Production of Cookies Enriched with Bioactive Compounds through the Partial Replacement of Wheat Flour by Cocoa Bean Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cocoa Bean Shell Flour Production and Characterization
2.2.1. Cocoa Bean Shell Flour Production
2.2.2. Proximate Analysis
2.2.3. Total Phenolic and Flavanol Contents
2.2.4. Protein Functionalities
2.3. Cookie Production
Cookie Characterization
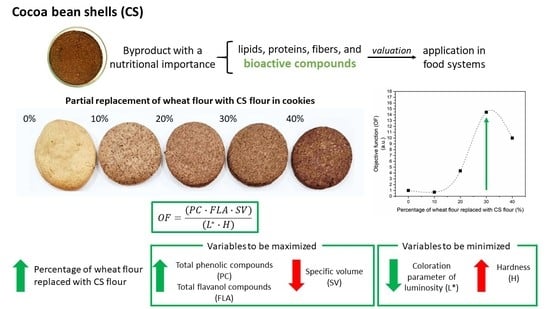
2.4. Optimized Objective Function (OF)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the CS Flour
3.1.1. Chemical Composition of the CS Flour
3.1.2. Total Phenolic and Flavanol Contents
3.1.3. Protein Functionalities
3.2. Characterization of the Cookies
3.2.1. Chemical Composition of the Cookies
3.2.2. Physical Properties of the Cookies
3.2.3. Optimized Objective Function (OF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa Shell and Its Compounds: Applications in the Food Industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Soares, I.D.; Okiyama, D.C.G.; Rodrigues, C.E.D.C. Simultaneous Green Extraction of Fat and Bioactive Compounds of Cocoa Shell and Protein Fraction Functionalities Evaluation. Food Res. Int. 2020, 137, 109622. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of Phenolic Compounds from Cocoa Shell: Modeling Using Response Surface Methodology and Artificial Neural Networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Balentić, J.P.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Cocoa Organization Production of Cocoa Beans 2022. Available online: https://www.icco.org/wp-content/uploads/Production_QBCS-XLVIII-No.-4.pdf (accessed on 19 December 2022).
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Babić, J.; Pajin, B.; Miličević, B.; Guberac, S.; Vrdoljak, A.; Šubarić, D. Cocoa Husk Application in the Enrichment of Extruded Snack Products. J. Food Process. Preserv. 2019, 43, e13866. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [Green Version]
- de Barros, H.E.A.; Natarelli, C.V.L.; Tavares, I.M.d.C.; de Oliveira, A.L.M.; Araújo, A.B.S.; Pereira, J.; Carvalho, E.E.N.; Boas, E.V.d.B.V.; Franco, M. Nutritional Clustering of Cookies Developed with Cocoa Shell, Soy, and Green Banana Flours Using Exploratory Methods. Food Bioprocess Technol. 2020, 13, 1566–1578. [Google Scholar] [CrossRef]
- Botella-Martinez, C.; Lucas-González, R.; Lorenzo, J.M.; Santos, E.M.; Rosmini, M.; Sepúlveda, N.; Teixeira, A.; Sayas-Barberá, E.; Pérez-Alvarez, J.A.; Fernandez-Lopez, J.; et al. Cocoa Coproducts-Based and Walnut Oil Gelled Emulsion as Animal Fat Replacer and Healthy Bioactive Source in Beef Burgers. Foods 2021, 10, 2706. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized Liquid Extraction of Flavanols and Alkaloids from Cocoa Bean Shell Using Ethanol as Solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of Cacao Pod Husk through Supercritical Fluid Extraction of Phenolic Compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Toda, T.A.; Oliveira, A.L.; Rodrigues, C.E.C. Effect of the Temperature on the Kinetics of Cocoa Bean Shell Fat Extraction Using Pressurized Ethanol and Evaluation of the Lipid Fraction and Defatted Meal. Ind. Crop. Prod. 2019, 130, 96–103. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa Bean Shell Waste Valorisation; Extraction from Lab to Pilot-Scale Cavitational Reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Barišić, V.; Petrović, J.; Lončarević, I.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Blažić, M.; Ačkar, Ð. Physical Properties of Chocolates Enriched with Untreated Cocoa Bean Shells and Cocoa Bean Shells Treated with High-Voltage Electrical Discharge. Sustainability 2021, 13, 2620. [Google Scholar] [CrossRef]
