A Combinational Optimization Method for Efficient Production of Indigo by the Recombinant Escherichia coli with Expression of Monooxygenase and Malate Dehydrogenase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymes and Chemicals
2.2. Strains, Plasmids and Culture Conditions
2.3. DNA Manipulation Techniques
2.4. Shaking Flask Fermentation of Indigo
2.5. Gene Expression Optimization of styAB and mdh by Using Different Promoters
2.6. RNA Preparation and Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) Analysis
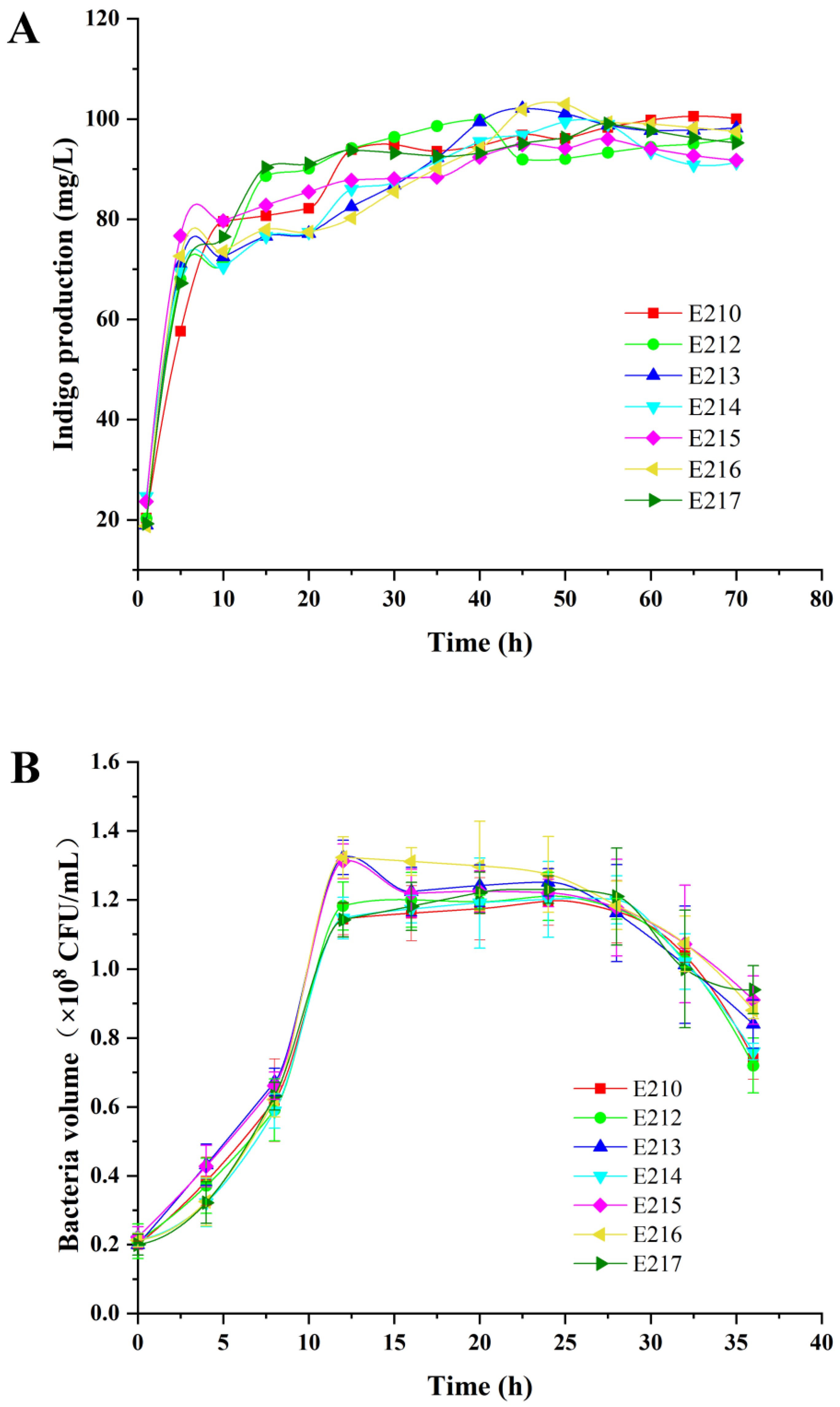
2.7. Measurement of Growth Curve
2.8. Enzyme Assays
2.9. Measurement of Indigo and Tryptophan
3. Results
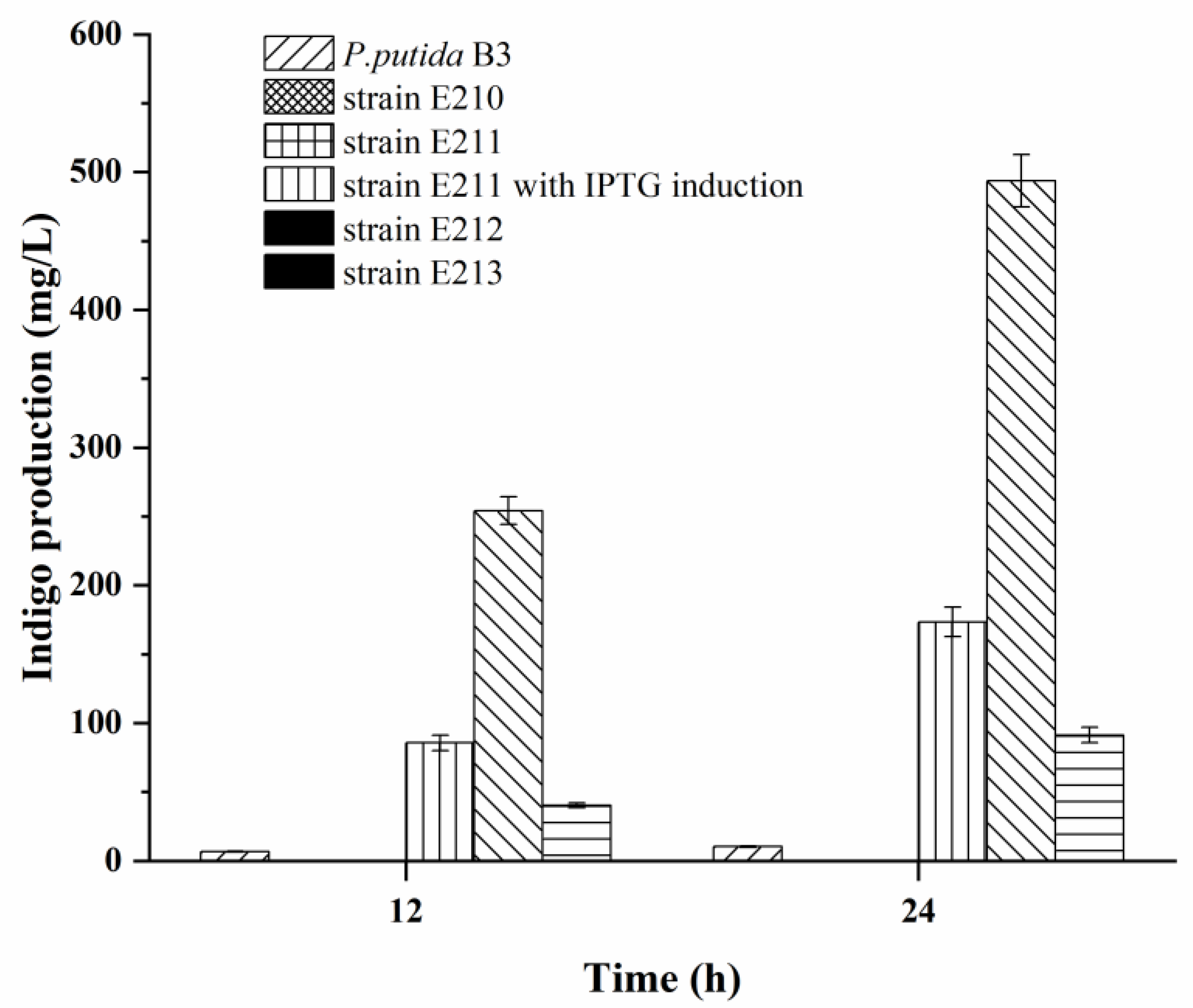
3.1. Introduction and Promoter Optimization of Indigo Biosynthetic Pathway Genes into E. coli
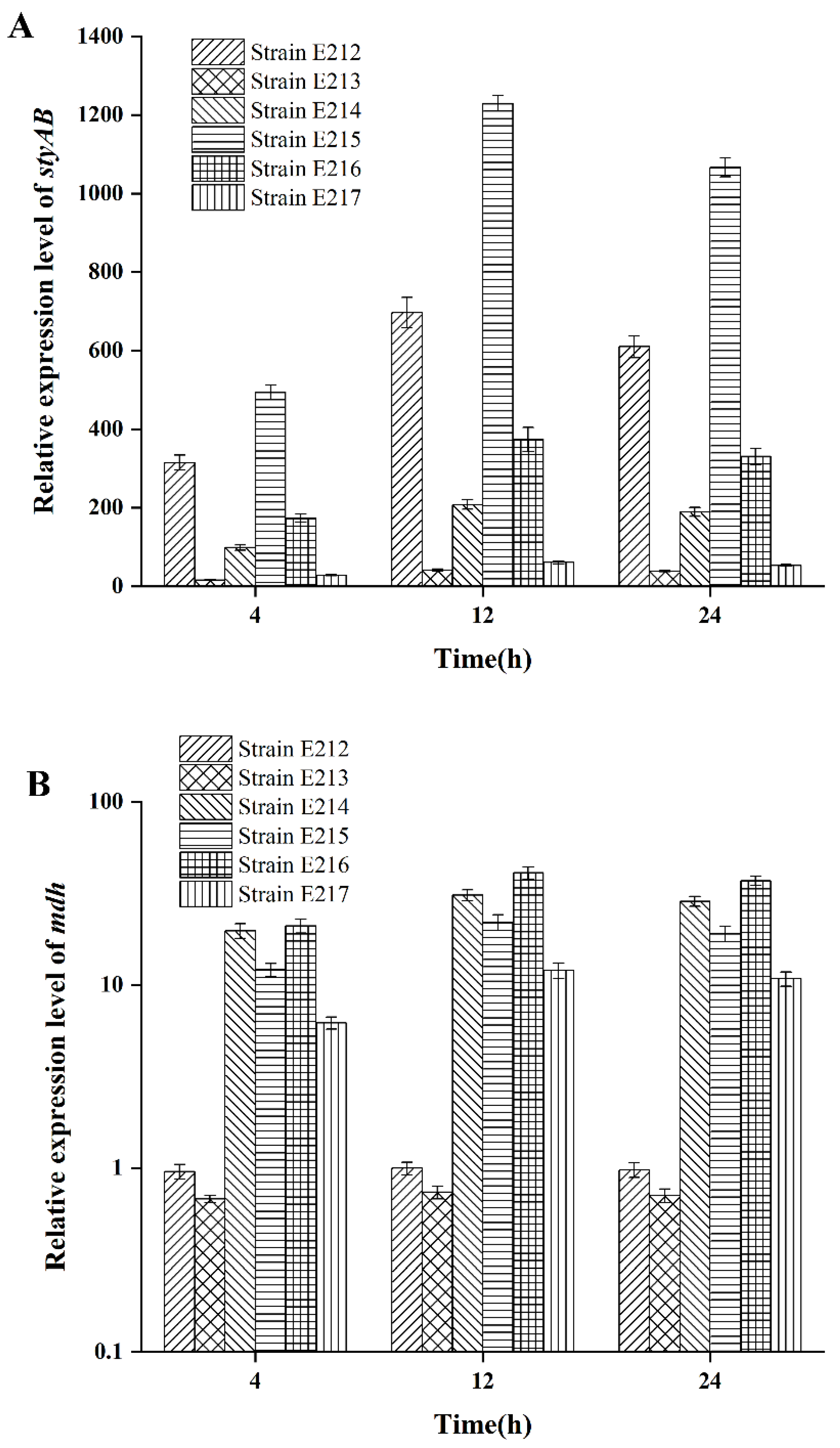
3.2. Regulation of styAB Expression by Redox-Cofactor Rebalancing with Promoter Combinational Optimization
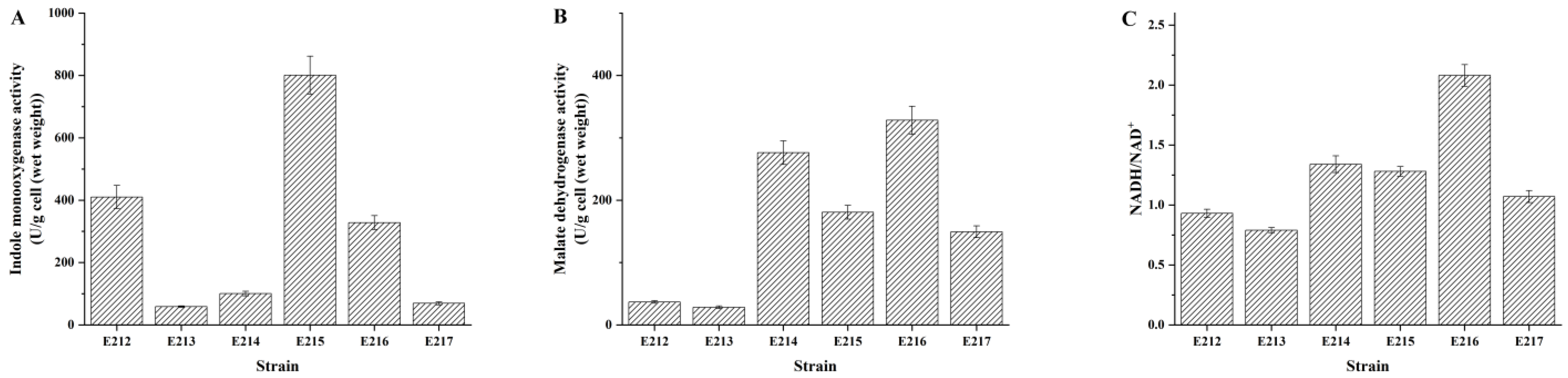
3.3. Activities of Indigo Biosynthesis Enzymes and Ratio of NADH/NAD+
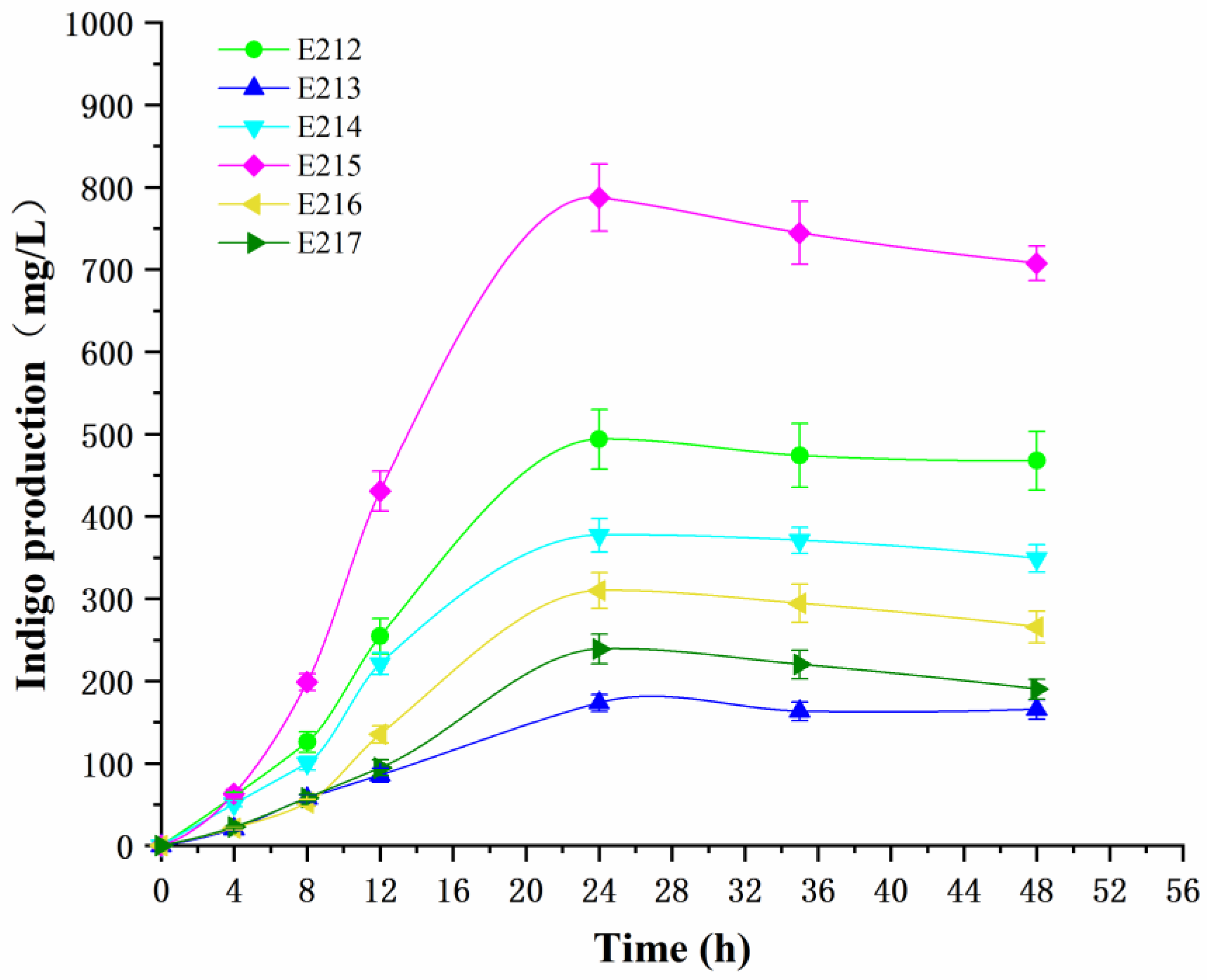
3.4. Indigo Production in Recombinant Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liquids 2020, 318, 114061. [Google Scholar] [CrossRef]
- Pattanaik, L.; Padhi, S.K.; Hariprasad, P.; Naik, S.N. Life cycle cost analysis of natural indigo dye production from Indigofera tinctoria L. plant biomass: A case study of India. Clean Technol. Environ. Policy 2020, 22, 1639–1654. [Google Scholar] [CrossRef]
