Effect of Cooking Method and Doneness Degree on Volatile Compounds and Taste Substance of Pingliang Red Beef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Different Cooking Methods of Pingliang Red Beef
2.3. HS-GC-IMS Analysis
2.4. Fatty Acid Analysis
2.5. Nucleotide-Related Compound Determination
2.6. Equivalent Umami Concentration (EUC)
2.7. Free Amino Acid Determination
2.8. Statistical Analysis
3. Results and Discussion
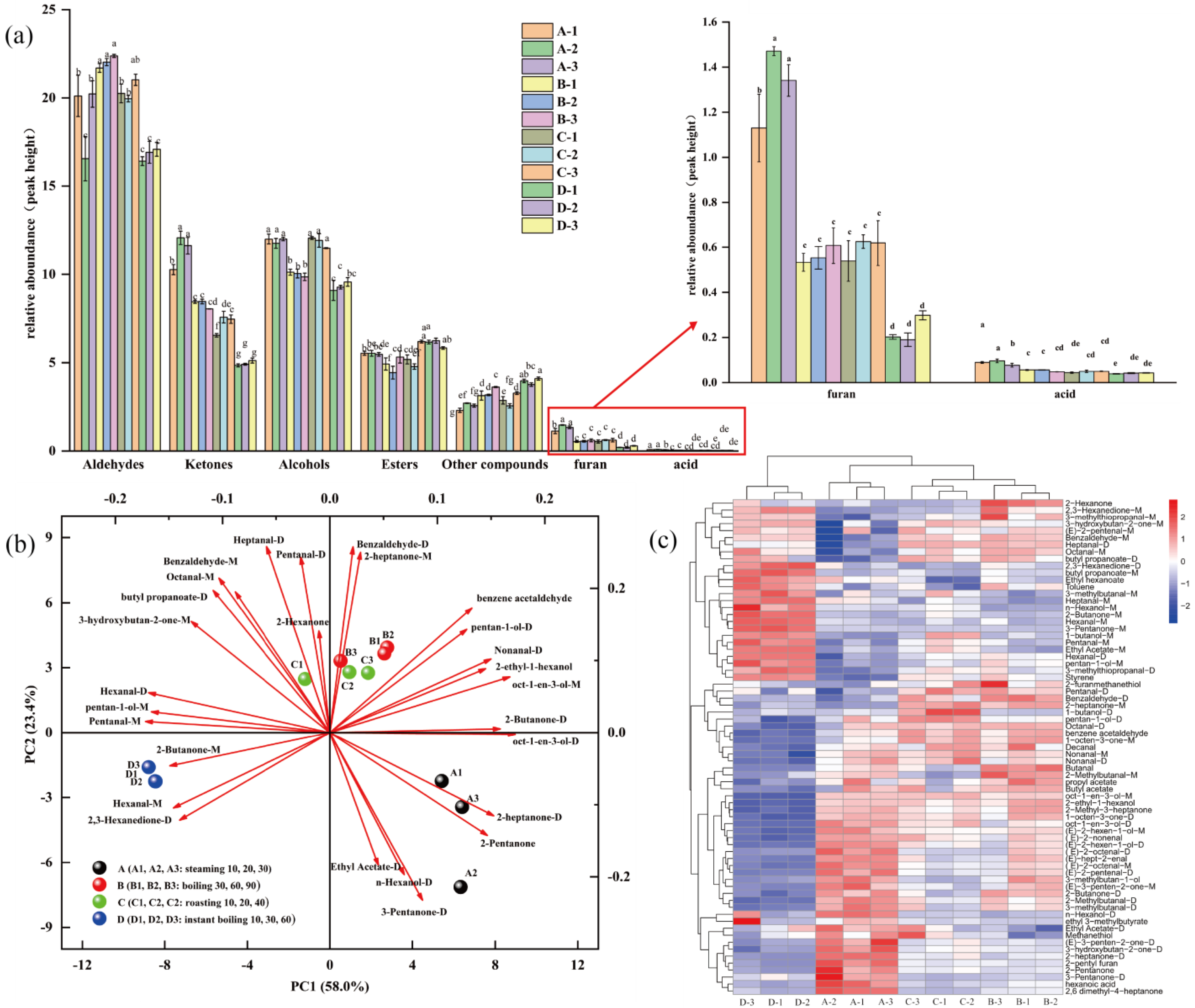
3.1. GC-IMS Fingerprint Analysis of Pingliang Red Beef under Different Cooking Methods
3.2. Volatile Compound Variation in Steaming, Roasting, Boiling, and Instant Boiling of Pingliang Red Beef
| Compound | CAS | Formula | MW | RI (s) | Dt | Sensory Descriptions [46] | |
|---|---|---|---|---|---|---|---|
| Aldehydes | Decanal | C112312 | C10H20O | 156.3 | 1277.1 | 1.53797 | Fresh grease, fruity, soapy |
| (E)-2-nonenal | C18829566 | C9H16O | 140.2 | 1188.1 | 1.4107 | Intense floral, fruity and oily aromas, soapy | |
| Nonanal-M | C124196 | C9H18O | 142.2 | 1109.1 | 1.47344 | Floral, aldehyde-like, citrus, soapy, fried fragrant, roasted fragrant | |
| Nonanal-D | C124196 | C9H18O | 142.2 | 1108.1 | 1.94666 | ||
| (E)-2-octenal-M | C2548870 | C8H14O | 126.2 | 1054.7 | 1.33541 | Meaty, nutty, grease, cucumber flavor, umami | |
| (E)-2-octenal-D | C2548870 | C8H14O | 126.2 | 1054.7 | 1.82835 | ||
| Octanal-M | C124130 | C8H16O | 128.2 | 1008.6 | 1.40712 | Grease, citrus, soapy | |
| Octanal-D | C124130 | C8H16O | 128.2 | 1004.5 | 1.82656 | ||
| Benzaldehyde-M | C100527 | C7H6O | 106.1 | 956.7 | 1.15172 | Almond, burnt sugar, sweet | |
| Benzaldehyde-D | C100527 | C7H6O | 106.1 | 957.1 | 1.47171 | ||
| benzene acetaldehyde | C122781 | C8H8O | 120.2 | 1039.3 | 1.25813 | Sweet, intense floral | |
| (E)-hept-2-enal | C18829555 | C7H12O | 112.2 | 952.3 | 1.67411 | ||
| Hexanal-M | C66251 | C6H12O | 100.2 | 791.9 | 1.25392 | Grassy, green, fresh, tallow, fat | |
| Hexanal-D | C66251 | C6H12O | 100.2 | 787.2 | 1.56558 | ||
| Heptanal-M | C111717 | C7H14O | 114.2 | 898.2 | 1.32579 | Grassy, green, fat, | |
| Heptanal-D | C111717 | C7H14O | 114.2 | 898.9 | 1.69956 | ||
| 2-Methylbutanal-D | C96173 | C5H10O | 86.1 | 654.4 | 1.40175 | Almond, chocolate, cocoa, fermented, hazelnut | |
| 2-Methylbutanal-M | C96173 | C5H10O | 86.1 | 652.8 | 1.15776 | ||
| 3-methylthiopropanal-M | C3268493 | C4H8OS | 104.2 | 905.2 | 1.08897 | ||
| 3-methylthiopropanal-D | C3268493 | C4H8OS | 104.2 | 904.7 | 1.40189 | ||
| Pentanal-M | C110623 | C5H10O | 86.1 | 693.4 | 1.18321 | Almond, chemical, green, malt, oil | |
| Pentanal-D | C110623 | C5H10O | 86.1 | 692.1 | 1.43008 | ||
| Butanal | C123728 | C4H8O | 72.1 | 540.3 | 1.28464 | Banana, green, pungent | |
| (E)-2-pentenal-D | C1576870 | C5H8O | 84.1 | 745.3 | 1.35813 | apple, floral, green, strawberry, tomato | |
| (E)-2-pentenal-M | C1576870 | C5H8O | 84.1 | 744.8 | 1.10603 | ||
| 3-methylbutanal-D | C590863 | C5H10O | 86.1 | 638.4 | 1.41016 | Almond, cheese, chocolate, malt | |
| 3-methylbutanal-M | C590863 | C5H10O | 86.1 | 635.1 | 1.17318 | ||
| Ketones | 2-Methyl-3-heptanone | C13019200 | C8H16O | 128.2 | 1085.8 | 1.27626 | |
| 1-octen-3-one-M | C4312996 | C8H14O | 126.2 | 979.4 | 1.27092 | Boiled mushroom, earth, green, metal, mushroom | |
| 1-octen-3-one-D | C4312996 | C8H14O | 126.2 | 978.7 | 1.68592 | ||
| 2-Pentanone | C107879 | C5H10O | 86.1 | 676.5 | 1.37584 | Burnt plastic, ether, fruit, kerosine, orange peel | |
| 2-Butanone-D | C78933 | C4H8O | 72.1 | 566.8 | 1.24553 | Butterscotch, ether, fragrant, fruit, pleasant | |
| 2-Butanone-M | C78933 | C4H8O | 72.1 | 565.1 | 1.06071 | ||
| 2-heptanone-M | C110430 | C7H14O | 114.2 | 891.8 | 1.25627 | Bell pepper, blue cheese, cinnamon, green, nut | |
| 2-heptanone-D | C110430 | C7H14O | 114.2 | 890.1 | 1.63201 | ||
| 2,3-Hexanedione-M | C3848246 | C6H10O2 | 114.1 | 778.3 | 1.09198 | ||
| 2,3-Hexanedione-D | C3848246 | C6H10O2 | 114.1 | 772.7 | 1.35966 | ||