- Barišić, V.; Stokanović, M.C.; Flanjak, I.; Doko, K.; Jozinović, A.; Babić, J.; Šubarić, D.; Miličević, B.; Cindrić, I.; Ačkar, Đ. Cocoa Shell as a Step Forward to Functional Chocolates—Bioactive Components in Chocolates with Different Composition. Molecules 2020, 25, 5470. [Google Scholar] [CrossRef]
- de Barros, H.E.A.; Natarelli, C.V.L.; de Abreu, D.J.M.; de Oliveira, A.L.M.; Lago, R.C.D.; Dias, L.L.d.C.; de Carvalho, E.E.N.; Bilal, M.; Ruiz, H.A.; Franco, M.; et al. Application of Chemometric Tools in the Development of Food Bars Based on Cocoa Shell, Soy Flour and Green Banana Flour. Int. J. Food Sci. Technol. 2021, 56, 5296–5304. [Google Scholar] [CrossRef]
- Handojo, L.; Triharyogi, H.; Indarto, A. Cocoa Bean Shell Waste as Potential Raw Material for Dietary Fiber Powder. Int. J. Recycl. Org. Waste Agric. 2019, 8, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Orden, D.; Stévigny, C.; Zeppa, G.; Bertolino, M. Physical Properties and Consumer Evaluation of Cocoa Bean Shell-Functionalized Biscuits Adapted for Diabetic Consumers by the Replacement of Sucrose with Tagatose. Foods 2020, 9, 814. [Google Scholar] [CrossRef]
- ASAE Method of Determining and Expressing Fineness of Feed Materials by Sieving 1995, S319.2. Available online: https://dokumen.tips/documents/asae-s3193-method-of-determining-and-expressing-fineness-of-feed-materials-by-sieving.html?page=1 (accessed on 10 January 2023).
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 3rd ed.; AOCS Press: Champaign, IL, USA, 1998. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and Validation of a Quantitative Liquid Chromatography—Tandem Mass Spectrometric Method Covering 295 Bacterial and Fungal Metabolites Including All Regulated Mycotoxins in Four Model Food Matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2009. [Google Scholar]
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of Procyanidins in Cocoa (Theobroma Cacao) and Chocolate Using High-Performance Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 1999, 47, 490–496. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.-P.; MacIà, A.; Reguant, J.; Anglès, N.; Morelló, J.-R.; Motilva, M.-J. Obtention and Characterization of Phenolic Extracts from Different Cocoa Sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Cuevas, M.S.; de Souza, P.T.; Rodrigues, C.E.d.C.; Meirelles, A.J.A. Quantification and Determination of Composition of Steryl Ferulates in Refined Rice Bran Oils Using an UPLC-MS Method. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 375–385. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Stuart, D.A.; Ou, B.; Fan, E.; Ji, H.; Kou, Y. Determination of Total Procyanidins in Selected Chocolate and Confectionery Products Using DMAC. J. AOAC In. 2010, 93, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Van Buren, J.P.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of Different Alcoholic Extraction Conditions on Soybean Oil Yield, Fatty Acid Composition and Protein Solubility of Defatted Meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Pareyt, B.; Delcour, J.A. The Role of Wheat Flour Constituents, Sugar, and Fat in Low Moisture Cereal Based Products: A Review on Sugar-Snap Cookies. Crit. Rev. Food Sci. Nutr. 2008, 48, 824–839. [Google Scholar] [CrossRef]
- Das Chagas, E.G.L.; Vanin, F.M.; Garcia, V.A.d.S.; Yoshida, C.M.P.; de Carvalho, R.A. Enrichment of Antioxidants Compounds in Cookies Produced with Camu-Camu (Myrciaria Dubia) Coproducts Powders. LWT 2021, 137, 110472. [Google Scholar] [CrossRef]
