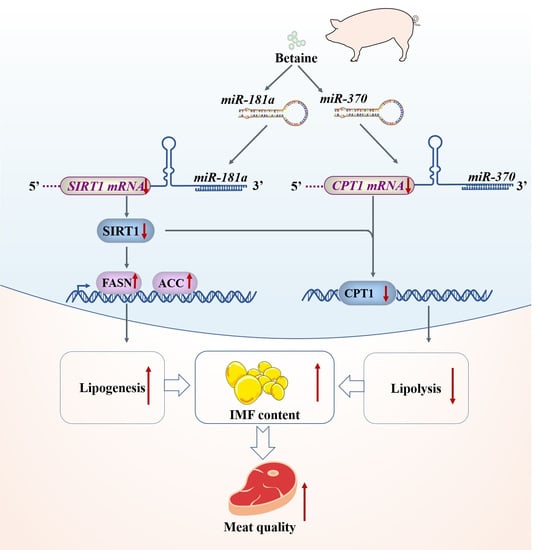
Long-Term Dietary Supplementation with Betaine Improves Growth Performance, Meat Quality and Intramuscular Fat Deposition in Growing-Finishing Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
2.2. Growth Performance
2.3. Samples Collection
2.4. Analysis of Carcass Characteristics
2.5. Measurement of Meat Quality
2.6. Determination of Serum Hormones and Biochemical Indicators
2.7. RNA Extraction and Real-Time RCR
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Carcass Characteristics
3.3. Meat Quality
3.4. Visceral Index, Hepatic and Muscle Lipid Contents
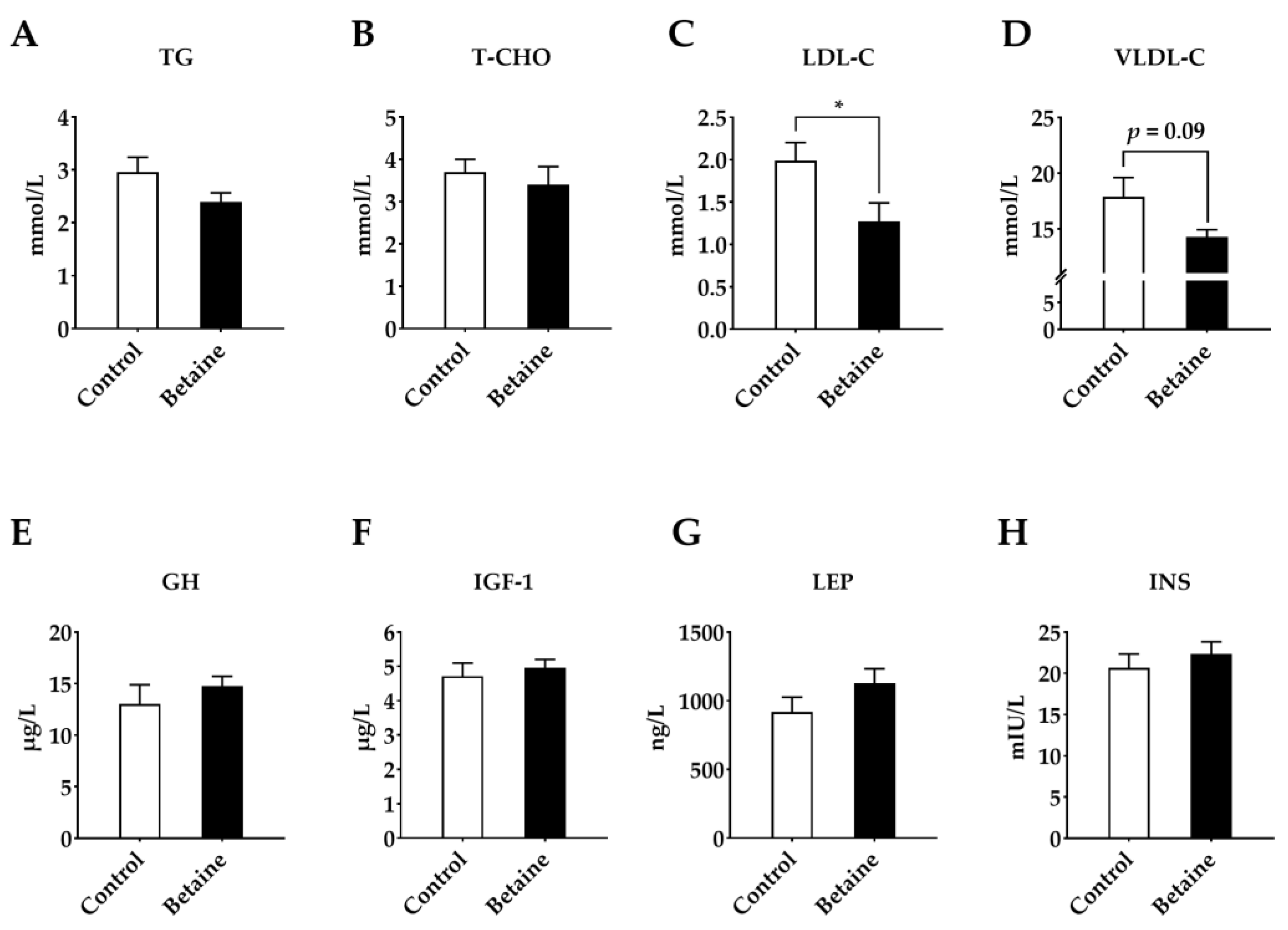
3.5. Serum Hormones and Biochemical Indicators
3.6. Expression of Lipogenic and Lipolysis Genes in Muscle
3.7. Lipid Metabolism-Related miRNAs Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emmans, G.C.; Kyriazakis, I. Issues arising from genetic selection for growth and body composition characteristics in poultry and pigs. BSAP Occas. Publ. 2000, 27, 39–53. [Google Scholar] [CrossRef]
- Madeira, M.S.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.; Coelho, D.; Fontes, C.M.G.A.; Prates, J.A.M. Ameliorating pork marbling and quality with novel feeding approaches. In Advances in Animal Health, Medicine and Production; Springer: Berlin/Heidelberg, Germany, 2020; pp. 161–177. [Google Scholar]
- Zhong, Y.; Yan, Z.; Song, B.; Zheng, C.; Duan, Y.; Kong, X.; Deng, J.; Li, F. Dietary supplementation with betaine or glycine improves the carcass trait, meat quality and lipid metabolism of finishing mini-pigs. Anim. Nutr. 2021, 7, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wen, C.; Chen, Y.; Zhuang, S.; Zhou, Y. Effects of betaine supplementation on growth performance, muscle amino acid contents, meat quality and antioxidant capacity in finishing pigs. J. Anim. Sci. 2019, 97, 349–350. [Google Scholar] [CrossRef]
- Mwangi, F.W.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Kinobe, R.T.; Malau-Aduli, A.E. Diet and genetics influence beef cattle performance and meat quality characteristics. Foods 2019, 8, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.H.; Lee, S.J.; Lee, E.Y.; Joo, S.T. Effects of carcass weight increase on meat quality and sensory properties of pork loin. J. Anim. Sci. Technol. 2020, 62, 753. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Alfaia, C.M.; Lopes, P.A.; Madeira, M.S.; Pestana, J.M.; Coelho, D.; Toldrá, F.; Prates, J.A.M. Current feeding strategies to improve pork intramuscular fat content and its nutritional quality. Adv. Food Nutr. Res. 2019, 89, 53–94. [Google Scholar]