| (E)-3-penten-2-one-D | C3102338 | C5H8O | 84.1 | 731.8 | 1.35105 | ||
| (E)-3-penten-2-one-M | C3102338 | C5H8O | 84.1 | 734.2 | 1.09263 | ||
| 2-Hexanone | C591786 | C6H12O | 100.2 | 779.5 | 1.18752 | Ether | |
| 2,6-dimethyl-4-heptanone | C108838 | C9H18O | 142.2 | 967.8 | 1.32856 | ||
| 3-Pentanone-D | C96220 | C5H10O | 86.1 | 692.6 | 1.35946 | Ether, sweet | |
| 3-Pentanone-M | C96220 | C5H10O | 86.1 | 693.2 | 1.10836 | ||
| Alcohols | 2-ethyl-1-hexanol | C104767 | C8H18O | 130.2 | 1026.9 | 1.26013 | Citrus, green, oil, rose |
| oct-1-en-3-ol-M | C3391864 | C8H16O | 128.2 | 986 | 1.15975 | earth, fat, floral, green, herb | |
| oct-1-en-3-ol-D | C3391864 | C8H16O | 128.2 | 979.4 | 1.60406 | ||
| n-Hexanol-M | C111273 | C6H14O | 102.2 | 861.9 | 1.32497 | Bread, flower, fruit, green, herb | |
| n-Hexanol-D | C111273 | C6H14O | 102.2 | 861.9 | 1.6385 | ||
| (E)-2-hexen-1-ol-M | C928950 | C6H12O | 100.2 | 846 | 1.18518 | Bell pepper, fat, geranium, green, spice | |
| (E)-2-hexen-1-ol-D | C928950 | C6H12O | 100.2 | 844 | 1.51752 | ||
| pentan-1-ol-M | C71410 | C5H12O | 88.1 | 762.4 | 1.25392 | Balsamic, fruit, green, medicine, yeast | |
| pentan-1-ol-D | C71410 | C5H12O | 88.1 | 764.4 | 1.51286 | ||
| 3-methylbutan-1-ol | C123513 | C5H12O | 88.1 | 727.3 | 1.48806 | Banana, cocoa, floral, fruit, fusel | |
| 3-hydroxybutan-2-one-M | C513860 | C4H8O2 | 88.1 | 712.8 | 1.05627 | Butter, cream | |
| 3-hydroxybutan-2-one-D | C513860 | C4H8O2 | 88.1 | 713.2 | 1.33282 | ||
| 1-butanol-M | C71363 | C4H10O | 74.1 | 654.3 | 1.1823 | Alcohol, fruit, medicine | |
| 1-butanol-D | C71363 | C4H10O | 74.1 | 653 | 1.37683 | ||
| Methanethiol | C74931 | CH4S | 48.1 | 441.9 | 1.0483 | Cabbage, garlic, gasoline, putrid, sulfur | |
| 2-furanmethanethiol | C98022 | C5H6OS | 114.2 | 912.8 | 1.11099 | Burnt, coffee, green, metal, roast | |
| Esters | Ethyl hexanoate | C123660 | C8H16O2 | 144.2 | 999.5 | 1.34258 | Apple peel, banana, brandy, cheese, overripe fruit |
| Ethyl Acetate-D | C141786 | C4H8O2 | 88.1 | 601.1 | 1.34402 | Balsamic, fruit, grape, pineapple | |
| Ethyl Acetate-M | C141786 | C4H8O2 | 88.1 | 599.1 | 1.0987 | ||
| butyl propanoate-M | C590012 | C7H14O2 | 130.2 | 907.3 | 1.28334 | Red fruit, strawberry | |
| butyl propanoate-D | C590012 | C7H14O2 | 130.2 | 907.1 | 1.72743 | ||
| Ethyl 3-methylbutyrate | C108645 | C7H14O2 | 130.2 | 842.7 | 1.65769 | Apple, citrus, pineapple, sour, sweet | |
| propyl acetate | C109604 | C5H10O2 | 102.1 | 703.6 | 1.47843 | Celery, floral, pear, red fruit | |
| Butyl acetate | C123864 | C6H12O2 | 116.2 | 816.1 | 1.61605 | Apple, banana, pungent, sweet | |
| Furan | 2-pentylfuran | C3777693 | C9H14O | 138.2 | 994.1 | 1.2552 | Floral, fruit, green, green bean |
| acid | hexanoic acid | C142621 | C6H12O2 | 116.2 | 994.1 | 1.30503 | Acid, cheese, goat, pungent, rancid |
| Hydrocarbons | Styrene | C100425 | C8H8 | 104.2 | 887.9 | 1.41987 | Balsamic, gasoline, plastic, rubber, solvent |
| Toluene | C108883 | C7H8 | 92.1 | 760.5 | 1.01492 | Bitter almond, glue, paint, solvent |
3.3. Nonvolatile Compounds
3.3.1. Fatty Acid
3.3.2. Nucleotide-Related Compounds
3.3.3. Free Amino Acids
3.3.4. Correlation between Non-Volatile Compounds and Umami-Taste Characteristics of Free Amino Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Ding, Y.C.; Gu, S.Q.; Zhu, S.C.; Zhou, X.X.; Ding, Y.T. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food. Res. Int. 2020, 137, 109339. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Wan, W.; Du, X.; Kong, B.; Liu, Q.; Lv, H.; Xia, X.; Li, F. Mechanisms of Change in Emulsifying Capacity Induced by Protein Denaturation and Aggregation in Quick-Frozen Pork Patties with Different Fat Levels and Freeze-Thaw Cycles. Foods 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Ellies-Oury, M.P.; Chriki, S.; Legrand, I.; Pogorzelski, G.; Wierzbicki, J.; Farmer, L.; Troy, D.; Polkinghorne, R.; Hocquette, J.F. Contributions of tenderness, juiciness and flavor liking to overall liking of beef in Europe. Meat Sci. 2020, 168, 108190. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.Q.; Wang, H.B.; Xi, B.; He, X.N.; Yang, X.L.; Li, W.H. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.Y.; Zhou, G.H.; Li, L.; Xu, X.L.; Yu, X.B.; Bai, Y.; Li, C.B. Effect of Cooking on in Vitro Digestion of Pork Proteins: A Peptidomic Perspective. J. Agric. Food Chem. 2015, 63, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, Y.; Jin, G.; Duan, J.; Zhang, Y.; Cao, J. The Texture Change of Chinese Traditional Pig Trotter with Soy Sauce during Stewing Processing: Based on a Thermal Degradation Model of Collagen Fibers. Foods 2022, 11, 1772. [Google Scholar] [CrossRef]
- Roldan, M.; Loebner, J.; Degen, J.; Henle, T.; Antequera, T.; Ruiz-Carrascal, J. Advanced glycation end products, physico-chemical and sensory characteristics of cooked lamb loins affected by cooking method and addition of flavour precursors. Food Chem. 2015, 168, 487–495. [Google Scholar] [CrossRef]
- Reblova, Z.; Aubourg, S.P.; Pokorny, J. The Effect of Different Freshness of Raw Material on Lipid Quality and Sensory Acceptance of Canned Sardines. Foods 2022, 11, 1987. [Google Scholar] [CrossRef]
- Xu, J.J.; Zhang, M.; Wang, Y.C.A.; Bhandari, B. Novel Technologies for Flavor Formation in the Processing of Meat Products: A Review. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Bi, S.; Wang, A.J.D.; Lao, F.; Shen, Q.; Liao, X.J.; Zhang, P.Z.; Wu, J.H. Effects of frying, roasting and boiling on aroma profiles of adzuki beans (Vigna angularis) and potential of adzuki bean and millet flours to improve flavor and sensory characteristics of biscuits. Food Chem. 2021, 339, 127878. [Google Scholar] [CrossRef]
- Devaere, J.; De Winne, A.; Dewulf, L.; Fraeye, I.; Soljic, I.; Lauwers, E.; de Jong, A.; Sanctorum, H. Improving the Aromatic Profile of Plant-Based Meat Alternatives: Effect of Myoglobin Addition on Volatiles. Foods 2022, 11, 1985. [Google Scholar] [CrossRef]
- Aliani, M.; Farmer, L.J. Precursors of chicken flavor. II. Identification of key flavor precursors using sensory methods. J. Agric. Food Chem. 2005, 53, 6455–6462. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products a review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xi, L.; Zhang, J.; Wu, R.; Wang, T.; Ding, W. Characterization of the Volatile Compounds of Zhenba Bacon at Different Process Stages Using GC-MS and GC-IMS. Foods 2021, 10, 2869. [Google Scholar] [CrossRef]
- Cerny, C.; Grosch, W. Evaluation of potent odorants in roasted beef by aroma extract dilution analysis. Eur. Food Res. Technol. 1992, 194, 322–325. [Google Scholar] [CrossRef]
- Gasser, U.; Grosch, W. Primary odorants of chicken broth: A comparative study with meat broths from cow and ox. Z. Lebensm. Unters. Forsch. 1990, 190, 3–8. [Google Scholar] [CrossRef]
- Zou, Y.; Kang, D.; Liu, R.; Qi, J.; Zhou, G.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochem. 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chen, H.T.; Sun, B.G. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chem. 2020, 320, 126656. [Google Scholar] [CrossRef]
- Rotola-Pukkila, M.K.; Pihlajaviita, S.T.; Kaimainen, M.T.; Hopia, A.I. Concentration of Umami Compounds in Pork Meat and Cooking Juice with Different Cooking Times and Temperatures. J. Food. Sci. 2015, 80, C2711–C2716. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Dong, S.C.; Li, F. A system dynamics model for analyzing the eco-agriculture system with policy recommendations. Ecol. Model. 2012, 227, 34–45. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano Essential Oils Promote Rumen Digestive Ability by Modulating Epithelial Development and Microbiota Composition in Beef Cattle. Front. Nutr. 2021, 8, 722557. [Google Scholar] [CrossRef] [PubMed]
- GB/T 19477-2018; Operating Procedure of Livestock and Poultry Slaughtering–Cattle. Standards Press of China: Beijing, China, 2018.
