Interrogating Raisin Associated Unsaturated Fatty Acid Derived Volatile Compounds Using HS–SPME with GC–MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Free-Form Volatiles
2.3. Optimization of pH for Model Reaction
2.4. UFAO Model Reaction
2.5. GC-MS Analysis
2.6. Quantification
2.7. Statistical Analysis
3. Results and Discussion
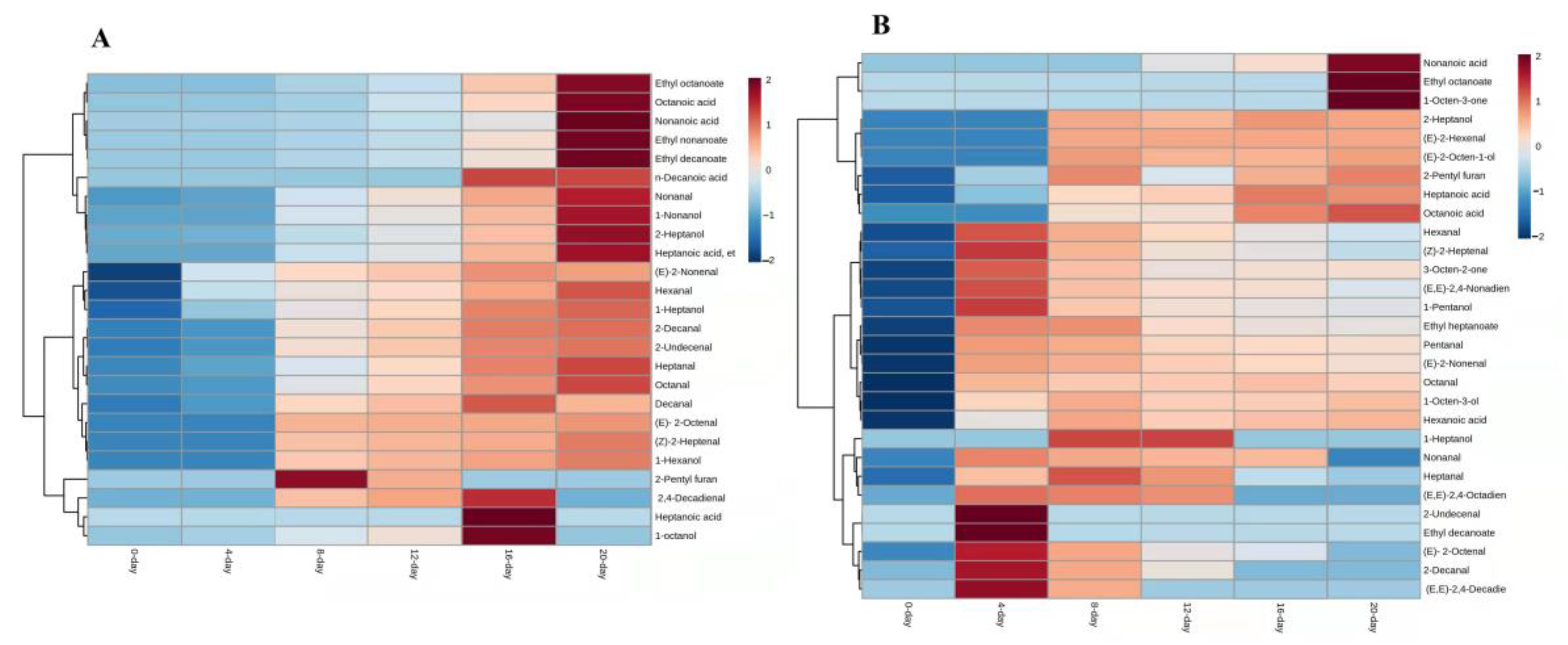
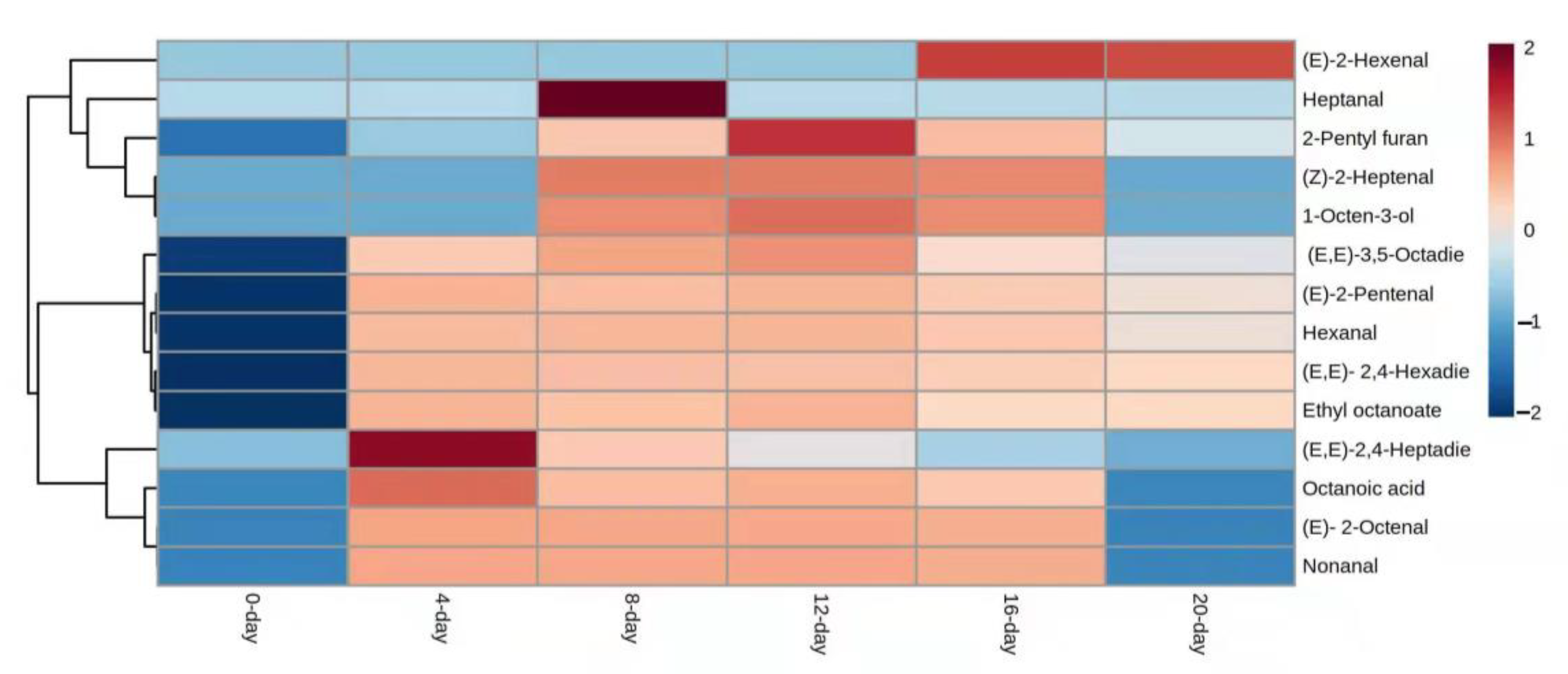
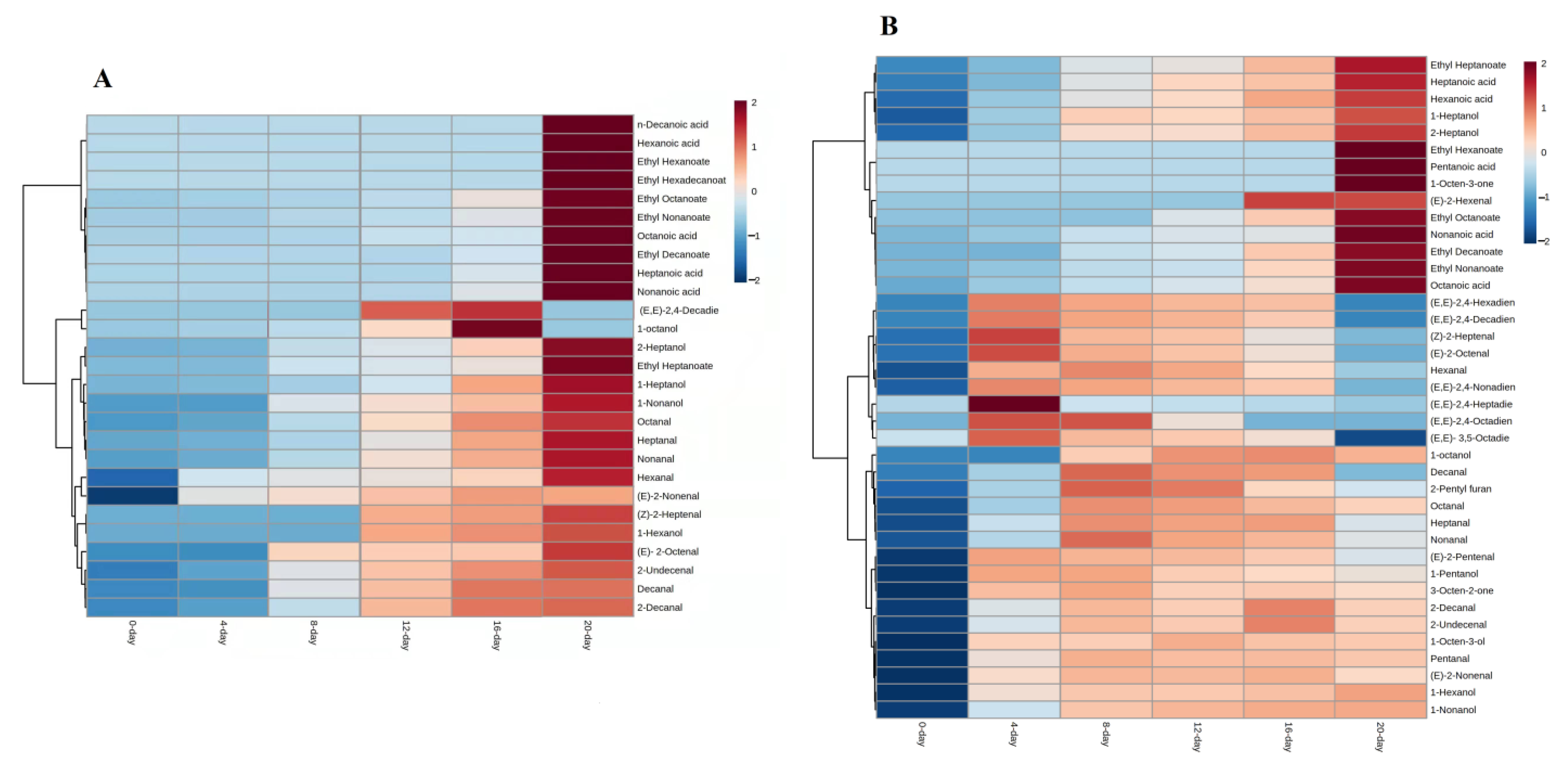
3.1. Free-Form Volatile Compounds Formed during Raisins Drying
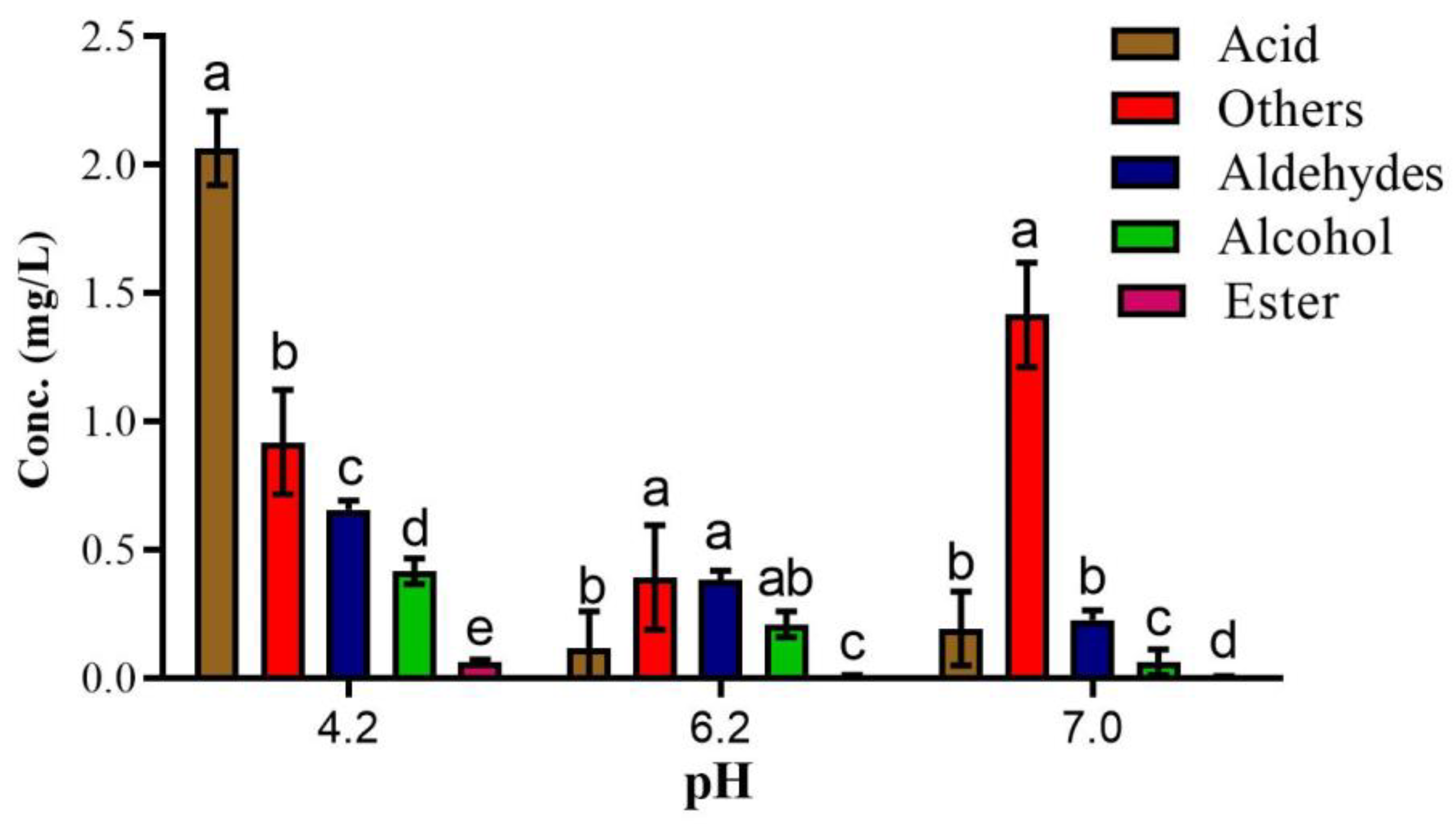
3.2. Optimization of pH for Model Reaction
3.3. UFAO Model Reaction
3.3.1. Control and Stearic Acid
3.3.2. Oleic Acid (C18:1)
3.3.3. Linoleic Acid (C18:2)
3.3.4. Linolenic Acid (C18:3)
3.3.5. Erucic Acid (C22:1)
3.3.6. All Fatty Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, L.P. Raisin Production Manual; UCANR publication: Oakland, CA, USA, 2000; Volume 3393, ISBN 978-1879906440. [Google Scholar]
- USDA. Raisins: World Markets and Trade; Foreign Agricultural Service: Washington, DC, USA, 2017. [Google Scholar]
- Inouye, A. China—Peoples Republic of Raisin Annual; Turpan; Foreign Agricultural Service: Washington, DC, USA, 2017. [Google Scholar]
- Khiari, R.; Zemni, H.; Mihoubi, D. Raisin processing: Physicochemical, nutritional and microbiological quality characteristics as affected by drying process. Food Rev. Int. 2018, 35, 246–298. [Google Scholar] [CrossRef]
- Javed, H.U.; Wang, D.; Wu, G.-F.; Kaleem, Q.M.; Duana, C.-Q.; Shi, Y. Post-storage changes of volatile compounds in air-and sun-dried raisins with different packaging materials using HS-SPME with GC/MS. Food Res. Int. 2019, 119, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Duan, C.-Q.; Shi, Y.; Zhu, B.-Q.; Javed, H.U.; Wang, J. Free and glycosidically bound volatile compounds in sun-dried raisins made from different fragrance intensities grape varieties using a validated HS-SPME with GC–MS method. Food Chem. 2017, 228, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pangavhane, D.R.; Sawhney, R.L. Review of research and development work on solar dryers for grape drying. Energy Convers. Manag. 2002, 43, 45–61. [Google Scholar] [CrossRef]
- Jelen, H.; Wasowicz, E. Lipid-Derived Flavor Compounds. In Food Flavors: Chemical, Sensory, and Technological Properties; Jelen, H., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 65–89. [Google Scholar]