- Teanglum, A.; Teanglum, S.; Saithong, A. Selection of Indigo Plant Varieties and Other Plants that Yield Indigo Dye. In Proceedings of the 3rd International Science, Social Science, Engineering and Energy Conference (ISEEC), Nakhon Pathom, Thailand, 2–5 February 2012; pp. 184–190. [Google Scholar]
- Choi, K.Y. A review of recent progress in the synthesis of bio-indigoids and their biologically assisted end-use applications. Dye. Pigment. 2020, 181, 108570. [Google Scholar] [CrossRef]
- Wambuguh, D.; Chianelli, R.R. Indigo dye waste recovery from blue denim textile effluent: A by-product synergy approach. New J. Chem. 2008, 32, 2189–2194. [Google Scholar] [CrossRef]
- Pattanaik, L.; Duraivadivel, P.; Hariprasad, P.; Naik, S.N. Utilization and re-use of solid and liquid waste generated from the natural indigo dye production process—A zero waste approach. Bioresour. Technol. 2020, 301, 122721. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Lopes, H.d.F.S.; Igarashi, K.; Yumoto, I. Characterization of the microbiota in long- and short-term natural indigo fermentation. J. Ind. Microbiol. Biotechnol. 2019, 46, 1657–1667. [Google Scholar] [CrossRef]
- Pathak, H.; Madamwar, D. Biosynthesis of Indigo Dye by Newly Isolated Naphthalene-Degrading Strain Pseudomonas sp. HOB1 and its Application in Dyeing Cotton Fabric. Appl. Biochem. Biotechnol. 2010, 160, 1616–1626. [Google Scholar] [CrossRef]