- Xiao, Z.; Luo, Y.; Wang, G.; Ge, C.; Zhou, G.; Zhang, W.; Liao, G. 1 H NMR-based water-soluble lower molecule characterization and fatty acid composition of boiled Wuding chicken during processing. J. Sci. Food Agric. 2018, 99, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Jayasena, D.D.; Jung, S.; Kim, H.J.; Bae, Y.S.; Yong, H.I.; Lee, J.H.; Kim, J.G.; Jo, C. Comparison of quality traits of meat from korean native chickens and broilers used in two different traditional korean cuisines. Asian Austral. J. Anim. 2013, 26, 1038–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Liu, D.-Y.; Zhou, G.-H.; Xu, X.-L. Characteristic Flavor of Traditional Soup Made by Stewing Chinese Yellow-Feather Chickens. J. Food Sci. 2017, 82, 2031–2040. [Google Scholar] [CrossRef]
- Sabikun, N.; Bakhsh, A.; Rahman, M.S.; Hwang, Y.-H.; Joo, S.-T. Volatile and nonvolatile taste compounds and their correlation with umami and flavor characteristics of chicken nuggets added with milkfat and potato mash. Food Chem. 2020, 343, 128499. [Google Scholar] [CrossRef]
- Bi, J.; Lin, Z.; Li, Y.; Chen, F.; Liu, S.; Li, C. Effects of different cooking methods on volatile flavor compounds of chicken breast. J. Food Biochem. 2021, 45, e13770. [Google Scholar] [CrossRef]
- Qian, M.; Zheng, M.; Zhao, W.; Liu, Q.; Zeng, X.; Bai, W. Effect of marinating and frying on the flavor of braised pigeon. J. Food Process. Pres. 2021, 45, e15219. [Google Scholar] [CrossRef]
- Sproston, M.J.; Akoh, C.C. Antioxidative Effects of a Glucose-Cysteine Maillard Reaction Product on the Oxidative Stability of a Structured Lipid in a Complex Food Emulsion. J. Food Sci. 2016, 81, C2923–C2931. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Liu, D.C.; Chen, M.T. The Effect of Roasting Temperature on the Formation of Volatile Compounds in Chinese-Style Pork Jerky. Asian Austral. J. Anim. 2002, 15, 427–431. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Hayat, K.; Liu, P.; Jia, C.; Xia, S.; Xiao, Z.; Tian, H.; Niu, Y. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 2011, 124, 203–209. [Google Scholar] [CrossRef]
- Rabe, S.; Krings, U.; Berger, R.G. Influence of oil-in-water emulsion characteristics on initial dynamic flavour release. J. Sci. Food Agric. 2003, 83, 1124–1133. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- Duan, Z.; Dong, S.; Dong, Y.; Gao, Q. Geographical origin identification of two salmonid species via flavor compound analysis using headspace-gas chromatography-ion mobility spectrometry combined with electronic nose and tongue. Food Res. Int. 2021, 145, 110385. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kitabayashi, K.; Fukusima, C.; Sugiyama, S.; Hashimoto, T. Effect of storage period before reheating on the volatile compound composition and lipid oxidation of steamed meat of yellowtail Seriola quinqueradiata. Fish. Sci. 2015, 81, 1145–1155. [Google Scholar] [CrossRef]
- Shahidi, F.; Samaranayaka, A.G.P.; Pegg, R.B. Cooking of Meat | Maillard Reaction and Browning. In Encyclopedia of Meat Sciences, 2nd ed.; Dikeman, M., Devine, C., Eds.; Academic Press: Oxford, UK, 2014; pp. 391–403. [Google Scholar] [CrossRef]
- Xie, J.; Sun, B.; Zheng, F.; Wang, S. Volatile flavor constituents in roasted pork of Mini-pig. Food Chem. 2008, 109, 506–514. [Google Scholar] [CrossRef]
- Xie, J.C.; Sun, B.G.; Wang, S.B. Aromatic Constituents from Chinese Traditional Smoke-cured Bacon of Mini-pig. Food Sci. Technol. Int. 2008, 14, 329–340. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Furst, P.; Gundert-Remy, U.; Gurtler, R.; Husoy, T.; et al. Scientific Opinion on Flavouring Group Evaluation 5, Revision 3 (FGE.05Rev3): Branched- and straight-chain unsaturated aldehydes, dienals, unsaturated and saturated carboxylic acids and related esters with saturated and unsaturated aliphatic alcohols and a phenylacetic acid related ester from chemical groups 1, 2, 3, 5 and 15. EFSA J. 2019, 17, e05761. [Google Scholar] [CrossRef] [Green Version]
- Minor, L.J.; Pearson, A.M.; Dawson, L.E.; Schweigert, B.S. Chicken Flavor: The Identification of Some Chemical Components and the Importance of Sulfur Compounds in the Cooked Volatile Fraction. J. Food Sci. 1965, 30, 686–696. [Google Scholar] [CrossRef]
- Ramirez, R.; Cava, R. Volatile profiles of dry-cured meat products from three different Iberian x Duroc genotypes. J. Agric. Food Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E.; Fadel, H.M. Investigation of the volatile aroma components of garlic leaves essential oil. Possibility of utilization to enrich garlic bulb oil. Eur. Food Res. Technol. 2014, 214, 105–107. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Cai, Z.; Ruan, Y.; He, J.; Dang, Y.; Cao, J.; Sun, Y.; Pan, D.; Tian, H. Effects of microbial fermentation on the flavor of cured duck legs. Poultry Sci. 2020, 99, 4642–4652. [Google Scholar] [CrossRef]