- Javed, H.U.; Wang, D.; Shi, Y.; Wu, G.F.; Xie, H.; Pan, Y.Q.; Duan, C.-Q. Changes of free-form volatile compounds in pre-treated raisins with different packaging materials during storage. Food Res. Int. 2018, 107, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cai, J.; Zhu, B.Q.; Wu, G.F.; Duan, C.-Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS-SPME with GC-MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Reineccius, G. Flavor Chemistry and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Xu, X.Q.; Wu, Y.W.; Duan, C.Q.; Pan, Q.H. Isolation and characterization of two hydroperoxide lyase genes from grape berries HPL isogenes in Vitis vinifera grapes. Mol. Biol. Rep. 2012, 39, 7443–7455. [Google Scholar] [CrossRef]
- Aydin, A. Determination of fatty acid compositions of some raisin cultivars in Turkey. Asian J. Chem. 2011, 23, 1819–1821. [Google Scholar]
- Wang, D.; Javed, H.U.; Kargar, M.; Ali, S.; Shi, Y.; Abdullah; Duan, C.Q. Effects of drying process and time of storage on fatty acid composition in raisins. J. Food Meas. Charact. 2021, 15, 2974–2983. [Google Scholar] [CrossRef]
- Wang, D.; Javed, H.U.; Shi, Y.; Naz, S.; Ali, S.; Duan, C.-Q. Impact of Drying Method on the Evaluation of Fatty Acids and Their Derived Volatile Compounds in ‘Thompson Seedless’ Raisins. Molecules 2020, 25, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, F.B.; Mottram, D.S. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Meeting, A.C.S.; Ho, C.-T.; Hartman, T.G. Lipids in Food Flavors; ACS Publications, American Chemical Society: Washington, DC, USA, 1994. [Google Scholar]
- Van Ba, H.; Amna, T.; Hwang, I. Significant influence of particular unsaturated fatty acids and pH on the volatile compounds in meat-like model systems. Meat Sci. 2013, 94, 480–488. [Google Scholar] [CrossRef]
- Meynier, A.; Mottram, D.S. The effect of pH on the formation of volatile compounds in meat-related model systems. Food Chem. 1995, 52, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Adams, A.; Kitryt, V.; Venskutonis, R.; Kimpe, N. De Model Studies on the Pattern of Volatiles Generated in Mixtures of Amino Acids, Lipid-Oxidation-Derived Aldehydes, and Glucose. J. Agric. Food Chem. 2011, 59, 1449–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, J.S.; Campo, M.M.; Enser, M.; Mottram, D.S. Effect of lipid composition on meat-like model systems containing cysteine, ribose, and polyunsaturated fatty acids. J. Agric. Food Chem. 2002, 50, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Q.; He, F.; Zhu, B.Q.; Lan, Y.B.; Pan, Q.H.; Li, C.Y.; Reeves, M.J.; Wang, J. Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Peacock, W.L. The raisin drying process. In Harvesting and Drying Raisin Grapes; UCDAVIS: Davis, CA, USA, 2013; pp. 207–215. [Google Scholar]