- O’Connor, K.E.; Dobson, A.D.; Hartmans, S. Indigo formation by microorganisms expressing styrene monooxygenase activity. Appl. Environ. Microbiol. 1997, 63, 4287–4291. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; Samanta, S.K.; Jain, R.K. Indigo production by naphthalene-degrading bacteria. Lett. Appl. Microbiol. 2000, 31, 5–9. [Google Scholar] [CrossRef]
- Alemayehu, D.; Gordon, L.M.; O’Mahony, M.M.; O’Leary, N.D.; Dobson, A.D.W. Cloning and functional analysis gene involved in indigo production by gene disruption of a novel and fluoranthene metabolism in Pseudomonas alcaligenes PA-10. FEMS Microbiol. Lett. 2004, 239, 285–293. [Google Scholar] [CrossRef]
- Doukyu, N.; Aono, R. Biodegradation of indole at high concentration by persolvent fermentation with Pseudomonas sp. ST-200. Extremophiles 1997, 1, 100–105. [Google Scholar] [CrossRef]
- Toda, H.; Itoh, N. Isolation and characterization of styrene metabolism genes from styrene-assimilating soil bacteria Rhodococcus sp. ST-5 and ST-10. J. Biosci. Bioeng. 2012, 113, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, X.; Ma, Q.; Ma, F.; Zhang, Q.; Li, X.; Zhou, H.; Zhou, J. Indigo biosynthesis by Comamonas sp. MQ. Biotechnol. Lett. 2012, 34, 353–357. [Google Scholar] [CrossRef]
- Lin, G.-H.; Chen, H.-P.; Huang, J.-H.; Liu, T.-T.; Lin, T.-K.; Wang, S.-J.; Tseng, C.-H.; Shu, H.