- Zhou, J.; Han, Y.; Zhuang, H.; Feng, T.; Xu, B. Influence of the Type of Extraction Conditions and Fiber Coating on the Meat of Sauced Duck Neck Volatile Compounds Extracted by Solid-Phase Microextraction (SPME). Food Anal. Method 2015, 8, 1661–1672. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, G.; Zhao, G.; Xu, X.; Peng, Z. Changes in flavor compounds of dry-cured Chinese Jinhua ham during processing. Meat Sci. 2005, 71, 291–299. [Google Scholar] [CrossRef]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.-M.; Lee, J.C.-Y.; Durand, T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B 2014, 964, 65–78. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, G.; Yin, X.; Ge, C.; Liao, G. Effects of different cooking methods on free fatty acid profile, water-soluble compounds and flavor compounds in Chinese Piao chicken meat. Food Res. Int. 2021, 149, 110696. [Google Scholar] [CrossRef]
- Wu, S.; Yang, J.; Dong, H.; Liu, Q.; Li, X.; Zeng, X.; Bai, W. Key aroma compounds of Chinese dry-cured Spanish mackerel (Scomberomorus niphonius) and their potential metabolic mechanisms. Food Chem. 2020, 342, 128381. [Google Scholar] [CrossRef]
- Kim, T.-K.; Hwang, K.-E.; Kim, Y.-B.; Jeon, K.-H.; Leem, K.-H.; Choi, Y.-S. Effects of Pre-cooking Methods on Quality Characteristics of Reheated Marinated Pork Loin. Korean J. Food Sci. An. 2018, 38, 970–980. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Jiang, X.; Chen, Q.; Zhang, H.; Sun, H.; Zhang, W.-M.; Li, C. Oxidative stabilities of olive and camellia oils: Possible mechanism of aldehydes formation in oleic acid triglyceride at high temperature. LWT Food Sci. Technol. 2019, 118, 108858. [Google Scholar] [CrossRef]
- Benet, I.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J.; Roura, E. Analysis of SPME or SBSE extracted volatile compounds from cooked cured pork ham differing in intramuscular fat profiles. LWT Food Sci. Technol. 2014, 60, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, X.-l.; Zhou, G.-h. Changes in taste compounds of duck during processing. Food Chem. 2007, 102, 22–26. [Google Scholar] [CrossRef]
- Ishiwatari, N.; Fukuoka, M.; Hamada-Sato, N.; Sakai, N. Decomposition kinetics of umami component during meat cooking. J. Food Eng. 2013, 119, 324–331. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the Relative Taste Intensity of Some L-A-Amino Acids And 5’-Nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Greenwood, D.A.; Kraybill, H.R.; Schweigert, B.S. Amino acid composition of fresh and cooked beef cuts. J. Biol. Chem. 1951, 193, 23–28. [Google Scholar] [CrossRef]
- Dinh, T.T.N.; Legako, J.F.; Miller, M.F.; Brooks, J.C. Effects of USDA quality grade and cooking on water-soluble precursors of beef flavor. Meat Sci. 2018, 146, 122–130. [Google Scholar] [CrossRef]
- Wu, H.-C.; Shiau, C.-Y. Proximate composition, Free amino acids and peptides contents in commercial chicken and other meat essences. J. Food Drug Anal. 2002, 10, 170–177. [Google Scholar] [CrossRef]
- Zhuang, K.; Wu, N.; Wang, X.; Wu, X.; Wang, S.; Long, X.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir sinensis). J. Food Sci. 2016, 81, S968–S981. [Google Scholar] [CrossRef]




| IDX | SN | Cooking Method | Internal T (°C) | Total Cooking Time (min) | Details |
|---|---|---|---|---|---|
| 1 | A-1 | Steaming for 10 min | About 45 °C | 10 min | 5 cm × 5 cm × 2.5 cm |
| 2 | A-2 | Steaming for 30 min | About 75 °C | 30 min | 5 cm × 5 cm × 2.5 cm |
| 3 | A-3 | Steaming for 60 min | About 98 °C | 60 min | 5 cm × 5 cm × 2.5 cm |
| 4 | B-1 | Boiling for 30 min | About 45 °C | 30 min | 5 cm × 5 cm × 2.5 cm |
| 5 | B-2 | Boiling for 60 min | About 75 °C | 60 min | 5 cm × 5 cm × 2.5 cm |
| 6 | B-3 | Boiling for 90 min | About 98 °C | 90 min | 5 cm × 5 cm × 2.5 cm |
| 7 | C-1 | Roasting for 10 min | About 45 °C | 10 min | 3 cm × 3 cm × 2.5 cm |
| 8 | C-2 | Roasting for 20 min | About 75 °C | 20 min | 3 cm × 5 cm × 2.5 cm |
| 9 | C-3 | Roasting for 40 min | About 98 °C | 40 min | 3 cm × 3 cm × 2.5 cm |
| 10 | D-1 | Instant boiling for 10 s | - | 10 s | 5 cm × 5 cm × 2 mm |
| 11 | D-2 | Instant boiling for 30 s | - | 30 s | 5 cm × 5 cm × 2 mm |
| 12 | D-3 | Instant boiling for 60 s | - | 60 s | 5 cm × 5 cm × 2 mm |