- Smewing, J. AACCI Approved Methods Technical Committee Report on the Collaborative Study for a New AACCI Method (10-16.01): Volumetric and Dimensional Profile Determination of Baked Products Using Laser Topography—VolScan Profiler Method. Cereal Foods World 2016, 61, 18–23. [Google Scholar] [CrossRef] [Green Version]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; International, M.A., Ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Bárcenas, M.E.; Rosell, C.M. Effect of HPMC Addition on the Microstructure, Quality and Aging of Wheat Bread. Food Hydrocoll. 2005, 19, 1037–1043. [Google Scholar] [CrossRef]
- De Castro, L.A.; Lizi, J.M.; Das Chagas, E.G.L.; De Carvalho, R.A.; Vanin, F.M. From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy. Foods 2020, 9, 593. [Google Scholar] [CrossRef]
- Rodrigues, C.E.D.C.; Oliveira, R. Response Surface Methodology Applied to the Analysis of Rice Bran Oil Extraction Process with Ethanol. Int. J. Food Sci. Technol. 2010, 45, 813–820. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple F and Multiple “F” Test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Arlorio, M.; Coïsson, J.D.; Travaglia, F.; Varsaldi, F.; Miglio, G.; Lombardi, G.; Martelli, A. Antioxidant and Biological Activity of Phenolic Pigments from Theobroma Cacao Hulls Extracted with Supercritical CO2. Food Res. Int. 2005, 38, 1009–1014. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary Fibre Composition, Antioxidant Capacity and Physico-Chemical Properties of a Fibre-Rich Product from Cocoa (Theobroma Cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Pereira, J.L.; Lemes, D.P.; Nakano, F.; Taniwaki, M.H. Determination of Aflatoxins in By-Products of Industrial Processing of Cocoa Beans. Food Addit. Contam. Part A Chem. Anal. Control. Exp. Risk Assess. 2012, 29, 972–978. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Nester, M.A.; Efraim, P.; Taniwaki, M.H. Occurrence of Ochratoxin A in Cocoa By-Products and Determination of Its Reduction during Chocolate Manufacture. Food Chem. 2013, 136, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Coulibaly, A.; Biego, G.H.M.; Dembélé, A.; Bohoussou, K.M.; Toure, A. Cocoa Beans and Cocoa Derivatives from Cote-D’Ivoire: Investigating Ochratoxin A Level and Assessing Dietary Intake Adults. Sustain. Agric. Res. 2012, 2, 173. [Google Scholar] [CrossRef] [Green Version]
- ANVISA. Instrução Normativa—IN N° 88, de 26 de Março de 2021. Available online: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-in-n-88-de-26-de-marco-de-2021-311655598 (accessed on 24 November 2022).
- European Commission. Regulamento (CE) n. o 1881/2006 Da Comissão, de 19 de Dezembro de 2006. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=celex:32006R1881 (accessed on 25 November 2022).
- Duda-Chodak, A.; Blaszczyk, U. The Impact of Nickel on Human Health. J. Elem. 2008, 13, 685–696. [Google Scholar]
- Ahamed, M.; Siddiqui, M.K.J. Environmental Lead Toxicity and Nutritional Factors. Clin. Nutr. 2007, 26, 400–408. [Google Scholar] [CrossRef]
- Bernard, A. Cadmium & Its Adverse Effects on Human Health. Indian J. Med. Res. 2008, 128, 55–564. [Google Scholar]
- Maji, P.; Mitra, R.; Ray, K.K. Effect of Cr on the Evolution of Microstructures in As-Cast Ternary Niobium-Silicide-Based Composites. Intermetallics 2017, 85, 34–47. [Google Scholar] [CrossRef]
- Dico, G.M.L.; Galvano, F.; Dugo, G.; D’ascenzi, C.; Macaluso, A.; Vella, A.; Giangrosso, G.; Cammilleri, G.; Ferrantelli, V. Toxic Metal Levels in Cocoa Powder and Chocolate by ICP-MS Method after Microwave-Assisted Digestion. Food Chem. 2018, 245, 1163–1168. [Google Scholar] [CrossRef]