- Wu, Y.; Pan, Q.; Qu, W.; Duan, C. Comparison of volatile profiles of nine litchi (Litchi chinensis Sonn.) cultivars from Southern China. J. Agric. Food Chem. 2009, 57, 9676–9681. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 9783540408185. [Google Scholar]
- Frankel, E.N. Lipid oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E.; Edward, S. Analysis of Autoxidized Fats by Gas Chromatography- Mass Spectrometry: VII. Volatile Thermal Decomposition Products of Pure Hydroperoxides from Autoxidized and Photosensitized Oxidized Methyl Oleate, Linoleate and Linolenate. Lipids 1981, 16, 279–285. [Google Scholar] [CrossRef]
- Horvat, R.I.; Mcfadden, W.H.; Ng, H.; Lane, W.G.; Lee, A.; Lundin, R.E.; Scherer, R. Identification of Some Acids From Autoxidation of Methyl Linoleate. J. Am. Oil Chem. Soc. 1968, 46, 94–96. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Yu, W.; Zhao, L.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, L.; Wang, S. Study on the volatile composition of table grapes of three aroma types. Lwt 2019, 115, 108450. [Google Scholar] [CrossRef]
- Buttery, R.G. Volatile Aroma/Flavor Components of Raisins (Dried Grapes). In Handbook of Fruit and Vegetable Flavors; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 549–556. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
| S.No | RI | Compound Name | ID | 0-Day | 4-Day | 8-Days | 12-Days | 16-Days | 20-Day |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 975 | Pentanal | 2 | 147.6 ± 20.86 c | 198.8 ± 9.24 b | 223.1 ± 19.20 a | 113.5 ± 6.61 d | 93.3 ± 11.44 e | 88.9 ± 2.88 e |
| 2 | 1066 | Hexanal | 1 | 5802.8 ± 193.9 a | 2036.6 ± 67.3 b | 1491.3 ± 126.3 c | 594.9 ± 39.94 d | 559.2 ± 18.45 e | 534.4 ± 14.85 f |
| 3 | 1132 | 3,4-Dimethyl-2,4,6-octatriene | 2 | 35.44 ± 3.65 a | 15.54 ± 0.72 b | 10.53 ± 0.90 c | 7.88 ± 0.16 d | 9.68 ± 0.46 cd | 16.76 ± 0.82 b |
| 4 | 1167 | 2,6-Dimethyl-4-heptanone | 2 | 139.93 ± 2.81 a | 19.68 ± 2.84 b | 5.26 ± 0.54 d | 1.91 ± 0.06 e | 0.87 ± 0.14 f | 7.53 ± 1.14 c |
| 5 | 1178 | Heptanal | 2 | 62.8 ± 6.18 b | 68.8 ± 5.20 b | 76.5 ± 8.82 a | 42.6 ± 2.90 c | 41.6 ± 2.44 c | 45.4 ± 3.52 c |
| 6 | 1205 | 1-Pentanol | 2 | 87.752 ± 16.38 a | 79.714 ± 1.853 ab | 69.93 ± 1.752 c | 35.408 ± 3.42 d | 16.386 ± 0.12 e | 9.42 ± 0.297 f |
| 7 | 1217 | (E)-2-Hexenal | 2 | 2892.9 ± 81.9 a | 505.1 ± 26.71 b | 104.1 ± 6.42 c | 31.84 ± 2.70 d | 16.39 ± 0.80 e | 13.36 ± 0.91 f |