| Gas-Phase Ion Mobility Spectrometry Unit | |
|---|---|
| Analysis time | 20 min |
| Column type | MXT-5 15 m ID: 0.53 mm |
| Column temperature | 60 °C |
| Carrier gas/drift gas | N2 |
| IMS temperature | 45 °C |
| Automatic headspace sampling unit | |
| Injection volume | 500 μL |
| Incubation time | 15 min |
| Incubation temperature | 60 °C |
| Syringe temperature | 65 °C |
| Incubation speed | 500 rpm |
| Traits | Raw | A-1 | A-2 | A-3 | B-1 | B-2 | B-3 | C-1 | C-2 | C-3 | D-1 | D-2 | D-3 | M 1 | N 2 | M × N 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid | ||||||||||||||||
| C10:0 | 0.28 ± 0.1 ab | 0.08 ± 0.01 bc | 0.06 ± 0.04 c | 0.05 ± 0.04 c | 0.29 ± 0.16 a | 0.15 ± 0.05 abc | 0.07 ± 0.004 c | 0.06 ± 0.01 c | 0.05 ± 0.03 c | 0.04 ± 0.002 c | 0.1 ± 0.01 abc | 0.09 ± 0.06 bc | 0.07 ± 0.04 c | ** | ** | * |
| C11:0 | 32.02 ± 1.54 d | 49.93 ± 0.93 a | 39.8 ± 0.98 bc | 34.48 ± 0.98 cd | 52.05 ± 1.83 a | 53.83 ± 4.33 a | 32.81 ± 1.67 d | 35.99 ± 0.88 cd | 37.37 ± 1.89 cd | 15.06 ± 0.03 e | 43.23 ± 1.76 b | 35.14 ± 0.29 cd | 54.62 ± 1.68 a | ** | ** | ** |
| C12:0 | 0.07 ± 0.04 | 0.04 ± 0.02 | 0.05 ± 0.03 | 0.06 ± 0.04 | 0.04 ± 0.01 | 0.07 ± 0.02 | 0.03 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.04 | 0.05 ± 0.001 | 0.04 ± 0.02 | 0.04 ± 0.03 | 0.04 ± 0.02 | NS | NS | NS |
| C13:0 | 2.84 ± 1.48 a | 0.08 ± 0.04 b | 0.08 ± 0.01 b | 0.11 ± 0.06 b | 0.06 ± 0.04 b | 0.05 ± 0.03 b | 0.03 ± 0.02 b | 0.2 ± 0.02 b | 0.32 ± 0.09 b | 0.5 ± 0.23 b | 0.34 ± 0.22 b | 0.41 ± 0.25 b | 0.69 ± 0.31 b | ** | * | NS |
| C14:0 | 1.28 ± 0.22 def | 1.1 ± 0.07 ef | 1.34 ± 0.06 def | 1.74 ± 0.56 bcd | 1.05 ± 0.11 ef | 1.31 ± 0.07 def | 2.1 ± 0.06 abc | 1.59 ± 0.07 bcde | 1.54 ± 0.04 cdef | 2.14 ± 0.01 ab | 1.22 ± 0.1 def | 0.97 ± 0.06 f | 2.57 ± 0.01 a | ** | ** | ** |
| C15:0 | 0.36 ± 0.14 bc | 0.16 ± 0.05 d | 0.26 ± 0.02 bcd | 0.28 ± 0.06 bcd | 0.22 ± 0.02 cd | 0.21 ± 0.01 cd | 0.43 ± 0.01 b | 0.27 ± 0.06 bcd | 0.35 ± 0.03 bc | 0.65 ± 0.031 a | 0.26 ± 0.1 bcd | 0.31 ± 0.01 bcd | 0.66 ± 0.05 a | ** | ** | ** |
| C16:0 | 16.89 ± 1.42 b | 13.45 ± 0.05 de | 15.84 ± 0.18 bcd | 15.91 ± 0.43 bcd | 9.02 ± 0.05 f | 12.15 ± 0.22 e | 18.1 ± 0.84 b | 16.08 ± 1.42 bc | 15.98 ± 0.23 bcd | 20.94 ± 1.54 a | 13.81 ± 0.34 cde | 13.11 ± 0.11 e | 18.37 ± 0.47 b | ** | ** | ** |
| C17:0 | 0.82 ± 0.4 ab | 0.32 ± 0.17 de | 0.51 ± 0.02 bcde | 0.32 ± 0.1 de | 0.36 ± 0.04 cde | 0.45 ± 0.01 bcde | 0.79 ± 0.01 bc | 0.76 ± 0.04 bc | 0.27 ± 0.01 e | 1.19 ± 0.002 a | 0.51 ± 0.01 bcde | 0.56 ± 0.02 bcde | 0.74 ± 0.03 bcd | ** | ** | ** |
| C18:0 | 14.19 ± 2.72 a | 8.93 ± 0.61 bcd | 9.66 ± 0.55 bcd | 9.5 ± 0.5 bcd | 7.35 ± 0.32 d | 7.98 ± 0.19 cd | 11.32 ± 0.42 b | 11.3 ± 0.38 b | 10.75 ± 0.21 bc | 15.11 ± 0.03 a | 8.82 ± 0.54 bcd | 10.49 ± 0.06 bc | 10.59 ± 0.09 bc | ** | ** | ** |
| C20:0 | 0.14 ± 0.07 | 0.04 ± 0.02 | 0.05 ± 0.04 | 0.09 ± 0.05 | 0.06 ± 0.03 | 0.09 ± 0.05 | 0.16 ± 0.03 | 0.02 ± 0.01 | 0.05 ± 0.03 | 0.05 ± 0.003 | 0.03 ± 0.02 | 0.06 ± 0.04 | 0.1 ± 0.06 | ** | ** | NS |
| C14:1 | 0.18 ± 0.07 b | 0.19 ± 0.06 b | 0.2 ± 0.03 b | 0.24 ± 0.13 b | 0.24 ± 0.05 b | 0.24 ± 0.01 b | 0.27 ± 0 b | 0.25 ± 0.01 b | 0.25 ± 0.04 b | 0.31 ± 0.03 b | 0.2 ± 0.03 b | 0.27 ± 0.06 b | 0.58 ± 0.02 a | ** | ** | ** |
| C16:1 | 1.78 ± 0.29 b | 1.89 ± 0.11 b | 2.58 ± 0.54 ab | 2.51 ± 0.5 ab | 0.89 ± 0.02 c | 1.84 ± 0.56 b | 2.29 ± 0.09 ab | 2.18 ± 0.28 ab | 2.24 ± 0 ab | 2.68 ± 0.08 ab | 1.81 ± 0.03 b | 1.94 ± 0.05 b | 3.05 ± 0.11 a | * | ** | ** |
| C20:1 | 0.57 ± 0.34 a | 0.08 ± 0.02 b | 0.1 ± 0.02 b | 0.11 ± 0.02 b | 0.17 ± 0.06 ab | 0.17 ± 0.05 ab | 0.26 ± 0.14 ab | 0.18 ± 0.06 ab | 0.19 ± 0.08 ab | 0.29 ± 0.11 ab | 0.09 ± 0.02 b | 0.17 ± 0.06 ab | 0.21 ± 0.1 ab | NS | * | NS |
| C18:1n9c | 26.01 ± 4.5 a | 16.99 ± 0.21 ab | 22.49 ± 0.64 ab | 20.99 ± 0.5 ab | 12.07 ± 1.25 bc | 16.71 ± 0.37 ab | 24.3 ± 0.24 ab | 24.83 ± 1.87 ab | 12.97 ± 12.94 abc | 1.2 ± 1.12 c | 18.83 ± 0.85 e | 22.29 ± 0.33 de | 0.84 ± 0.6 c | * | ** | ** |
| C18:1n9t | 41.02 ± 7 a | 9.16 ± 2.15 cde | 10.51 ± 2.53 cde | 15.71 ± 3.2 cde | 12.97 ± 5.38 cde | 14.72 ± 2.74 cde | 16.55 ± 4.84 cde | 18.9 ± 4.54 bcd | 21.01 ± 3.01 bc | 30.01 ± 1.34 ab | 4.83 ± 2.09 bc | 7.45 ± 1.74 ab | 8.39 ± 1.6 cde | ** | ** | * |
| C18:2n6c | 3.32 ± 1.1 ef | 7.73 ± 0.7 bc | 8.6 ± 0.48 abc | 9.62 ± 0.6 ab | 12.1 ± 0.37 a | 6.87 ± 0.12 bcd | 3.64 ± 3.43 def | 7.89 ± 0.52 bc | 0.33 ± 0.02 f | 7.25 ± 0.52 bc | 7.48 ± 0.37 bc | 10.06 ± 0.34 a | 5.42 ± 0.17 bc | ** | ** | ** |
| C18:2 n6t | 1.25 ± 0.16 bc | 3.12 ± 2 bc | 2.07 ± 0.96 bc | 1.23 ± 0.19 bc | 1.45 ± 0.42 bc | 4.4 ± 1.2 b | 0.05 ± 0.01 c | 2.71 ± 0.85 bc | 9.5 ± 2.32 a | 4.46 ± 1.28 b | 2.53 ± 0.86 b | 1.57 ± 0.9 b | 5.19 ± 2.03 b | ** | ** | ** |