- Fu, R.; Wang, Q.; Kong, C.; Liu, K.; Si, H.; Sui, S. Mechanism of action and the uses betaine in pig production. J. Anim. Physiol. An. N. 2022, 3, 528–536. [Google Scholar] [CrossRef]
- Scheibler, C. Ueber das betain, eine im safte der zuckerrüben (beta vulgaris) vorkommende pflanzenbase. Ber. Dtsch. Chem. Ges. 1869, 2, 292–295. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Guimaraes-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef]
- Preedy, V.R. Betaine: Chemistry, Analysis, Function and Effects; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 4–5. [Google Scholar]
- Sahebi-Ala, F.; Hassanabadi, A.; Golian, A. Effect of replacement different methionine levels and sources with betaine on blood metabolites, breast muscle morphology and immune response in heat-stressed broiler chickens. Ital. J. Anim. Sci. 2021, 20, 33–45. [Google Scholar] [CrossRef]
- Cadogan, D.; Campbell, R.; Harrison, D.; Edwards, A. The effects of betaine on the growth performance and carcass characteristics of female pigs. Manip. Pig Prod. 1993, IV, 219. [Google Scholar]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.; Warner, R.D.; Dunshea, F.R. Dietary betaine reduces the negative effects of cyclic heat exposure on growth performance, blood gas status and meat quality in broiler chickens. Agriculture 2020, 10, 176. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Ji, Y.; Lin, X.; Zhao, Y. Effect of betaine diet on growth performance, carcass quality and fat deposition in finishing ningxiang pigs. Animals 2021, 11, 3408. [Google Scholar] [CrossRef]
- Mendoza, S.; Boyd, R.; Zier-Rush, C.; Ferket, P.; Haydon, K.; van Heugten, E. Effect of natural betaine and ractopamine HCL on whole-body and carcass growth in pigs housed under high ambient temperatures. J. Anim. Sci. 2017, 95, 3047–3056. [Google Scholar] [CrossRef]