-Y. Identification and characterization of an indigo-producing oxygenase involved in indole 3-acetic acid utilization by Acinetobacter baumannii. Antonie Van Leeuwenhoek 2012, 101, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ruiz, E.; Hernaez, M.J.; Martinez-Perez, O.; Santero, E. Identification and functional characterization of Sphingomonas macrogolitabida strain TFA genes involved in the first two steps of the tetralin catabolic pathway. J. Bacteriol. 2003, 185, 2026–2030. [Google Scholar] [CrossRef] [Green Version]
- Son, K.; Shin, Y.; Yoo, D.I. Effect of pH Condition on Natural Indigo (Indigofera tinctoria) Reduction by Yeast (Saccharomyces cerevisiae). Fibers Polym. 2019, 20, 2570–2580. [Google Scholar] [CrossRef]
- Ensley, B.D.; Ratzkin, B.J.; Osslund, T.D.; Simon, M.J.; Wackett, L.P.; Gibson, D.T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 1983, 222, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Takahashi, S.; Ishii, Y.; Kino, K.; Kirimura, K. Cloning of a gene encoding flavin reductase coupling with dibenzothiophene monooxygenase through coexpression screening using indigo production as selective indication. Biochem. Biophys. Res. Commun. 2004, 313, 570–575. [Google Scholar] [CrossRef]
- Inoue, S.; Morita, R.; Minami, Y. An indigo-producing plant, Polygonum tinctorium, possesses a flavin-containing monooxygenase capable of oxidizing indole. Biochem. Biophys. Res. Commun. 2021, 534, 199–205. [Google Scholar] [CrossRef]
- Cheng, L.; Yue, J.; Yin, S.; Ren, M.; Wang, C. Expression of styAB is regulated by a two-component system during indigo biosynthesis in Pseudomonas putida. Biochem. Biophys. Res. Commun. 2019, 519, 198–203. [Google Scholar] [CrossRef]
