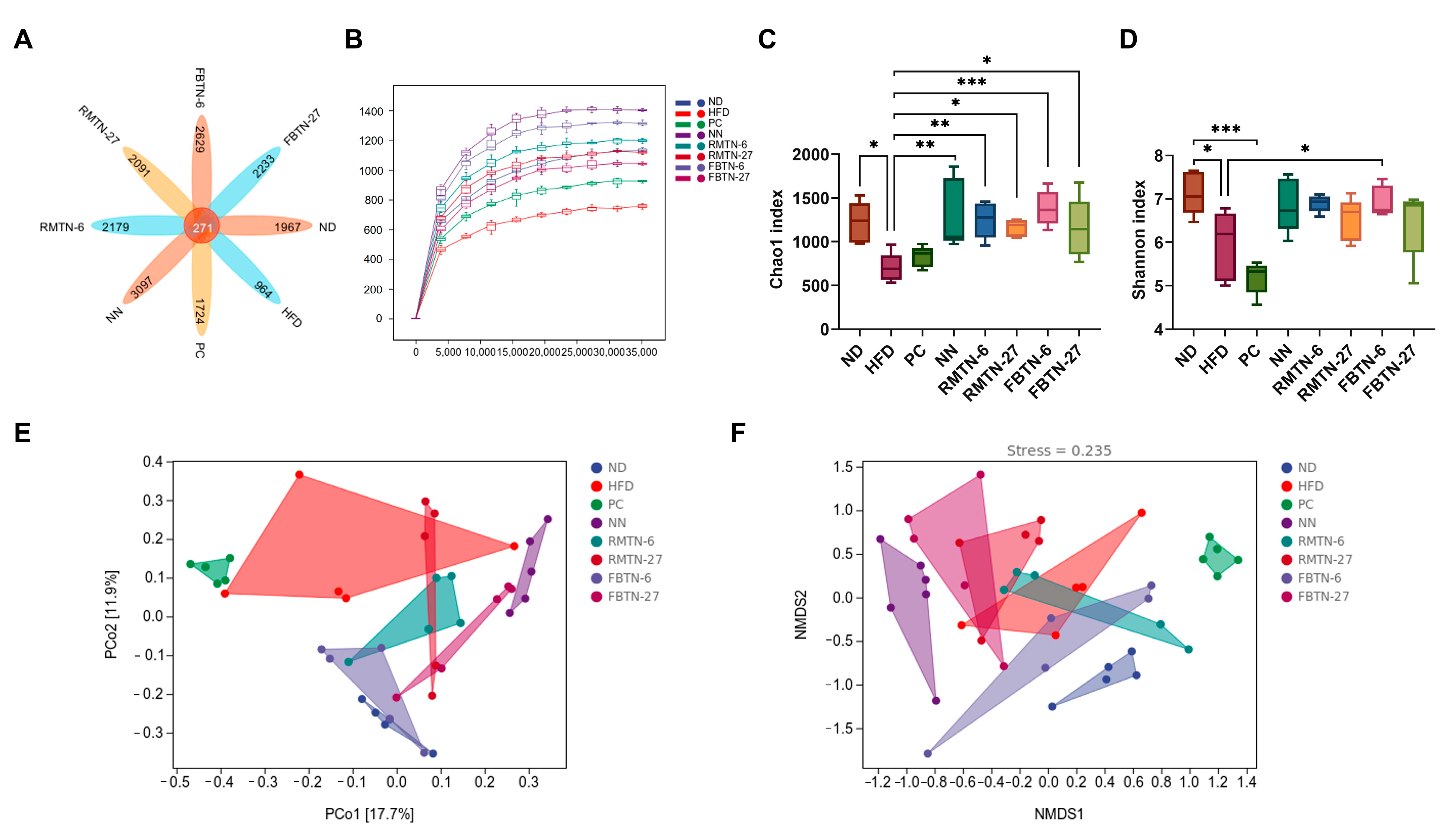
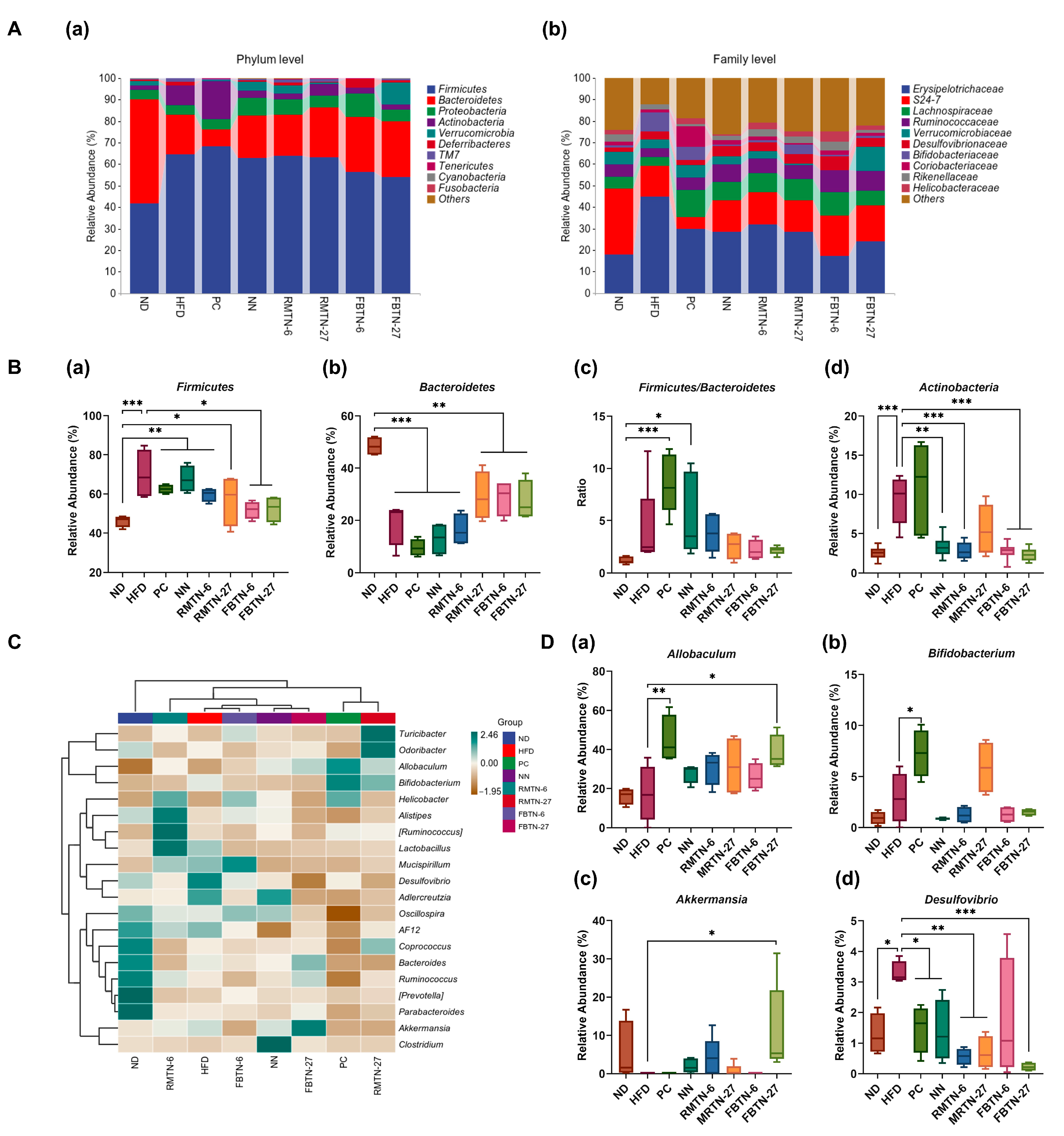
3.5. Effect of FBTN on the Composition and Structure of the Gut Microbiota of HFD-Fed Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Han, Y.; Zhao, H.; Zhang, H.; Yan, B.; Pei, S.; He, X.; Li, Y.; Meng, X.; Chen, L.; et al. Punicalagin alleviates renal injury via the gut-kidney axis in high-fat diet-induced diabetic mice. Food Funct. 2022, 13, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. New therapeutic approaches for the treatment of obesity. Sci. Transl. Med. 2016, 8, 323rv2. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-J.; Wei, X.-L.; Liu, H.-Y.; Li, H.; Xia, Y.; Wu, D.-T.; Zhang, P.-Z.; Gandhi, G.R.; Hua-Bin, L.; Gan, R.-Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Wen, J.; Liu, P.; Liu, Z.; Li, Z. Fungal community associated with fermentation and storage of Fuzhuan brick-tea. Int. J. Food Microbiol. 2011, 146, 14–22. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Li, Y.; Zhang, Y.; Luo, Y.; Chen, Y.; Lin, H.; Wang, K.; Liu, Z. Fungal community succession and major components change during manufacturing process of Fu brick tea. Sci. Rep. 2017, 7, 6947. [Google Scholar] [CrossRef]
- Qi, B.; Ren, D.; Li, T.; Niu, P.; Zhang, X.; Yang, X.; Xiao, J. Fu Brick Tea Manages HFD/STZ-Induced Type 2 Diabetes by Regulating the Gut Microbiota and Activating the IRS1/PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2022, 70, 8274–8287. [Google Scholar] [CrossRef]
- Yoo, A.; Jung Kim, M.; Ahn, J.; Hwa Jung, C.; Deok Seo, H.; Yung Ly, S.; Youl Ha, T. Fuzhuan brick tea extract prevents diet-induced obesity via stimulation of fat browning in mice. Food Chem. 2022, 377, 132006. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, A.; Du, H.; Liu, Y.; Qi, B.; Yang, X. Theabrownin from Fu Brick Tea Exhibits the Thermogenic Function of Adipocytes in High-Fat-Diet-Induced Obesity. J. Agric. Food Chem. 2021, 69, 11900–11911. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, Y.L.; Zhang, X.; Wang, K.B.; Huang, J.A.; Liu, Z.H.; Zhu, M.Z. Polyphenols from Fu Brick Tea Reduce Obesity via Modulation of Gut Microbiota and Gut Microbiota-Related Intestinal Oxidative Stress and Barrier Function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zeng, Z.; Xie, M.; Peng, Y.; Zhou, W.; Xu, W.; Sun, Y.; Zeng, X.; Liu, Z. Fermentation characteristics and probiotic activity of a purified fraction of polysaccharides from Fuzhuan brick tea. Food Sci. Hum. Wellness 2022, 11, 727–737. [Google Scholar] [CrossRef]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef]