- Madeira, M.; Alfaia, C.; Costa, P.; Lopes, P.; Martins, S.; Lemos, J.; Moreira, O.; Santos-Silva, J.; Bessa, R.; Prates, J. Effect of betaine and arginine in lysine-deficient diets on growth, carcass traits, and pork quality. J. Anim. Sci. 2015, 93, 4721–4733. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, A.; Neves, J.A.; Redondeiro, M.; Laranjo, M.; Felix, M.; Freitas, A.; Tirapicos, J.L.; Martins, J.M. Long term betaine supplementation regulates genes involved in lipid and cholesterol metabolism of two muscles from an obese pig breed. Meat Sci. 2017, 124, 25–33. [Google Scholar] [CrossRef]
- Huang, Q.C.; Han, X.Y.; Xu, Z.R.; Yang, X.Y.; Chen, T.; Zheng, X.T. Betaine suppresses carnitine palmitoyltransferase Ⅰ in skeletal muscle but not in liver of finishing pigs. Livest. Sci. 2009, 126, 130–135. [Google Scholar] [CrossRef]
- Martins, J.M.; Neves, J.A.; Freitas, A.; Tirapicos, J.L. Effect of long-term betaine supplementation on chemical and physical characteristics of three muscles from the Alentejano pig. J. Sci. Food Agr. 2012, 92, 2122–2127. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, H.; Wang, X.; Wang, Y.; Feng, J. Betaine affects muscle lipid metabolism via regulating the fatty acid uptake and oxidation in finishing pig. J. Anim. Sci. Biotechno. 2017, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Agbu, P.; Carthew, R.W. Microrna-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Bio. 2021, 22, 425–438. [Google Scholar] [CrossRef]
- Wu, C.; Gong, Y.; Yuan, J.; Zhang, W.; Zhao, G.; Li, H.; Sun, A.; Zou, Y.; Ge, J. Microrna-181a represses ox-LDL-stimulated inflammatory response in dendritic cell by targeting c-Fos. J. Lipid Res. 2012, 53, 2355–2363. [Google Scholar] [CrossRef] [Green Version]
- Iliopoulos, D.; Drosatos, K.; Hiyama, Y.; Goldberg, I.J.; Zannis, V.I. Microrna-370 controls the expression of microrna-122 and cpt1α and affects lipid metabolism. J. Lipid Res. 2010, 51, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Liu, H.; Yuan, M.; Pan, S.; Jia, Y.; Zhao, R. Expression of hepatic mirnas targeting porcine glucocorticoid receptor (GR) 3’UTR in the neonatal piglets under a maternal gestational betaine supplementation. Data Brief 2016, 6, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NRC (National Academy of Sciences-National Research Council). Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; pp. 212–213.