- Cheng, L.; Yin, S.; Chen, M.; Sun, B.; Hao, S.; Wang, C. Enhancing Indigo Production by Over-Expression of the Styrene Monooxygenase in Pseudomonas putida. Curr. Microbiol. 2016, 73, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Dong, S.; Liu, Y.; Pei, X.-Q.; Lin, H.; Wu, Z.-L. A new clade of styrene monooxygenases for (R)-selective epoxidation. Catal. Sci. Technol. 2021, 11, 2195–2201. [Google Scholar] [CrossRef]
- Eaton, R.W.; Chapman, P.J. Formation of indigo and related compounds from indolecarboxylic acids by aromatic acid-degrading bacteria: Chromogenic reactions for cloning genes encoding dioxygenases that act on aromatic acids. J. Bacteriol. 1995, 177, 6983–6988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doukyu, N.; Toyoda, K.; Aono, R. Indigo production by Escherichia coli carrying the phenol hydroxylase gene from Acinetobacter sp. strain ST-550 in a water-organic solvent two-phase system. Appl. Microbiol. Biotechnol. 2003, 60, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Du, L.Y.; Yue, J.M.; Zhu, Y.Y.; Yin, S. Production of Indigo by Recombinant Escherichia coli with Expression of Monooxygenase, Tryptophanase, and Molecular Chaperone. Foods 2022, 11, 2117. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
| Strains and Plasmids | Related Properties or Functions | Source |
|---|---|---|
| Strains | ||
| Pseudomonas putida B3 | Wild type, used to offer styAB gene | Laboratory collection |