| C18:3n3 | 1.02 ± 0.15 d | 1.38 ± 0.11 bc | 1.61 ± 0.07 ab | 1.72 ± 0.05 a | 0.54 ± 0.01 e | 1.34 ± 0.01 c | 1.45 ± 0.05 bc | 1.56 ± 0.12 abc | 1.73 ± 0.01 a | 1.4 ± 0.07 bc | 1.39 ± 0.03 def | 1.78 ± 0.02 b | 1.39 ± 0.05 g | ** | ** | ** |
| C20:3n6 | 3.04 ± 1.29 a | 0.21 ± 0.1 b | 0.18 ± 0.09 b | 0.15 ± 0.04 b | 0.11 ± 0.04 b | 0.09 ± 0.02 b | 0.04 ± 0.01 b | 0.63 ± 0.19 b | 0.89 ± 0.31 b | 0.95 ± 0.62 b | 0.42 ± 0.35 b | 0.08 ± 0.01 b | 0.08 ± 0.05 a | * | NS | NS |
| C20:4n6 | 1.85 ± 0.3 ef | 2.63 ± 0.32 bcd | 2.55 ± 0.21 bcde | 2.94 ± 0.18 bc | 4.02 ± 0.32 a | 1.97 ± 0.05 def | 2.1 ± 0.17 def | 2.25 ± 0.22 cdef | 2.44 ± 0.16 cdef | 1.79 ± 0.172 fg | 2.09 ± 0.19 def | 3.19 ± 0.08 b | 1.18 ± 0.11 g | ** | ** | ** |
| C20:5 | 0.27 ± 0.13 b | 0.42 ± 0.05 b | 0.42 ± 0.04 b | 0.48 ± 0.05 b | 0.69 ± 0.08 b | 0.46 ± 0.05 b | 0.28 ± 0.01 b | 0.34 ± 0.03 b | 0.39 ± 0.05 b | 0.16 ± 0.13 b | 0.31 ± 0.03 b | 0.84 ± 0.03 b | 1.84 ± 0.71 a | ** | * | ** |
| C22:6n3 | 0.33 ± 0.21 bc | 0.15 ± 0.05 bc | 0.11 ± 0.02 bc | 0.05 ± 0.03 c | 0.3 ± 0.03 bc | 0.21 ± 0.1 bc | 0.14 ± 0.04 bc | 0.18 ± 0.07 bc | 0.42 ± 0.03 b | 0.92 ± 0.24 a | 0.19 ± 0.08 bc | 0.12 ± 0.03 bc | 0.1 ± 0.07 bc | ** | ** | ** |
| SFA 1 | 68.88 ± 1.3 cde | 74.13 ± 1.96 bc | 67.64 ± 0.19 def | 62.52 ± 0.26 ef | 70.51 ± 1.6 bcd | 76.28 ± 4.11 b | 65.83 ± 2.9 def | 66.3 ± 2.71 def | 66.73 ± 1.45 def | 55.72 ± 1.75 g | 68.35 ± 0.47 cde | 61.17 ± 0.23 fg | 88.43 ± 1.44 a | ** | * | ** |
| MUFA 2 | 69.56 ± 2.6 a | 28.29 ± 2.3 c | 35.87 ± 2.63 bc | 39.57 ± 3.31 bc | 26.33 ± 6.75 cd | 33.67 ± 3.59 bc | 43.66 ± 4.37 b | 46.34 ± 2.45 b | 36.66 ± 9.89 bc | 34.48 ± 2.33 bc | 25.76 ± 2.96 cd | 32.13 ± 1.24 bc | 13.07 ± 1.01 d | ** | * | ** |
| PUFA 3 | 11.07 ± 2.36 bc | 15.63 ± 3.32 ab | 15.53 ± 0.3 ab | 16.18 ± 1.06 ab | 19.2 ± 0.3 a | 15.34 ± 1.19 ab | 7.7 ± 3.28 c | 15.55 ± 0.16 ab | 15.69 ± 2.43 ab | 16.93 ± 3.02 ab | 14.4 ± 0.31 ab | 17.63 ± 1.18 ab | 15.18 ± 2.51 ab | NS | * | ** |
| FFAs 4 | 149.5 ± 3.67 a | 118.05 ± 7.57 bc | 119.04 ± 2.14 bc | 118.26 ± 2.51 bc | 116.04 ± 5.45 bc | 125.29 ± 0.67 b | 117.19 ± 4.74 bc | 128.19 ± 0.42 b | 119.07 ± 6 bc | 107.13 ± 2.44 c | 108.5 ± 2.8 c | 110.92 ± 0.17 c | 116.68 ± 4.96 bc | * | * | ** |
| 5′-nucleotide | ||||||||||||||||
| GMP | 0.14 ± 0.03 | 0.23 ± 0.1 | 0.32 ± 0.12 | 0.4 ± 0.12 | 0.4 ± 0.15 | 0.4 ± 0.24 | 0.37 ± 0.21 | 0.39 ± 0.18 | 0.26 ± 0.12 | 0.21 ± 0.06 | 0.18 ± 0.12 | 0.19 ± 0.12 | 0.3 ± 0.12 | NS | NS | NS |
| AMP | 0.12 ± 0.03 a | 0.03 ± 0.02 b | 0.03 ± 0.02 b | 0.02 ± 0.01 b | 0.03 ± 0.02 b | 0.03 ± 0.02 b | 0.02 ± 0.01 b | 0.05 ± 0.03 b | 0.04 ± 0.01 b | 0.02 ± 0.01 b | 0.03 ± 0.01 b | 0.03 ± 0.02 b | 0.03 ± 0.02 b | NS | * | NS |
| Hypoxanthine | 0.47 ± 0.08 a | 0.02 ± 0.01 b | 0.03 ± 0.01 b | 0.04 ± 0.01 b | 0.02 ± 0.01 b | 0.03 ± 0.02 b | 0.04 ± 0.01 b | 0.03 ± 0.01 b | 0.03 ± 0.01 b | 0.03 ± 0.02 b | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 0.03 ± 0.01 b | NS | NS | NS |
| ADP | 0.38 ± 0.19 a | 0.05 ± 0.01 b | 0.06 ± 0.02 b | 0.06 ± 0.01 b | 0.05 ± 0.02 b | 0.06 ± 0.02 b | 0.08 ± 0.02 b | 0.07 ± 0.03 b | 0.08 ± 0.02 b | 0.08 ± 0.02 b | 0.06 ± 0.02 b | 0.05 ± 0.01 b | 0.08 ± 0.01 b | NS | NS | NS |
| IMP | 1.01 ± 0.13 a | 0.3 ± 0.02 cd | 0.13 ± 0.02 e | 0.13 ± 0.02 e | 0.36 ± 0.04 bc | 0.31 ± 0.07 bcd | 0.17 ± 0.02 de | 0.48 ± 0.02 b | 0.35 ± 0.02 bc | 0.36 ± 0.01 bc | 0.33 ± 0.03 bcd | 0.3 ± 0.03 cd | 0.16 ± 0.08 de | ** | ** | ** |
| Total 5 | 2.12 ± 0.33 a | 0.63 ± 0.07 b | 0.56 ± 0.13 b | 0.65 ± 0.15 b | 0.85 ± 0.16 b | 0.82 ± 0.33 b | 0.67 ± 0.26 b | 1.02 ± 0.23 b | 0.76 ± 0.12 b | 0.7 ± 0.05 b | 0.61 ± 0.16 b | 0.59 ± 0.13 b | 0.6 ± 0.17 b | NS | NS | NS |
| Free amino acid | ||||||||||||||||
| Aspartic | 0.39 ± 0.11 | 0.32 ± 0.12 | 0.34 ± 0.14 | 0.16 ± 0.1 | 0.4 ± 0.21 | 0.27 ± 0.08 | 0.3 ± 0.09 | 0.31 ± 0.1 | 0.26 ± 0.05 | 0.15 ± 0.05 | 0.35 ± 0.1 | 0.37 ± 0.12 | 0.33 ± 0.12 | NS | ** | * |
| Threonine | 0.17 ± 0.1 | 0.13 ± 0.08 | 0.14 ± 0.1 | 0.12 ± 0.08 | 0.17 ± 0.03 | 0.11 ± 0.03 | 0.12 ± 0.05 | 0.13 ± 0.05 | 0.11 ± 0.03 | 0.11 ± 0.02 | 0.14 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.02 | NS | * | NS |
| Serine | 0.14 ± 0.09 ab | 0.11 ± 0.07 ab | 0.12 ± 0.08 ab | 0.36 ± 0.17 a | 0.13 ± 0.04 ab | 0.09 ± 0.04 b | 0.09 ± 0.06 b | 0.1 ± 0.07 ab | 0.09 ± 0.06 ab | 0.34 ± 0.11 ab | 0.11 ± 0.04 ab | 0.16 ± 0.05 ab | 0.1 ± 0.02 ab | NS | ** | ** |