| 8 | 1224 | 2-Pentyl furan | 2 | 34.09 ± 2.66 f | 65.92 ± 4.30 e | 241.47 ± 21.31 a | 220.56 ± 18.62 ab | 159.53 ± 15.84 c | 116.34 ± 8.02 d |
| 9 | 1227 | Ethyl hexanoate | 2 | 41.105 ± 2.923 a | 18.01 ± 0.92 b | 11.75 ± 0.07 c | 8.14 ± 0.05 d | 7.59 ± 0.91 e | 7.68 ± 0.58 e |
| 10 | 1292 | Octanal | 2 | 353.2 ± 84.02 a | 147.98 ± 24.162 b | 69.3 ± 2.86 c | 66.2 ± 1.48 c | 61.2 ± 1.77 c | 52.5 ± 1.05 d |
| 11 | 1325 | (E)-2-Heptenal | 2 | 23.32 ± 9.27 e | 50.94 ± 22.67 d | 365.6 ± 91.98 a | 121.8 ± 4.24 b | 78.1 ± 3.47 c | 49.08 ± 2.86 d |
| 12 | 1349 | 1-Hexanol | 1 | 491.7 ± 43.57 a | 267.5 ± 53.79 b | 153.6 ± 11.38 c | 76.2 ± 13.85 d | 51.8 ± 13.83 e | 40.5 ± 7.17 f |
| 13 | 1378 | Ethyl octanoate | 2 | 0.68 ± 0.042 a | 0.57 ± 0.043 b | 0.49 ± 0.094 c | 0.44 ± 0.13 d | 0.34 ± 0.044 e | 0.34 ± 0.027 e |
| 14 | 1393 | Nonanal | 1 | 5.32 ± 0.77 a | 3.917 ± 0.52 b | 3.46 ± 0.76 b | 2.391 ± 0.22 c | 2.15 ± 0.14 c | 1.75 ± 0.18 d |
| 15 | 1395 | (Z)-3-Hexen-1-ol | 2 | 188.5 ± 9.96 c | 271.7 ± 5.84 b | 304.6 ± 27.02 a | 170.2 ± 13.01 c | 141.9 ± 19.81 d | 104.5 ± 7.32 e |
| 16 | 1411 | 2-Octanol | 2 | 0.893 ± 0.42 a | NF | NF | NF | NF | NF |
| 17 | 1416 | 3-Octen-2-one | 2 | 3.93 ± 0.59 e | 4.13 ± 0.28 e | 34.85 ± 4.02 a | 17.45 ± 0.54 b | 11.34 ± 0.144 c | 10.40 ± 0.358 cd |
| 18 | 1434 | (E)-2-Octenal | 2 | 24.90 ± 0.65 d | 36.41 ± 2.14 b | 81.61 ± 11.56 a | 36.82 ± 2.84 b | 30.31 ± 0.68 c | 19.98 ± 0.18 e |
| 19 | 1449 | 1-Octen-3-ol | 1 | 13.95 ± 1.16 e | 43.76 ± 3.03 c | 202.04 ± 29.17 a | 52.03 ± 3.73 b | 40.64 ± 1.33 c | 34.18 ± 0.71 d |
| 20 | 1453 | 1-Heptanol | 2 | 5.56 ± 0.62 c | 9.18 ± 0.48 b | 15.60 ± 2.81 a | 9.23 ± 0.89 b | 5.19 ± 0.63 c | 5.46 ± 0.282 d |
| 21 | 1487 | 2-Ethyl-1-hexanol | 1 | 10.58 ± 0.53 b | 16.84 ± 0.56 a | 11.63 ± 1.34 b | 4.40 ± 0.73 c | 2.06 ± 0.29 d | 1.77 ± 0.04 e |
| 22 | 1488 | 2-Nonanol | 2 | 1.59 ± 0.12 a | NF | NF | NF | NF | NF |
| 23 | 1497 | (E,E)-2,4-Heptadienal | 2 | 2266.1 ± 135.4 a | 858.6 ± 114.3 b | 238.3 ± 5.9 c | 160.1 ± 8.8 d | 107.9 ± 5.16 e | 113.1 ± 3.82 e |
| 24 | 1501 | Decanal | 1 | 6.76 ± 0.17 a | 4.92 ± 0.66 b | 4.74 ± 0.13 b | 4.19 ± 0.27 bc | 3.31 ± 0.17 c | 2.92 ± 0.66 cd |
| 25 | 1539 | (E)-2-Nonenal | 2 | 10.79 ± 1.75 d | 21.10 ± 0.67 a | 19.57 ± 0.46 b | 16.38 ± 2.20 c | 11.66 ± 2.40 d | 14.50 ± 1.59 c |
| 26 | 1555 | 1-Octanol | 1 | 4.31 ± 0.44 cd | 4.07 ± 0.35 cd | 12.51 ± 1.28 a | 6.67 ± 0.19 b | 4.98 ± 0.16 c | 4.67 ± 0.05 c |
| 27 | 1570 | Methyl octanoate | 2 | 5.75 ± 0.21 a | 4.47 ± 0.30 b | 4.72 ± 0.69 b | 2.31 ± 0.22 c | 2.51 ± 0.33 c | 2.44 ± 0.27 c |