| E. coli-BL21 (DE3) | Used as host strain | Tiangen Biotech, Beijing, China |
| E210 | E. coli-BL21 (DE3) harboring pET-28a(+) | This work |
| E211 | E. coli-BL21 (DE3) harboring pET-28a(+)-styAB | This work |
| E212 | E. coli-BL21 (DE3) harboring pETPT7-styAB | This work |
| E213 | E. coli-BL21 (DE3) harboring pETPcat-styAB | This work |
| E214 | E. coli-BL21 (DE3) harboring pETPT7-styAB-PT7-mdh | This work |
| E215 | E. coli-BL21 (DE3) harboring pETPT7-styAB-Pcat-mdh | This work |
| E216 | E. coli-BL21 (DE3) harboring pETPcat-styAB-PT7-mdh | This work |
| E217 | E. coli-BL21 (DE3) harboring pETPcat-styAB-Pcat-mdh | This work |
| Plasmids | ||
| pET-28a(+) | Heterologous expression vector | MiaoLingPlasmid, Wuhan, China |
| pHS-avc | Used to offer Pcat promoter | Laboratory collection |
| pET-28a(+)-styAB | pET-28a(+) carrying styAB gene originated from Pseudomonas putida B3, inducible expression | This work |
| pETPT7-styAB | pET-28a(+) carrying styAB gene ligated with PT7 promoter, constitutive expression | This work |
| pETPcat-styAB | pET-28a(+) carrying styAB gene ligated with Pcat promoter, constitutive expression | This work |
| pETPT7-styAB-PT7-mdh | pET-28a(+) carrying styAB gene ligated with PT7 promoter and mdh gene ligated with PT7 promoter, constitutive expression | This work |
| pETPT7-styAB-Pcat-mdh | pET-28a(+) carrying styAB gene ligated with PT7 promoter and mdh gene ligated with Pcat promoter, constitutive expression | This work |