- Kruszewski, B.; Obiedziński, M.W.; Kowalska, J. Nickel, Cadmium and Lead Levels in Raw Cocoa and Processed Chocolate Mass Materials from Three Different Manufacturers. J. Food Compos. Anal. 2018, 66, 127–135. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Lucas-González, R.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Martuscelli, M.; Chaves-López, C. Bioactive Compounds and Techno-Functional Properties of High-Fiber Co-Products of the Cacao Agro-Industrial Chain. Heliyon 2021, 7, e06799. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Nsor-Atind, J.; Zhong, F.; Mothibe, K.J.; Bangoura, M.L.; Lagnika, C. Quantification of Total Polyphenolic Content and Antimicrobial Activity of Cocoa (Theobroma Cacao L.) Bean Shells. Pak. J. Nutr. 2012, 11, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Kim, C.; Neilson, A.P.; Griffin, L.E.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Comparison of Common Analytical Methods for the Quantification of Total Polyphenols and Flavanols in Fruit Juices and Ciders. J. Food Sci. 2019, 84, 2147–2158. [Google Scholar] [CrossRef] [Green Version]
- Bonvehí, J.S.; Coll, F.V. Protein Quality Assessment in Cocoa Husk. Food Res. Int. 1999, 32, 201–208. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P. Improved Solubility and Emulsification of Wet-Milled Corn Germ Protein Recovered by Ultrafiltration—Diafiltration. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1623–1631. [Google Scholar] [CrossRef]
- Chandi, G.K.; Sogi, D.S. Functional Properties of Rice Bran Protein Concentrates. J. Food Eng. 2007, 79, 592–597. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Vojvodić, A.; Bušić, A.; Keppler, J.; Steffen-Heins, A.; Komes, D. Encapsulation Templated Approach to Valorization of Cocoa Husk, Poppy and Hemp Macrostructural and Bioactive Constituents. Ind. Crop. Prod. 2018, 112, 402–411. [Google Scholar] [CrossRef]
- Rosell, C.M. Enzymatic Manipulation of Gluten-Free Breads. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Greco, E.; Winquist, A.; Lee, T.J.; Collins, S.; Lebovic, Z.; Zerbe-Kessinger, T.; Mihan, A.J. The Role of Source of Protein in Regulation of Food Intake, Satiety, Body Weight and Body Composition. J. Nutr. Health Food Eng. 2017, 6, 186–193. [Google Scholar] [CrossRef] [Green Version]
- ANVISA. Resolução Da Diretoria Colegiada—RDC No 54, de 12 de Novembro de 2012. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0054_12_11_2012.html (accessed on 6 December 2022).
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Ktenioudaki, A.; Gallagher, E. Recent Advances in the Development of High-Fibre Baked Products. Trends Food Sci. Technol. 2012, 28, 4–14. [Google Scholar] [CrossRef]
- Jan, K.N.; Panesar, P.S.; Singh, S. Optimization of Antioxidant Activity, Textural and Sensory Characteristics of Gluten-Free Cookies Made from Whole Indian Quinoa Flour. Lwt 2018, 93, 573–582. [Google Scholar] [CrossRef]
- Guiné, R.P.F. Textural Properties of Bakery Products: A Review of Instrumental and Sensory Evaluation Studies. Appl. Sci. 2022, 12, 8628. [Google Scholar] [CrossRef]



| di (µm) | di + 1 (µm) | CS Flour Mass (g) | Frequency (%) | Average Particle Size (µm) |
|---|---|---|---|---|
| 1680 | 2280 | 0.74 ± 0.04 | 2.9 ± 0.2 | 303 ± 42 |
| 1190 | 1680 | 0.78 ± 0.07 | 2.9 ± 0.3 | |
| 841 | 1190 | 2.06 ± 0.03 | 8.04 ± 0.05 | |
| 595 | 841 | 2.5 ± 0.3 | 10 ± 1 | |
| 420 | 595 | 8.2 ± 0.6 | 32 ± 3 | |
| 297 | 420 | 5 ± 1 | 19 ± 5 | |
| <297 | 297 | 6 ± 2 | 25 ± 6 |
| Characterization | Wheat Flour | CS Flour | Cookie Formulation | ||||
|---|---|---|---|---|---|---|---|