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Latimer Junior, G. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MO, USA, 2016. [Google Scholar]
- Fu, R.Q.; Liang, C.; Chen, D.W.; Yan, H.; Tian, G.; Zheng, P.; He, J.; Yu, J.; Mao, X.B.; Huang, Z.Q.; et al. Effects of dietary bacillus coagulans and yeast hydrolysate supplementation on growth performance, immune response and intestinal barrier function in weaned piglets. J. Anim. Physiol. An. N. 2021, 105, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Arce, R.; Barros, S.; Wacker, B.; Peters, B.; Moss, K.; Offenbacher, S. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta 2009, 30, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.C.; Xu, Z.R.; Han, X.Y.; Li, W.F. Effect of betaine on growth hormone pulsatile secretion and serum metabolites in finishing pigs. J. Anim. Physiol. An. N. 2007, 91, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xu, Z.; Li, W. Effects of betaine on growth performance and carcass characteristics in growing pigs. Asian-Australas. J. Anim. Sci. 2004, 17, 1700–1704. [Google Scholar] [CrossRef]
- Siljander-Rasi, H.; Peuranen, S.; Tiihonen, K.; Virtanen, E.; Kettunen, H.; Alaviuhkola, T.; Simmins, P. Effect of equi-molar dietary betaine and choline addition on performance, carcass quality and physiological parameters of pigs. Anim. Sci. 2003, 76, 55–62. [Google Scholar] [CrossRef]
- Nakev, J.; Popova, T.; Vasileva, V. Influence of dietary betaine supplementation on the growth performance and carcass characteristics in male and female growing-finishing pigs. Bulg. J. Agric. Sci. 2009, 15, 263–268. [Google Scholar]
- Wray-Cahen, D.; Fernandez-Fıgares, I.; Virtanen, E.; Steele, N.C.; Caperna, T.J. Betaine improves growth, but does not induce whole body or hepatic palmitate oxidation in swine (sus scrofa domestica). Comp. Biochem. Phys. A 2004, 137, 131–140. [Google Scholar] [CrossRef]
- Huang, Q.C.; Xu, Z.R.; Han, X.Y.; Li, W.F. Changes in hormones, growth factor and lipid metabolism in finishing pigs fed betaine. Livest. Sci. 2006, 105, 78–85. [Google Scholar] [CrossRef]
- Byrne, C.J.; Fair, S.; English, A.M.; Urh, C.; Sauerwein, H.; Crowe, M.A.; Lonergan, P.; Kenny, D.A. Effect of breed, plane of nutrition and age on growth, scrotal development, metabolite concentrations and on systemic gonadotropin and testosterone concentrations following a GnRH challenge in young dairy bulls. Theriogenology 2017, 96, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.; Southern, L.; Pontif, J.; Higbie, A.; Bidner, T. Interactive effects of betaine, crude protein, and net energy in finishing pigs. J. Anim. Sci. 1998, 76, 2444–2455. [Google Scholar] [CrossRef]
- Fernández-Fígares, I.; Conde-Aguilera, J.A.; Nieto, R.; Lachica, M.; Aguilera, J. Synergistic effects of betaine and conjugated linoleic acid on the growth and carcass composition of growing Iberian pigs. J. Anim. Sci. 2008, 86, 102–111. [Google Scholar] [CrossRef]
- Mancini, R.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Hur, S.J.; Park, G.B.; Joo, S.T. Effects of dietary glycine betaine on blood characteristics and pork quality. J. Muscle Foods 2010, 21, 87–101. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Xiong, G.; Shi, L.; Li, X.; Ding, A.; Qiao, Y.; Yang, Y.; Wu, W. Influence of bleeding on myoglobin and meat quality changes of channel catfish muscle during freeze-thaw cycles. J. Food Process Pres. 2021, 45, e15877. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xu, B.C.; Lei, H.M.; Xin, L.; Zhu, L.X.; Zhang, Y.M.; Mao, Y.W.; Liang, R.R. Effects of grape seed extract on meat color and premature browning of meat patties in high-oxygen packaging. J. Integr. Agr. 2022, 21, 2445–2455. [Google Scholar] [CrossRef]