| pETPcat-styAB-PT7-mdh | pET-28a(+) carrying styAB gene ligated with Pcat promoter and mdh gene ligated with PT7 promoter, constitutive expression | This work |
| pETPcat-styAB-Pcat-mdh | pET-28a(+) carrying styAB gene ligated with Pcat promoter and mdh gene ligated with Pcat promoter, constitutive expression | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Tao, D.; Ren, M.; Cheng, L. A Combinational Optimization Method for Efficient Production of Indigo by the Recombinant Escherichia coli with Expression of Monooxygenase and Malate Dehydrogenase. Foods 2023, 12, 502. https://doi.org/10.3390/foods12030502
Pan Z, Tao D, Ren M, Cheng L. A Combinational Optimization Method for Efficient Production of Indigo by the Recombinant Escherichia coli with Expression of Monooxygenase and Malate Dehydrogenase. Foods. 2023; 12(3):502. https://doi.org/10.3390/foods12030502
Chicago/Turabian StylePan, Zijing, Dejiang Tao, Mingjing Ren, and Lei Cheng. 2023. "A Combinational Optimization Method for Efficient Production of Indigo by the Recombinant Escherichia coli with Expression of Monooxygenase and Malate Dehydrogenase" Foods 12, no. 3: 502. https://doi.org/10.3390/foods12030502
APA StylePan, Z., Tao, D., Ren, M., & Cheng, L. (2023). A Combinational Optimization Method for Efficient Production of Indigo by the Recombinant Escherichia coli with Expression of Monooxygenase and Malate Dehydrogenase. Foods, 12(3), 502. https://doi.org/10.3390/foods12030502