| 28 | 1570 | Ethyl nonanoate | 1 | NF | 0.31 ± 0.03 a | 0.25 ± 0.01 b | 0.181 ± 0.008 c | 0.184 ± 0.016 c | 0.185 ± 0.023 c |
| 29 | 1614 | (E)-2-Octen-1-ol | 2 | 3.29 ± 0.267 d | 5.876 ± 0.795 c | 30.403 ± 3.094 a | 12.281 ± 1.54 b | 6.219 ± 1.253 c | 5.687 ± 0.132 c |
| 30 | 1636 | Butyrolactone | 2 | NF | 3.31 ± 0.12 c | 9.70 ± 1.32 a | 7.34 ± 0.20 b | 7.19 ± 0.29 b | 7.09 ± 0.27 b |
| 31 | 1657 | 1-Nonanol | 1 | 1.37 ± 0.105 bc | 1.53 ± 0.059 b | 2.40 ± 0.209 a | 1.49 ± 0.114 b | 1.14 ± 0.022 cd | 1.05 ± 0.08 d |
| 32 | 1740 | Pentanoic acid | 2 | 71.409 ± 4.741 e | 95.02 ± 5.60 d | 194.4 ± 14.15 b | 256.3 ± 15.81 a | 205.7 ± 7.49 b | 181.6 ± 6.67 bc |
| 33 | 1750 | (E,E)-2,4-Nonadienal | 1 | 266.4 ± 35.7 c | 268.7 ± 39.5 c | 673.8 ± 204.7 a | 621.2 ± 23.45 ab | 477.1 ± 72.70 b | 297.9 ± 9.41 c |
| 34 | 1847 | Hexanoic acid | 1 | 284.1 ± 24.71 d | 393.6 ± 8.35 c | 468.8 ± 74.12 b | 633.6 ± 29.01 a | 498.0 ± 22.30 b | 390.7 ± 7.68 c |
| 35 | 1953 | Heptanoic acid | 1 | 1.52 ± 0.33 e | 2.20 ± 0.12 d | 5.06 ± 0.66 c | 7.23 ± 0.67 a | 5.96 ± 0.29 b | 5.02 ± 0.23 c |
| 36 | 2035 | γ-Nonalactone | 2 | 2.49 ± 0.12 f | 5.40 ± 0.33 c | 10.84 ± 1.17 a | 9.36 ± 1.29 ab | 4.35 ± 0.27 d | 3.99 ± 0.13 e |
| 37 | 2060 | Octanoic acid | 1 | 1.37 ± 0.20 e | 1.85 ± 0.20 d | 4.86 ± 0.41 bc | 5.18 ± 0.37 b | 5.42 ± 0.46 b | 7.26 ± 0.81 a |
| 38 | 2163 | Methyl hexadecanoate | 2 | 0.392 ± 0.009 a | 0.173 ± 0.014 b | 0.132 ± 0.023 c | 0.079 ± 0.002 d | 0.072 ± 0.009 d | 0.086 ± 0.004 d |
| 39 | 2484 | Dodecanoic acid | 2 | NF | NF | NF | 14.15 ± 1.13 a | 12.77 ± 2.12 b | 8.52 ± 1.65 c |
| 10-Days | 20-Days | |||||||
|---|---|---|---|---|---|---|---|---|
| S.No | Compounds | 4.2 | 6.2 | 7.0 | 4.2 | 6.2 | 7.0 | |
| 1 | Pentanal | Aldehyde | 3060.5 ± 193.6 | 1521.3 ± 59.3 | 835.05 ± 25.2 | 3283.5 ± 110.9 | 1834.2 ± 111.3 | 1632.0 ± 62.7 |
| 2 | Hexanal | Aldehyde | 20,851.8 ± 361.2 | 15,511.1 ± 576.9 | 9977.3 ± 233.9 | 16,535.1 ± 647.2 | 14,231.2 ± 326.1 | 14,814.0 ± 46.8 |
| 3 | Heptanal | Aldehyde | 1644.1 ± 72.5 | 708.3 ± 65.4 | 510.4 ± 22.1 | 1499.0 ± 76.8 | 976.1 ± 113.8 | 305.4 ± 70.0 |
| 4 | Octanal | Aldehyde | 4377.1 ± 35.0 | 1289.3 ± 76.4 | 909.8 ± 52.8 | 4309.4 ± 36.1 | 2048.5 ± 103.4 | 1577.1 ± 104.6 |
| 5 | Nonanal | Aldehyde | 11,677.7 ± 105.4 | 1993.2 ± 88.2 | 1333.1 ± 32.5 | 7830.0 ± 62.8 | 1704.6 ± 86.1 | 1606.4 ± 49.6 |
| 6 | Decanal | Aldehyde | 865.0 ± 4.2 | NF | 1592.2 ± 118.4 | 546.3 ± 44.8 | 116.1 ± 7.0 | 55.9 ± 7.8 |
| 7 | (E)-2-Hexenal | Aldehyde | 21,025.7 ± 552.4 | 70,294.4 ± 3647.2 | NF | 19,286.5 ± 260.2 | 22,939.2 ± 320.3 | NF |