- Hur, S.J.; Yang, H.S.; Park, G.B.; Joo, S.T. Effects of dietary glycine betaine on pork quality in different muscle types. Asian-Australas. J. Anim. Sci. 2007, 20, 1754–1760. [Google Scholar] [CrossRef]
- Jeleníková, J.; Pipek, P.; Miyahara, M. The effects of breed, sex, intramuscular fat and ultimate ph on pork tenderness. Eur. Food Res. Technol. 2008, 227, 989–994. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, D.W. Factors affecting the water holding capacity of red meat products: A review of recent research advances. Crit. Rev. Food Sci. 2008, 48, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Motoyama, M.; Nakajima, I.; Sasaki, K. Relationship between water-holding capacity and intramuscular fat content in japanese commercial pork loin. Asian-Australas. J. Anim. Sci. 2018, 31, 914. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Cano, M.; Lara, L.; Lachica, M.; Aguilera, J.; Fernández-Fígares, I. Influence of betaine and conjugated linoleic acid on development of carcass cuts of Iberian pigs growing from 20 to 50 kg body weight. Meat Sci. 2011, 88, 525–530. [Google Scholar] [CrossRef]
- Fortin, A.; Robertson, W.M.; Tong, A.K.W. The eating quality of Canadian pork and its relationship with intramuscular fat. Meat Sci. 2005, 69, 297–305. [Google Scholar] [CrossRef]
- Dunshea, F.; D’souza, D. A review-fat deposition and metabolism in the pig. Manip. Pig Prod. 2003, IX, 127–150. [Google Scholar]
- Remmerie, A.; Scott, C.L. Macrophages and lipid metabolism. Cell. Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef]
- Mikl, C.; Peters, J.; Trapp, M.; Kornmueller, K.; Schneider, W.J.; Prassl, R. Softness of atherogenic lipoproteins: A comparison of very low density lipoprotein (VLDL) and low density lipoprotein (LDL) using elastic incoherent neutron scattering (EINS). J. Chem. Soc. 2011, 133, 13213–13215. [Google Scholar] [CrossRef]
- Jacobs, K.A.; Krauss, R.M.; Fattor, J.A.; Horning, M.A.; Friedlander, A.L.; Bauer, T.A.; Hagobian, T.A.; Wolfel, E.E.; Brooks, G.A. Endurance training has little effect on active muscle free fatty acid, lipoprotein cholesterol, or triglyceride net balances. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E656–E665. [Google Scholar] [CrossRef]
- Malgwi, I.H.; Halas, V.; Grünvald, P.; Schiavon, S.; Jócsák, I. Genes related to fat metabolism in pigs and intramuscular fat content of pork: A focus on nutrigenetics and nutrigenomics. Animals 2022, 12, 150. [Google Scholar] [CrossRef]
- Kelly, D.M.; Nettleship, J.E.; Akhtar, S.; Muraleedharan, V.; Sellers, D.J.; Brooke, J.C.; McLaren, D.S.; Channer, K.S.; Jones, T.H. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci. 2014, 109, 95–103. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, L.; Chen, L.; Zhang, X.; Cheng, M.; Li, W.; Zhang, Y.; Gao, S. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids 2009, 44, 1029–1037. [Google Scholar] [CrossRef]
- Yao, H.; Tao, X.; Xu, L.; Qi, Y.; Yin, L.; Han, X.; Xu, Y.; Zheng, L.; Peng, J. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway. Pharmacol. Res. 2018, 131, 51–60. [Google Scholar] [CrossRef]
- Ruixia, Z.; Lu, G.; Shuang, M.; Qiang, Z.; Xiaofang, T.; Yinggui, B. Studies on the effects of hypothermia combined with hypoxia on rat skeletal muscle and lipid metabolism based on AMPK/PGC1α pathway. J. Orthop. Surg. Res. 2021, 16, 712. [Google Scholar] [CrossRef]
- Hussein, R.M.; Al-Dalain, S.M. Betaine downregulates microRNA 34a expression via a p53-dependent manner in cisplatin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxic. 2021, 35, e22856. [Google Scholar] [CrossRef]