| C0 | C10 | C20 | C30 | C40 | |||
| Moisture (mass %) | n.d. | 7.6 ± 0.1 B | 7.7 ± 0.4 B | 8.5 ± 0.3 A | 8.6 ± 0.3 A | 8.6 ± 0.3 A | 8.3 ± 0.3 A |
| Lipid a (mass %) | n.d. | 6.6 ± 0.3 BCD | 6.4 ± 0.3 CD | 6.0 ± 0.5 D | 7.1 ± 0.3 BC | 7.3 ± 0.5 B | 8.3 ± 0.3 A |
| Proteins a,b (mass %) | 15.43 ± 0.08 A | 16.0 ± 0.4 A | 9.5 ± 0.4 C | 10.1 ± 0.4 BC | 9.9 ± 0.7 BC | 9.8 ± 0.1 BC | 10.7 ± 0.5 B |
| PC (mg GAE/g sample) | n.d. | 10.8 ± 0.1 A | 0.68 ± 0.04 F | 1.05 ± 0.05 E | 1.44 ± 0.08 D | 2.1 ± 0.1 C | 2.37 ± 0.07 B |
| FLA (mg EE/g sample) | n.d. | 1.38 ± 0.05 A | 0.105 ± 0.004 C | 0.121 ± 0.003 C | 0.13 ± 0.01 C | 0.170 ± 0.005 B | 0.19 ± 0.01 B |
| Aflatoxins (B1, B2, G1, and G2) | n.d. | <LOQ c | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ochratoxin A (OTA) (μg/kg) | n.d. | 1.2 ± 0.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cadmium (Cd) (mg/kg) | n.d. | 0.177 ± 0.001 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Chromium (Cr) (mg/kg) | n.d. | 2.2 ± 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Nickel (Ni) (mg/kg) | n.d. | 7.89 ± 0.05 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lead (Pb) (mg/kg) | n.d. | 0.355 ± 0.001 | n.d. | n.d. | n.d. | n.d. | n.d. |
| NSI (%) in water | 87 ± 4 A | 38 ± 1 B | n.d. | n.d. | n.d. | n.d. | n.d. |
| NSI (%) in 0.1 M NaCl solution | 86 ± 5 A | 40 ± 5 B | n.d. | n.d. | n.d. | n.d. | n.d. |
| WAC (g of water/g sample) | 0.86 ± 0.01 B | 5.6 ± 0.4 A | n.d. | n.d. | n.d. | n.d. | n.d. |
| OAC (g of oil/g sample) | 0.954 ± 0.001 B | 2.0 ± 0.2 A | n.d. | n.d. | n.d. | n.d. | n.d. |
| EAI (m2/g) | n.d. | 60 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. |
| ESI (%) d | n.d. | 81 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Characterization | Cookie Formulation | |||||
|---|---|---|---|---|---|---|
| C0 | C10 | C20 | C30 | C40 | ||
| Coloration | L* | 64 ± 1 A | 50 ± 1 B | 43 ± 1 C | 40 ± 1 D | 36 ± 2 E |
| a* | 7.1 ± 0.6 B | 7.2 ± 0.2 B | 8.4 ± 0.4 A | 8.8 ± 0.4 A | 9.0 ± 0.5 A | |
| b* | 27.0 ± 0.5 A | 18 ± 1 B | 16.1 ± 0.6 C | 14.8 ± 0.3 D | 13.8 ± 0.7 D | |
| °Hue | 75 ± 1 A | 67.8 ± 0.8 B | 64 ± 1 C | 61.6 ± 0.6 D | 59 ± 1 E | |
| Spreading factor | 5.6 ± 0.7 B | 5.9 ± 0.5 AB | 6.0 ± 0.4 AB | 6.1 ± 0.3 AB | 6.7 ± 0.3 A | |
| Specific volume (mL/g) | 1.36 ± 0.06 A | 1.17 ± 0.05 BC | 1.26 ± 0.04 AB | 1.19 ± 0.06 BC | 1.10 ± 0.07 C | |
| Hardness (N) | 353 ± 25 B | 432 ± 46 A | 452 ± 32 A | 451 ± 41 A | 472 ± 76 A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, I.D.; Cirilo, M.E.M.; Junqueira, I.G.; Vanin, F.M.; Rodrigues, C.E.d.C. Production of Cookies Enriched with Bioactive Compounds through the Partial Replacement of Wheat Flour by Cocoa Bean Shells. Foods 2023, 12, 436. https://doi.org/10.3390/foods12030436
Soares ID, Cirilo MEM, Junqueira IG, Vanin FM, Rodrigues CEdC. Production of Cookies Enriched with Bioactive Compounds through the Partial Replacement of Wheat Flour by Cocoa Bean Shells. Foods. 2023; 12(3):436. https://doi.org/10.3390/foods12030436
Chicago/Turabian StyleSoares, Ingrid Denardi, Marcela Eduarda Marchi Cirilo, Isabela Gayola Junqueira, Fernanda Maria Vanin, and Christianne Elisabete da Costa Rodrigues. 2023. "Production of Cookies Enriched with Bioactive Compounds through the Partial Replacement of Wheat Flour by Cocoa Bean Shells" Foods 12, no. 3: 436. https://doi.org/10.3390/foods12030436
APA StyleSoares, I. D., Cirilo, M. E. M., Junqueira, I. G., Vanin, F. M., & Rodrigues, C. E. d. C. (2023). Production of Cookies Enriched with Bioactive Compounds through the Partial Replacement of Wheat Flour by Cocoa Bean Shells. Foods, 12(3), 436. https://doi.org/10.3390/foods12030436