| 8 | (E)-2-Heptenal | Aldehyde | 3822.6 ± 69.6 | 2006.0 ± 50.1 | 986.1 ± 75.7 | 2753.5 ± 96.7 | 642.8 ± 38.4 | 458.3 ± 46.0 |
| 9 | (E)-2-Octenal | Aldehyde | 8388.9 ± 189.6 | 2161.1 ± 85.6 | 2580.6 ± 43.3 | 4583.4 ± 126.4 | 522.3 ± 11.1 | 439.9 ± 26.6 |
| 10 | (E)-2-Nonenal | Aldehyde | 773.3 ± 20.6 | 1275.4 ± 27.7 | 7582.4 ± 401.5 | 537.8 ± 7.8 | 367.6 ± 45.2 | 245.0 ± 31.0 |
| 11 | (E,E)-2,4-Heptadienal | Aldehyde | 6032.3 ± 143.0 | NF | NF | 2745. ± 149.1 | 1682.9 ± 91.2 | 1535.6 ± 176.0 |
| 12 | (E,E)-2,4-Nonadienal | Aldehyde | 875.8 ± 63.3 | 609.5 ± 6.6 | 113.1 ± 2.0 | 393.2 ± 2.4 | 209.8 ± 7.0 | 232.9 ± 13.8 |
| 13 | 1-Pentanol | Alcohol | 34,425.3 ± 1551.2 | 20,350.9 ± 69.4 | 11,424.4 ± 319.7 | 26,752.6 ± 1114.4 | 25,294.4 ± 492.0 | 22,243.6 ± 62.1 |
| 14 | 1-Hexanol | Alcohol | 1487.4 ± 70.7 | NF | NF | NF | NF | NF |
| 15 | 1-Heptanol | Alcohol | 694.3 ± 11.9 | 514.9 ± 64.1 | NF | 797.1 ± 11.8 | 929.1 ± 10.1 | 588.6 ± 10.1 |
| 16 | 1-Octanol | Alcohol | 3189.3 ± 114.5 | 1984.4 ± 47.6 | NF | 3028.0 ± 56.1 | 1795.8 ± 52.1 | 1544.1 ± 47.3 |
| 17 | 1-Octen-3-ol | Alcohol | 1768.1 ± 27.5 | 1747.6 ± 46.7 | NF | 1647.7 ± 61.4 | 1434.3 ± 83.3 | 1132.3 ± 2.2 |
| 18 | 2-Octanol | Alcohol | NF | NF | 204.9 ± 10.0 | NF | NF | NF |
| 19 | (E)-2-Octen-1-ol | Alcohol | 209.72 ± 2.2 | NF | NF | 193.6 ± 4.3 | NF | NF |
| 20 | Pentanoic acid | Acid | 3176.5 ± 101.0 | NF | 31,214.3 ± 219.8 | NF | NF | NF |
| 21 | Hexanoic acid | Acid | 5821.4 ± 74.6 | 188.3 ± 37.8 | NF | 4775.0 ± 87.0 | 586.2 ± 1.7 | 181.7 ± 13.7 |
| 22 | Heptanoic acid | Acid | 3368.9 ± 75.1 | NF | NF | 3064.4 ± 7.9 | NF | NF |
| 23 | Octanoic acid | Acid | 3888.9 ± 79.7 | 289.5 ± 7.4 | 363.0 ± 28.2 | 5299.7 ± 436.7 | NF | NF |
| 24 | Nonanoic acid | Acid | 4377.1 ± 35.0 | 1389.3 ± 76.4 | 909.8 ± 52.8 | 4409.4 ± 177.6 | 2048.5 ± 103.4 | 1527.1 ± 175.3 |
| 25 | n-Decanoic acid | Acid | NF | NF | NF | 193.1 ± 7.07 | NF | NF |
| 26 | 2-Pentyl furan | Furan | 27,093.8 ± 388.4 | 19,343.9 ± 393.4 | 20,022.4 ± 95.7 | 25,569.7 ± 274.1 | 7054.2 ± 142.6 | 15,284.7 ± 3161.8 |
| 27 | 1-Octen-3-one | Ketone | NF | 785.4 ± 29.3 | NF | NF | NF | NF |
| S.No | CAS # | Final Compound Name | Source | Aromatic Series | Precursor |
|---|---|---|---|---|---|
| Unsaturated Fatty Acid | |||||
| 1 | 110-62-3 | Pentanal | Aldehyde | Fat, Green f | Linoleic acid, Mixture |
| 2 | 66-25-1 | Hexanal | Aldehyde | Green f | Oleic acid, Linoleic acid, Linolenic acid, Erucic acid, Mixture |