- Ang, K.; Bourgy, C.; Fenton, H.; Regina, A.; Newberry, M.; Diepeveen, D.; Lafiandra, D.; Grafenauer, S.; Hunt, W.; Solah, V. Noodles Made from High Amylose Wheat Flour Attenuate Postprandial Glycaemia in Healthy Adults. Nutrients 2020, 12, 2171. [Google Scholar] [CrossRef]
- AOAC International; Association of Official Analytical Chemists. Official Methods of Analysis; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol. 2010, 5, 145–171. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, N.; Zhou, W.; Chen, S.; Wang, Q.; Gao, H.; Xue, X.; Wu, L.; Cao, W. Honey Polyphenols Ameliorate DSS-Induced Ulcerative Colitis via Modulating Gut Microbiota in Rats. Mol. Nutr. Food Res. 2019, 63, e1900638. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Mao, X.; Zhang, X. Glucose-lowering activity of dark tea protein extract by modulating spleen-brain axis of diabetic mice. Br. J. Nutr. 2021, 126, 961–969. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Laforest, S.; Labrecque, J.; Michaud, A.; Cianflone, K.; Tchernof, A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit. Rev. Clin. Lab. Sci. 2015, 52, 301–313. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Zhonghua, L. Fuzhuan Brick Tea Attenuates High-Fat Diet-Induced Obesity and Associated Metabolic Disorders by Shaping Gut Microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhu, M.Z.; Tang, J.Y.; Ou-Yang, J.; Shang, B.H.; Liu, C.W.; Wang, J.; Liu, Q.; Huang, J.A.; Liu, Z.H. Six types of tea extracts attenuated high-fat diet-induced metabolic syndrome via modulating gut microbiota in rats. Food Res. Int. 2022, 161, 111788. [Google Scholar] [CrossRef]
- Hussain, K.; Yang, Y.; Wang, J.; Bian, H.; Lei, X.; Chen, J.; Li, Q.; Wang, L.; Zhong, Q.; Fang, X.; et al. Comparative study on the weight loss and lipid metabolism by tea polyphenols in diet induced obese C57BL/6J pseudo germ free and conventionalized mice. Food Sci. Hum. Wellness 2022, 11, 697–710. [Google Scholar] [CrossRef]