- Kida, K.; Nakajima, M.; Mohri, T.; Oda, Y.; Takagi, S.; Fukami, T.; Yokoi, T. Pparα is regulated by mir-21 and mir-27b in human liver. Pharmacol. Res. 2011, 28, 2467. [Google Scholar] [CrossRef]






| Items | Phase | |||
|---|---|---|---|---|
| 25–50 kg | 50–75 kg | 75–100 kg | 100–125 kg | |
| Ingredients, % | ||||
| Corn | 76.55 | 80.30 | 84.00 | 88.74 |
| Soybean meal | 16.71 | 15.43 | 10.87 | 6.20 |
| Wheat bran | 0.00 | 0.00 | 1.00 | 1.20 |
| Fish meal | 2.70 | 0.00 | 0.00 | 0.00 |
| Soybean oil | 1.40 | 1.40 | 1.50 | 1.40 |
| Limestone | 0.73 | 0.70 | 0.63 | 0.59 |
| Dicalcium phosphate | 0.47 | 0.66 | 0.53 | 0.43 |
| L-Lysine·HCL | 0.49 | 0.51 | 0.48 | 0.46 |
| DL-Methionine | 0.08 | 0.07 | 0.07 | 0.06 |
| L-Threonine | 0.15 | 0.16 | 0.15 | 0.15 |
| L-Tryptophan | 0.04 | 0.04 | 0.04 | 0.04 |
| NaCl | 0.30 | 0.35 | 0.35 | 0.35 |
| Chloride choline | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin premix 1 | 0.03 | 0.03 | 0.03 | 0.03 |
| Mineral premix 2 | 0.20 | 0.20 | 0.20 | 0.20 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated values | ||||
| Digestible energy, MJ/kg | 14.23 | 14.23 | 14.23 | 14.23 |
| Moisture, % | 12.31 | 11.88 | 11.73 | 11.71 |
| Crude protein, % | 15.69 | 13.75 | 12.13 | 10.44 |
| Crude fat, % | 4.48 | 4.48 | 4.66 | 4.63 |
| Crude fiber, % | 2.22 | 2.19 | 2.05 | 1.87 |
| Calcium | 0.66 | 0.59 | 0.52 | 0.46 |
| Available phosphorus | 0.31 | 0.27 | 0.24 | 0.21 |
| Lysine, % | 1.03 | 0.90 | 0.78 | 0.66 |
| Methionine, % | 0.30 | 0.25 | 0.23 | 0.20 |
| Threonine, % | 0.62 | 0.55 | 0.49 | 0.43 |
| Tryptophan, % | 0.18 | 0.16 | 0.14 | 0.12 |
| Items | Control | Betaine | SEM | p-Value |
|---|---|---|---|---|
| Dressing percentage, % | 74.49 | 74.62 | 0.57 | 0.86 |
| Carcass length, cm | 103.33 | 104.67 | 1.68 | 0.57 |
| Backfat thickness, cm | 1.79 | 1.84 | 0.14 | 0.80 |
| Loin muscle area, cm2 | 45.82 | 48.14 | 3.50 | 0.64 |
| Items | Control | Betaine | SEM | p-Value |
|---|---|---|---|---|
| Color parameters | ||||
| L*45 min (lightness) | 44.03 | 43.25 | 0.55 | 0.34 |
| a*45 min (redness) | 4.04 | 5.11 | 0.34 | <0.05 |
| b*45 min (yellowness) | 2.85 | 2.82 | 0.32 | 0.92 |
| L*24 h (lightness) | 53.84 | 53.00 | 1.00 | 0.54 |
| a*24 h (redness) | 6.45 | 6.62 | 0.38 | 0.74 |
| b*24 h (yellowness) | 4.26 | 3.52 | 0.28 | <0.05 |
| pH45 min | 6.36 | 6.31 | 0.16 | 0.70 |
| pH24 h | 5.49 | 5.53 | 0.07 | 0.57 |
| Cooking loss, % | 36.35 | 32.82 | 0.98 | <0.05 |
| Shear force, N 1 | 45.18 | 38.20 | 2.06 | 0.09 |
| Marbling 2 | 1.42 | 2.30 | - | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, R.; Zhang, H.; Chen, D.; Tian, G.; Zheng, P.; He, J.; Yu, J.; Mao, X.; Huang, Z.; Pu, J.; et al. Long-Term Dietary Supplementation with Betaine Improves Growth Performance, Meat Quality and Intramuscular Fat Deposition in Growing-Finishing Pigs. Foods 2023, 12, 494. https://doi.org/10.3390/foods12030494
Fu R, Zhang H, Chen D, Tian G, Zheng P, He J, Yu J, Mao X, Huang Z, Pu J, et al. Long-Term Dietary Supplementation with Betaine Improves Growth Performance, Meat Quality and Intramuscular Fat Deposition in Growing-Finishing Pigs. Foods. 2023; 12(3):494. https://doi.org/10.3390/foods12030494
Chicago/Turabian StyleFu, Runqi, Hengzhi Zhang, Daiwen Chen, Gang Tian, Ping Zheng, Jun He, Jie Yu, Xiangbing Mao, Zhiqing Huang, Junning Pu, and et al. 2023. "Long-Term Dietary Supplementation with Betaine Improves Growth Performance, Meat Quality and Intramuscular Fat Deposition in Growing-Finishing Pigs" Foods 12, no. 3: 494. https://doi.org/10.3390/foods12030494