| 3 | 111-71-7 | Heptanal | Aldehyde | Dry fish, solvent, smoky f | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 4 | 124-13-0 | Octanal | Aldehyde | Honey, Green, Fatty f | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 5 | 124-19-6 | Nonanal | Aldehyde | Green, Fruity f | Oleic acid, Linoleic acid, Linolenic acid, Erucic acid, Mixture |
| 6 | 112-31-2 | Decanal | Aldehyde | Sweet, citrus, green d | Oleic acid, Erucic acid, Mixture |
| 7 | 1576-87-0 | (E)-2-Pentenal | Aldehyde | Apple, green b | Linolenic acid, Mixture |
| 8 | 505-57-7 | (E)-2-Hexenal | Aldehyde | Green f | Linoleic acid, Linolenic acid, Mixture |
| 9 | 2463-63-0 | (E)-2-Heptenal | Aldehyde | Fatty, soapy, tallow f | Oleic acid, Linoleic acid, Linolenic acid, Erucic acid, Mixture |
| 10 | 2548-87-0 | (E)-2-Octenal | Aldehyde | Green, fatty, nut f | Oleic acid, Linoleic acid, Linolenic acid, Erucic acid, Mixture |
| 11 | 18829-56-6 | (E)-2-Nonenal | Aldehyde | Green, fat a | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 12 | 3913-81-3 | (E)-2-Decanal | Aldehyde | Green, fat | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 13 | 1337-83-3 | 2-Undecenal | Aldehyde | Sweet a | Oleic acid, Erucic acid, Mixture |
| 14 | 142-83-6 | (E,E)-2,4-Hexadienal | Aldehyde | Green a | Linolenic acid, Mixture |
| 15 | 4313--03-5 | (E,E)-2,4-Heptadienal | Aldehyde | Fatty, hay b | Linolenic acid, Mixture |
| 16 | 30361-28-5 | (E,E)-2,4-Octadienal | Aldehyde | Green, cucumber a | Linoleic acid |
| 17 | 5910-87-2 | (E,E)-2,4-Nonadienal | Aldehyde | Fatty, oily b | Linoleic acid, Mixture |
| 18 | 25152-84-5 | (E,E)-2,4-Decadienal | Aldehyde | Seaweed a | Oleic acid, Erucic acid, Mixture |
| 19 | 74-41-0 | 1-Pentanol | Alcohol | Balsamic, almond c | Linoleic acid, Mixture |
| 20 | 111-27-3 | 1-Hexanol | Alcohol | green b | Oleic acid, Erucic acid, Mixture |
| 21 | 111-70-6 | 1-Heptanol | Alcohol | Grape, sweet c | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 22 | 111-87-5 | 1-Octanol | Alcohol | Citrus, rose c | Linoleic acid, Erucic acid, Mixture |
| 23 | 143-08-8 | 1-Nonanol | Alcohol | Floral b | Oleic acid, Erucic acid, Mixture |
| 24 | 3391-86-4 | 1-Octen-3-ol | Alcohol | Mushroom, fruity b,f | Linolenic acid, Mixture |
| 25 | 104-76-7 | 2-Ethyl-1-hexanol | Alcohol | Floral, sweet fruity c | Linolenic acid |
| 26 | 543-49-7 | 2-Heptanol | Alcohol | Herbaceous, lemon | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 27 | 18409-17-1 | (E)-2-Octen-1-ol | Alcohol | Fatty, rancid b | Linoleic acid, Mixture |
| 28 | 628-97-7 | Ethyl Hexadecanoate | Ester | Wax a | Oleic acid, Erucic acid |
| 29 | 123-66-0 | Ethyl Hexanoate | Ester | Fruity, apple like a,f | Erucic acid, Mixture |