- Chen, S.; Guan, X.; Yong, T.; Gao, X.; Xiao, C.; Xie, Y.; Chen, D.; Hu, H.; Wu, Q. Structural characterization and hepatoprotective activity of an acidic polysaccharide from Ganoderma lucidum. Food Chem. X 2022, 13, 100204. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nie, S.P.; Zhu, K.X.; Ding, Q.; Li, C.; Xiong, T.; Xie, M.Y. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014, 5, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, Y.; Lin, L.; Yuan, D.; Peng, Y.; Li, L.; Xiao, W.; Gong, Z. Hypoglycemic effects of black brick tea with fungal growth in hyperglycemic mice model. Food Sci. Hum. Wellness 2022, 11, 711–718. [Google Scholar] [CrossRef]
- Darwish, A.M.; Mabrouk, D.M.; Desouky, H.M.; Khattab, A.E. Evaluation of the effectiveness of two new strains of Lactobacillus on obesity-induced kidney diseases in BALB/c mice. J. Genet. Eng. Biotechnol. 2022, 20, 148. [Google Scholar] [CrossRef]
- Han, F.; Kan, C.; Wu, D.; Kuang, Z.; Song, H.; Luo, Y.; Zhang, L.; Hou, N.; Sun, X. Irisin protects against obesity-related chronic kidney disease by regulating perirenal adipose tissue function in obese mice. Lipids Health Dis. 2022, 21, 115. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Ao, T.; Chen, Y.; Xie, J.; Hu, X.; Yu, Q. Exploration of the role of bound polyphenols on tea residues dietary fiber improving diabetic hepatorenal injury and metabolic disorders. Food Res. Int. 2022, 162, 112062. [Google Scholar] [CrossRef]
- Song, M.; Tan, D.; Li, B.; Wang, Y.; Shi, L. Gypenoside ameliorates insulin resistance and hyperglycemia via the AMPK-mediated signaling pathways in the liver of type 2 diabetes mellitus mice. Food Sci. Hum. Wellness 2022, 11, 1347–1354. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Chen, J.J.; Ji, X.M.; Hu, X.; Ling, T.J.; Zhang, Z.Z.; Bao, G.H.; Wan, X.C. Changes of major tea polyphenols and production of four new B-ring fission metabolites of catechins from post-fermented Jing-Wei Fu brick tea. Food Chem. 2015, 170, 110–117. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wei, Y.; Xu, L.; Peng, L.; Wang, Y.; Wei, X. Gut Microbiota Differentially Mediated by Qingmao Tea and Qingzhuan Tea Alleviated High-Fat-Induced Obesity and Associated Metabolic Disorders: The Impact of Microbial Fermentation. Foods 2022, 11, 3210. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Jiang, H.; Zhou, F.; Ben, A.; Wang, R.; Hua, C. Chicory polysaccharides alleviate high-fat diet-induced non-alcoholic fatty liver disease via alteration of lipid metabolism- and inflammation-related gene expression. Food Sci. Hum. Wellness 2022, 11, 954–964. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Xie, Y.; Li, N.; Liu, Y.; Wen, J.; Zhang, M.; Feng, W.; Huang, J.; Guo, Y.; et al. Clitoria ternatea blue petal extract protects against obesity, oxidative stress, and inflammation induced by a high-fat, high-fructose diet in C57BL/6 mice. Food Res. Int. 2022, 162, 112008. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, A.; Chen, M.; Wang, J.; Li, Z.; Sun, Z.; Ye, Y.; Nan, J.; Yu, S.; Chen, M.; et al. Impacts of selenium enrichment on nutritive value and obesity prevention of Cordyceps militaris: A nutritional, secondary metabolite, and network pharmacological analysis. Food Chem. X 2023, 19, 100788. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARalpha in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Tao, W.; Cao, W.; Yu, B.; Chen, H.; Gong, R.; Luorong, Q.; Luo, J.; Yao, L.; Zhang, D. Hawk tea prevents high-fat diet-induced obesity in mice by activating the AMPK/ACC/SREBP1c signaling pathways and regulating the gut microbiota. Food Funct. 2022, 13, 6056–6071. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, W.; Guo, Y.; Lin, Z.; Wang, D.; Guo, Q.; Zhou, Y. Apoptosis induction in human prostate cancer cells related to the fatty acid metabolism by wogonin-mediated regulation of the AKT-SREBP1-FASN signaling network. Food Chem. Toxicol. 2022, 169, 113450. [Google Scholar] [CrossRef]
- Gu, Y.; Duan, S.; Ding, M.; Zheng, Q.; Fan, G.; Li, X.; Li, Y.; Liu, C.; Sun, R.; Liu, R. Saikosaponin D attenuates metabolic associated fatty liver disease by coordinately tuning PPARalpha and INSIG/SREBP1c pathway. Phytomedicine 2022, 103, 154219. [Google Scholar] [CrossRef]
- Jin, L.; Wang, M.; Yang, B.; Ye, L.; Zhu, W.; Zhang, Q.; Lou, S.; Zhang, Y.; Luo, W.; Liang, G. A small-molecule JNK inhibitor JM-2 attenuates high-fat diet-induced non-alcoholic fatty liver disease in mice. Int. Immunopharmacol. 2022, 115, 109587. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Meng, L.; Chen, Y.; Wang, Q.; Zhang, Y.; Xi, X.; Kang, W. Lignans from Patrinia scabiosaefolia improve insulin resistance by activating PI-3K/AKT pathway and promoting GLUT4 expression. Food Sci. Hum. Wellness 2023, 12, 2014–2021. [Google Scholar] [CrossRef]