| Glutamic | 3.56 ± 1.55 b | 8.6 ± 1.29 ab | 8.81 ± 1.19 ab | 9.01 ± 1.3 ab | 10.03 ± 1.69 a | 7.11 ± 2 ab | 7.76 ± 1.78 ab | 8.24 ± 2.25 ab | 7.06 ± 2.77 ab | 5.44 ± 1.35 ab | 9.15 ± 2.04 ab | 8.98 ± 1.72 ab | 8.43 ± 1.22 ab | NS | ** | ** |
| EUC | 39.18 ± 29.51 | 86.59 ± 24.05 | 85.73 ± 18.43 | 90.15 ± 21.12 | 131.05 ± 51.32 | 70.5 ± 42.08 | 73.54 ± 35.96 | 100.9 ± 54.57 | 68.91 ± 47.08 | 36.85 ± 17.29 | 99.92 ± 39.81 | 95.2 ± 35.15 | 81.34 ± 27.22 | NS | ** | ** |
| Glycine | 0.21 ± 0.1 ab | 0.25 ± 0.12 ab | 0.19 ± 0.07 ab | 0.16 ± 0.06 ab | 0.22 ± 0.09 ab | 0.14 ± 0.04 ab | 0.09 ± 0.05 b | 0.77 ± 0.09 a | 0.35 ± 0.1 ab | 0.15 ± 0.1 ab | 0.44 ± 0.15 b | 0.25 ± 0.04 ab | 0.22 ± 0.06 ab | ** | ** | ** |
| Cystine | 0.25 ± 0.12 a | 0.01 ± 0 b | 0.09 ± 0.05 b | 0.11 ± 0.07 b | 0.01 ± 0 b | 0.01 ± 0 b | 0.01 ± 0 b | 0.01 ± 0 b | 0.01 ± 0 | 0.02 ± 0.01 b | 0.01 ± 0 b | 0.01 ± 0 b | 0.01 ± 0 b | * | ** | ** |
| Valine | 0.06 ± 0.04 | 0.18 ± 0.04 | 0.18 ± 0.01 | 0.18 ± 0.04 | 0.21 ± 0.07 | 0.16 ± 0.05 | 0.16 ± 0.02 | 0.17 ± 0.02 | 0.14 ± 0.03 | 0.16 ± 0.04 | 0.19 ± 0.07 | 0.22 ± 0.08 | 0.18 ± 0.07 | NS | NS | NS |
| Methionine | 0.04 ± 0.03 ab | 0.02 ± 0.01 b | 0.03 ± 0.02 ab | 0.03 ± 0.01 ab | 0.03 ± 0.02 ab | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 0.03 ± 0.02 ab | 0.02 ± 0.01 b | 0.03 ± 0.02 ab | 0.07 ± 0.02 a | 0.02 ± 0.01 b | NS | ** | ** |
| Isoleucine | 1.08 ± 0.51 a | 0.19 ± 0.02 b | 0.19 ± 0.04 b | 0.18 ± 0.02 b | 0.23 ± 0.07 b | 0.15 ± 0.05 b | 0.16 ± 0.04 b | 0.18 ± 0.06 b | 0.15 ± 0.04 b | 0.17 ± 0.06 b | 0.19 ± 0.08 b | 0.21 ± 0.09 b | 0.19 ± 0.07 b | NS | ** | ** |
| Leucine | 0.18 ± 0.06 | 0.41 ± 0.2 | 0.43 ± 0.22 | 0.41 ± 0.2 | 0.51 ± 0.3 | 0.34 ± 0.23 | 0.38 ± 0.17 | 0.39 ± 0.18 | 0.33 ± 0.12 | 0.38 ± 0.07 | 0.44 ± 0.13 | 0.45 ± 0.14 | 0.43 ± 0.12 | NS | * | * |
| Tyrosine | 0.07 ± 0.04 | 0.13 ± 0.07 | 0.13 ± 0.05 | 0.12 ± 0.03 | 0.16 ± 0.05 | 0.1 ± 0.06 | 0.11 ± 0.07 | 0.12 ± 0.03 | 0.1 ± 0.01 | 0.11 ± 0.02 | 0.13 ± 0.04 | 0.12 ± 0.04 | 0.13 ± 0.05 | NS | NS | NS |
| Phenylalanine | 0.07 ± 0.05 | 0.14 ± 0.07 | 0.15 ± 0.09 | 0.14 ± 0.08 | 0.18 ± 0.06 | 0.12 ± 0.06 | 0.13 ± 0.07 | 0.14 ± 0.08 | 0.12 ± 0.06 | 0.13 ± 0.06 | 0.16 ± 0.08 | 0.14 ± 0.06 | 0.15 ± 0.07 | NS | ** | ** |
| Lysine | 1.98 ± 0.53 a | 0.28 ± 0.17 b | 0.28 ± 0.14 b | 0.28 ± 0.14 b | 0.33 ± 0.19 b | 0.23 ± 0.09 b | 0.25 ± 0.08 b | 0.26 ± 0.09 b | 0.22 ± 0.08 b | 0.25 ± 0.04 b | 0.29 ± 0.08 b | 0.33 ± 0.09 b | 0.28 ± 0.14 b | NS | NS | NS |
| Histidine | 0.09 ± 0.06 | 0.11 ± 0.08 | 0.1 ± 0.07 | 0.1 ± 0.07 | 0.11 ± 0.08 | 0.09 ± 0.06 | 0.1 ± 0.07 | 0.1 ± 0.07 | 0.09 ± 0.06 | 0.1 ± 0.06 | 0.11 ± 0.07 | 0.14 ± 0.07 | 0.1 ± 0.03 | * | NS | * |
| Arginine | 0.09 ± 0.04 | 0.23 ± 0.12 | 0.23 ± 0.1 | 0.22 ± 0.09 | 0.29 ± 0.1 | 0.19 ± 0.1 | 0.19 ± 0.1 | 0.22 ± 0.11 | 0.18 ± 0.07 | 0.21 ± 0.09 | 0.24 ± 0.12 | 0.28 ± 0.13 | 0.23 ± 0.12 | NS | NS | NS |
| Umami 6 | 3.95 ± 1.66 b | 8.92 ± 1.17 ab | 9.14 ± 1.05 ab | 9.17 ± 1.2 ab | 10.43 ± 1.89 a | 7.38 ± 2.08 ab | 8.06 ± 1.87 ab | 8.54 ± 2.34 ab | 7.32 ± 2.82 ab | 5.59 ± 1.3 ab | 9.5 ± 2.14 ab | 9.34 ± 1.83 ab | 8.76 ± 1.34 ab | NS | ** | ** |
| Bitter 7 | 1.58 ± 0.55 | 1.27 ± 0.48 | 1.29 ± 0.41 | 1.25 ± 0.38 | 1.55 ± 0.43 | 1.06 ± 0.16 | 1.11 ± 0.14 | 1.19 ± 0.13 | 1.04 ± 0.09 | 1.15 ± 0.01 | 1.33 ± 0.01 | 1.49 ± 0.05 | 1.29 ± 0.05 | NS | ** | ** |
| FAAs 8 | 8.34 ± 2.72 | 11.09 ± 0.97 | 11.36 ± 0.88 | 11.55 ± 0.78 | 12.99 ± 2.18 | 9.1 ± 2.31 | 9.81 ± 2.15 | 11.09 ± 2.61 | 9.23 ± 2.82 | 7.7 ± 1.28 | 11.93 ± 2.22 | 11.81 ± 1.58 | 10.9 ± 1.09 | NS | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bu, X.; Yang, D.; Deng, D.; Lei, Z.; Guo, Z.; Ma, X.; Zhang, L.; Yu, Q. Effect of Cooking Method and Doneness Degree on Volatile Compounds and Taste Substance of Pingliang Red Beef. Foods 2023, 12, 446. https://doi.org/10.3390/foods12030446
Wang Y, Bu X, Yang D, Deng D, Lei Z, Guo Z, Ma X, Zhang L, Yu Q. Effect of Cooking Method and Doneness Degree on Volatile Compounds and Taste Substance of Pingliang Red Beef. Foods. 2023; 12(3):446. https://doi.org/10.3390/foods12030446
Chicago/Turabian StyleWang, Yujiao, Xinrong Bu, Dawei Yang, Dan Deng, Zhaomin Lei, Zhaobin Guo, Xiaotong Ma, Li Zhang, and Qunli Yu. 2023. "Effect of Cooking Method and Doneness Degree on Volatile Compounds and Taste Substance of Pingliang Red Beef" Foods 12, no. 3: 446. https://doi.org/10.3390/foods12030446
APA StyleWang, Y., Bu, X., Yang, D., Deng, D., Lei, Z., Guo, Z., Ma, X., Zhang, L., & Yu, Q. (2023). Effect of Cooking Method and Doneness Degree on Volatile Compounds and Taste Substance of Pingliang Red Beef. Foods, 12(3), 446. https://doi.org/10.3390/foods12030446