| 30 | 106-30-9 | Ethyl Heptanoate | Ester | Fruit a | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 31 | 106-32-1 | Ethyl Octanoate | Ester | Fruity, citrus like a | Oleic acid, Linoleic acid, Linolenic acid, Erucic acid, Mixture |
| 32 | 123-29-5 | Ethyl Nonanoate | Ester | Fruity, floral b | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 33 | 110-38-3 | Ethyl Decanoate | Ester | Grape a | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 34 | 104-61-0 | γ-Nonalactone | Ester | Coconut, peach a | Oleic acid, Linoleic acid, Mixture |
| 35 | 109-52-4 | Pentanoic acid | Acid | Sweet a | Mixture |
| 36 | 142-62-1 | Hexanoic acid | Acid | Rancid, Chees, Fatty f | Linoleic acid, Erucic acid, Mixture |
| 37 | 111-14-8 | Heptanoic acid | Acid | Sweety, cheesy e | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 38 | 124-07-2 | Octanoic acid | Acid | Rancid, Chees, Fatty f | Oleic acid, Linoleic acid, Linolenic acid, Erucic acid, Mixture |
| 39 | 67762-36-1 | Nonanoic acid | Acid | Green, fat a | Oleic acid, Linoleic acid, Erucic acid, Mixture |
| 40 | 334-48-5 | n-Decanoic acid | Acid | Raincid a | Erucic acid |
| 41 | 4312-99-6 | 1-Octen-3-one | Ketone | Mushroom, metal a | Linoleic acid, Mixture |
| 42 | 1669-44-9 | 3-Octen-2-one | Ketone | Green, fruity b | Linoleic acid, Mixture |
| 43 | 108-83-8 | 2,6-Dimethyl-4-heptanone | Ketone | NF | Control, Stearic acid, Oleic acid, Linoleic acid, Linolenic acid, Erucic acid, Mixture |
| 44 | 3777-69-3 | 2-Pentyl furan | Furan | Fruity, green, sweet f | Oleic acid, Linoleic acid, Linolenic acid, Mixture |
| 45 | 30086-02-3 | (E,E)-3,5-Octadien-2-one | NF | Linolenic acid, Mixture | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, H.U.; Wang, D.; Abdullah; Hasan, M.; Zeng, L.-Y.; Lan, Y.; Shi, Y.; Duan, C.-Q. Interrogating Raisin Associated Unsaturated Fatty Acid Derived Volatile Compounds Using HS–SPME with GC–MS. Foods 2023, 12, 428. https://doi.org/10.3390/foods12030428
Javed HU, Wang D, Abdullah, Hasan M, Zeng L-Y, Lan Y, Shi Y, Duan C-Q. Interrogating Raisin Associated Unsaturated Fatty Acid Derived Volatile Compounds Using HS–SPME with GC–MS. Foods. 2023; 12(3):428. https://doi.org/10.3390/foods12030428
Chicago/Turabian StyleJaved, Hafiz Umer, Dong Wang, Abdullah, Murtaza Hasan, Li-Yan Zeng, Yibin Lan, Ying Shi, and Chang-Qing Duan. 2023. "Interrogating Raisin Associated Unsaturated Fatty Acid Derived Volatile Compounds Using HS–SPME with GC–MS" Foods 12, no. 3: 428. https://doi.org/10.3390/foods12030428