- Choi, H.; Dikalova, A.; Stark, R.J.; Lamb, F.S. c-Jun N-terminal kinase attenuates TNFalpha signaling by reducing Nox1-dependent endosomal ROS production in vascular smooth muscle cells. Free Radic. Biol. Med. 2015, 86, 219–227. [Google Scholar] [CrossRef]
- Meng, H.; Song, J.; Fan, B.; Li, Y.; Zhang, J.; Yu, J.; Zheng, Y.; Wang, M. Monascus vinegar alleviates high-fat-diet-induced inflammation in rats by regulating the NF-κB and PI3K/AKT/mTOR pathways. Food Sci. Hum. Wellness 2022, 11, 943–953. [Google Scholar] [CrossRef]
- Wang, Q.M.; Wang, H.; Li, Y.F.; Xie, Z.Y.; Ma, Y.; Yan, J.J.; Gao, Y.F.; Wang, Z.M.; Wang, L.S. Inhibition of EMMPRIN and MMP-9 Expression by Epigallocatechin-3-Gallate through 67-kDa Laminin Receptor in PMA-Induced Macrophages. Cell Physiol. Biochem. 2016, 39, 2308–2319. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, F.; Wang, Y.; Li, F.; Wang, L.; Wang, Q.; Ren, Z.; Wang, Y. In vitro and in vivo anti-inflammatory effects of theaflavin-3,3′-digallate on lipopolysaccharide-induced inflammation. Eur. J. Pharmacol. 2017, 794, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.X.; Xu, S.; Yu, L.J.; Zhou, Y.F.; Zhou, Y.; Liu, Z.H. Eurotium cristatum Fermented Loose Dark Tea Ameliorates Cigarette Smoke-Induced Lung Injury by MAPK Pathway and Enhances Hepatic Metabolic Detoxification by PXR/AhR Pathway in Mice. Oxid. Med. Cell. Longev. 2021, 2021, 6635080. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, W.; Yuan, T.; Shi, C.; Jin, T.; Chong, Y.; Ji, J.; Lin, L.; Xu, J.; Zhang, Y.; et al. Platycodon grandiflorus root extract activates hepatic PI3K/PIP3/Akt insulin signaling by enriching gut Akkermansia muciniphila in high fat diet fed mice. Phytomedicine 2023, 109, 154595. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, M.; Zhou, H.; Cheng, L.; Wei, X.; Wang, Y. Liubao brick tea activates the PI3K-Akt signaling pathway to lower blood glucose, metabolic disorders and insulin resistance via altering the intestinal flora. Food Res. Int. 2021, 148, 110594. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, F.; Yang, C.; Zhao, C.; Cheng, N.; Cao, W.; Zhao, H. Alternative to Sugar, Honey Does Not Provoke Insulin Resistance in Rats Based on Lipid Profiles, Inflammation, and IRS/PI3K/AKT Signaling Pathways Modulation. J. Agric. Food Chem. 2022, 70, 10194–10208. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Singh, J.; Boparai, R.K.; Zhu, J.; Mantri, S.; Khare, P.; Khardori, R.; Kondepudi, K.K.; Chopra, K.; Bishnoi, M. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol. Res. 2017, 123, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pang, B.; Shao, D.; Jiang, C.; Hu, X.; Shi, J. Artemisia sphaerocephala Krasch polysaccharide mediates lipid metabolism and metabolic endotoxaemia in associated with the modulation of gut microbiota in diet-induced obese mice. Int. J. Biol. Macromol. 2020, 147, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Q.; Lin, F.; Zheng, B.; Huang, Y.; Yang, Y.; Xue, C.; Xiao, M.; Ye, J. Neoagarotetraose alleviates high fat diet induced obesity via white adipocytes browning and regulation of gut microbiota. Carbohydr. Polym. 2022, 296, 119903. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boque, N.; Macarulla, M.T.; Portillo, M.P.; Martinez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef]
- Lan, Y.; Ning, K.; Ma, Y.; Zhao, J.; Ci, C.; Yang, X.; An, F.; Zhang, Z.; An, Y.; Cheng, M. High-Density Lipoprotein Cholesterol as a Potential Medium between Depletion of Lachnospiraceae Genera and Hypertension under a High-Calorie Diet. Microbiol. Spectr. 2022, 10, e0234922. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Yuan, J.; Liu, Z.; Ye, C.; Qin, S. Undaria pinnatifida improves obesity-related outcomes in association with gut microbiota and metabolomics modulation in high-fat diet-fed mice. Appl. Microbiol. Biotechnol. 2020, 104, 10217–10231. [Google Scholar] [CrossRef] [PubMed]
- Botchlett, R.; Woo, S.-L.; Liu, M.; Pei, Y.; Guo, X.; Li, H.; Wu, C. Nutritional approaches for managing obesity-associated metabolic diseases. J. Endocrinol. 2017, 233, R145–R171. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, L.; Wang, S.; Wang, J.; Su, C.; Zhang, L.; Li, C.; Liu, S. Phenolics from noni (Morinda citrifolia L.) fruit alleviate obesity in high fat diet-fed mice via modulating the gut microbiota and mitigating intestinal damage. Food Chem. 2023, 402, 134232. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Hijona, E.; Aguirre, L.; Milagro, F.I.; Bujanda, L.; Rimando, A.M.; Martinez, J.A.; Portillo, M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Hu, Y.; Ma, H.; Zou, Z.; Xiao, Y.; Yang, Y.; Feng, M.; Li, X.; Ye, X. Rhizoma Coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. Biochim. Biophys. Acta 2016, 1862, 1696–1709. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Wang, Q.; Huang, H.; Zhao, Y.; Du, F.; Chen, Y.; Shen, J.; Luo, H.; Zhao, Q.; et al. Pueraria lobata starch regulates gut microbiota and alleviates high-fat high-cholesterol diet induced non-alcoholic fatty liver disease in mice. Food Res. Int. 2022, 157, 111401. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Z.; Zhang, X.; Zhu, F.; Liu, Y.; Jin, C.; Du, X.; Xu, C.; Chen, Y.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, C.; Fang, W.; Tang, Q.; Zhan, L.; Shi, Y.; Tang, M.; Liu, Z.; Zhang, S.; Liu, A. Research progress on the lipid-lowering and weight loss effects of tea and the mechanism of its functional components. J. Nutr. Biochem. 2023, 112, 109210. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1700485. [Google Scholar] [CrossRef] [PubMed]


| Nutritional Component | Sample | ||||
|---|---|---|---|---|---|
| NN | RMTN-6 | RMTN-27 | FBTN-6 | FBTN-27 | |
| Energy (KJ) | 1403.04 ± 0.52 | 1494.36 ± 0.12 *** | 1272.77 ± 0.43 *** | 1408.42 ± 0.52 | 1281.98 ± 0.14 *** |
| Protein (g) | 14.78 ± 0.13 | 15.66 ± 0.11 *** | 17.63 ± 0.08 *** | 15.37 ± 0.04 *** | 17.12 ± 0.09 * |
| Fat (g) | 1.31 ± 0.15 | 0.89 ± 0.21 *** | 0.87 ± 0.01 *** | 0.91 ± 0.02 *** | 0.59 ± 0.01 *** |
| Carbohydrate (g) | 61.89 ± 0.41 | 67.73 ± 0.35 *** | 49.79 ± 0.29 *** | 63.34 ± 0.46 *** | 50.51 ± 0.37 *** |
| Dietary fiber (g) | 8.48 ± 1.24 | 7.93 ± 0.57 *** | 13.70 ± 0.01 *** | 7.06 ± 0.81 *** | 15.30 ± 1.05 *** |
| Ash (g) | 1.01 ± 0.01 | 1.02 ± 0.00 | 2.01 ± 0.01 *** | 1.10 ± 0.00 *** | 2.11 ± 0.02 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Zhao, H.; Guo, L.; Liu, X.; Liang, Y.; Liu, Q.; Cao, W.; Chen, X.; Gao, X. Fu Brick Tea as a Staple Food Supplement Attenuates High Fat Diet Induced Obesity in Mice. Foods 2023, 12, 4488. https://doi.org/10.3390/foods12244488
Wu D, Zhao H, Guo L, Liu X, Liang Y, Liu Q, Cao W, Chen X, Gao X. Fu Brick Tea as a Staple Food Supplement Attenuates High Fat Diet Induced Obesity in Mice. Foods. 2023; 12(24):4488. https://doi.org/10.3390/foods12244488
Chicago/Turabian StyleWu, Daying, Haoan Zhao, Lei Guo, Xiukun Liu, Yan Liang, Qian Liu, Wei Cao, Xueyan Chen, and Xin Gao. 2023. "Fu Brick Tea as a Staple Food Supplement Attenuates High Fat Diet Induced Obesity in Mice" Foods 12, no. 24: 4488. https://doi.org/10.3390/foods12244488






