Pleurotus ostreatus Grown on Agro-Industrial Residues: Studies on Microbial Contamination and Shelf-Life Prediction under Different Packaging Types and Storage Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Total Microbial Count (TMC)
2.4. Physicochemical Properties and Nutritional Composition Variation in Mushrooms during Storage
2.5. Statistical Analysis
3. Results and Discussion
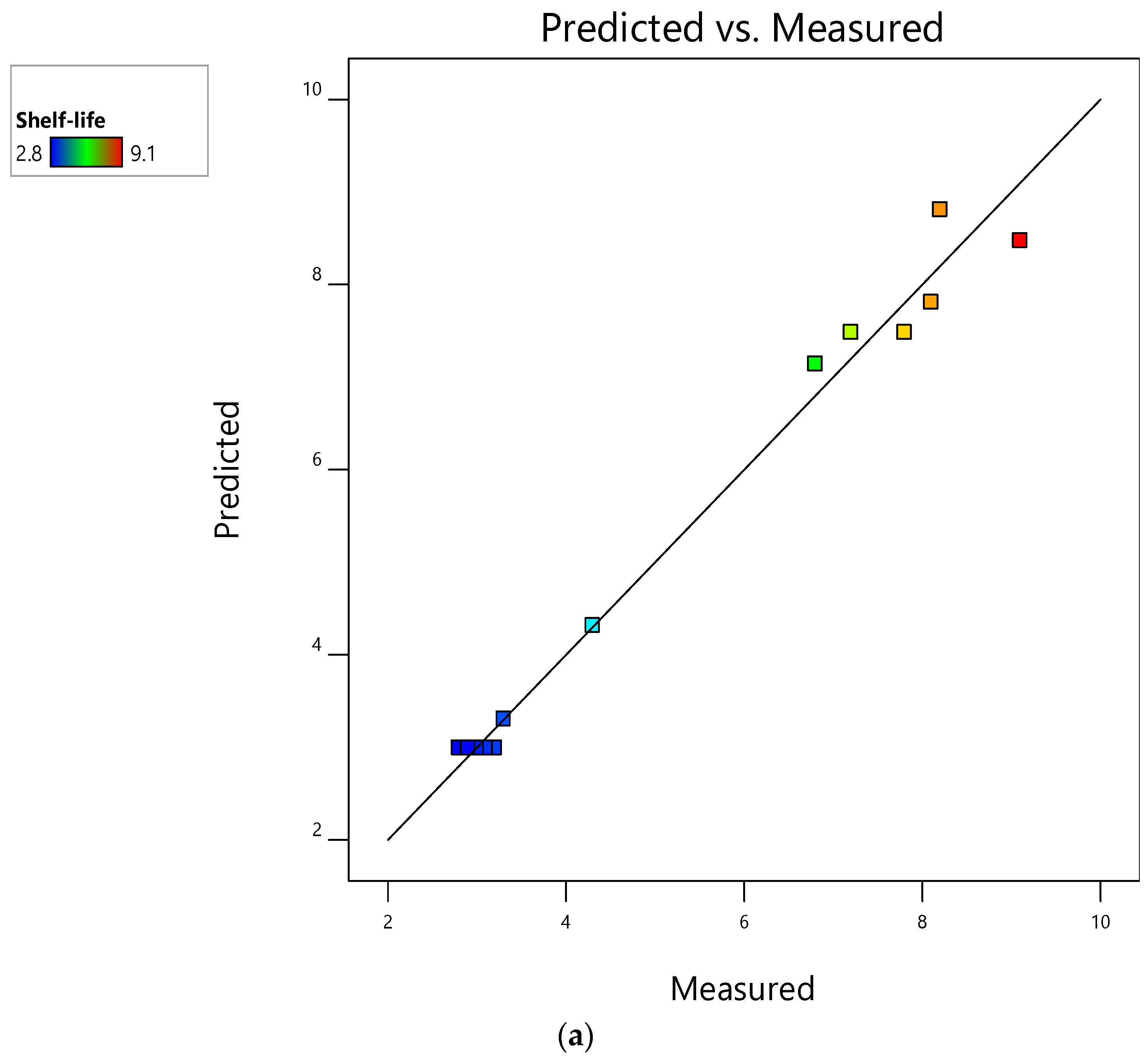
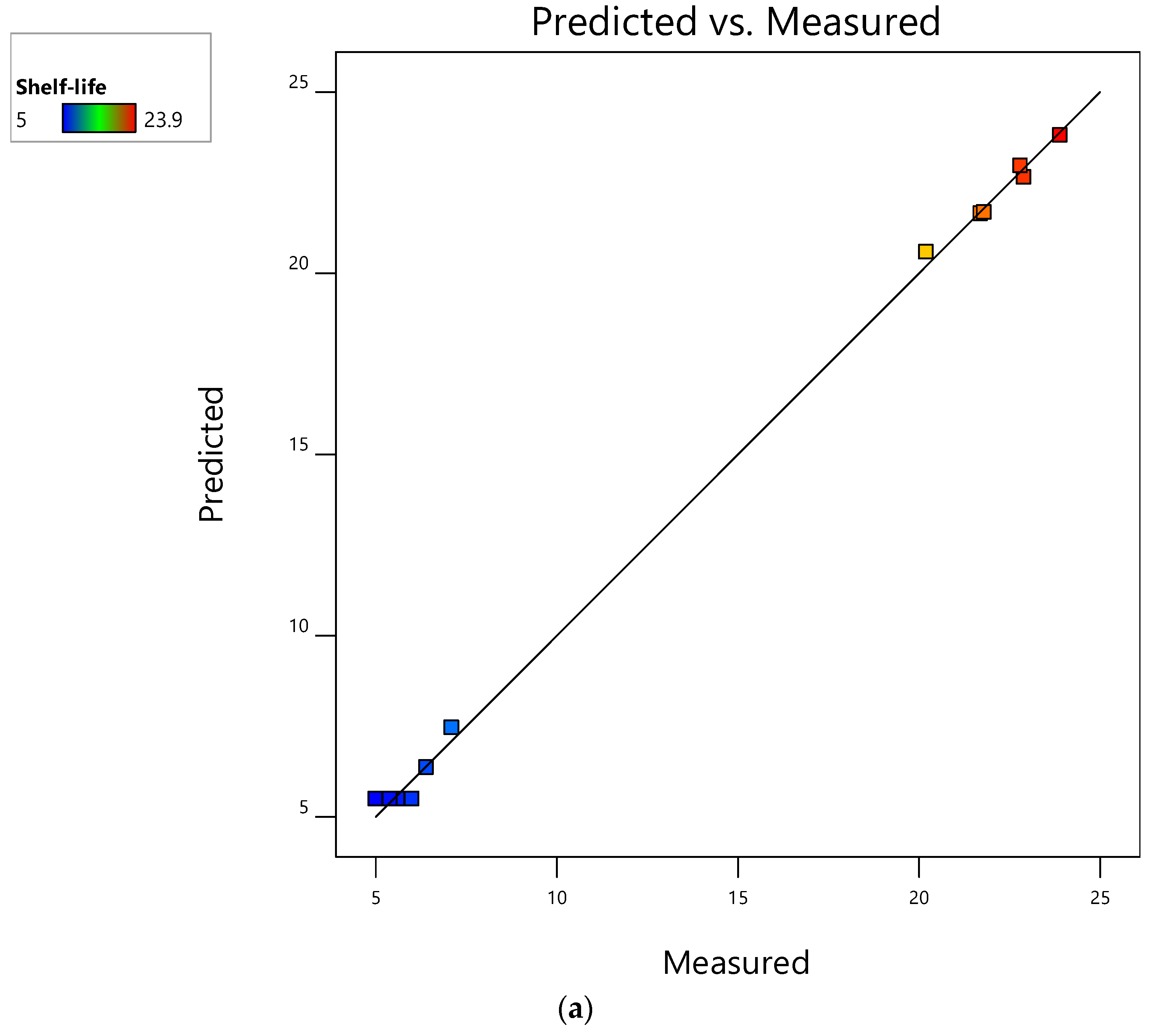
3.1. Evaluation of RSM Models and Their Performance for Mushroom Shelf-Life Extension during Storage at Ambient Temperature
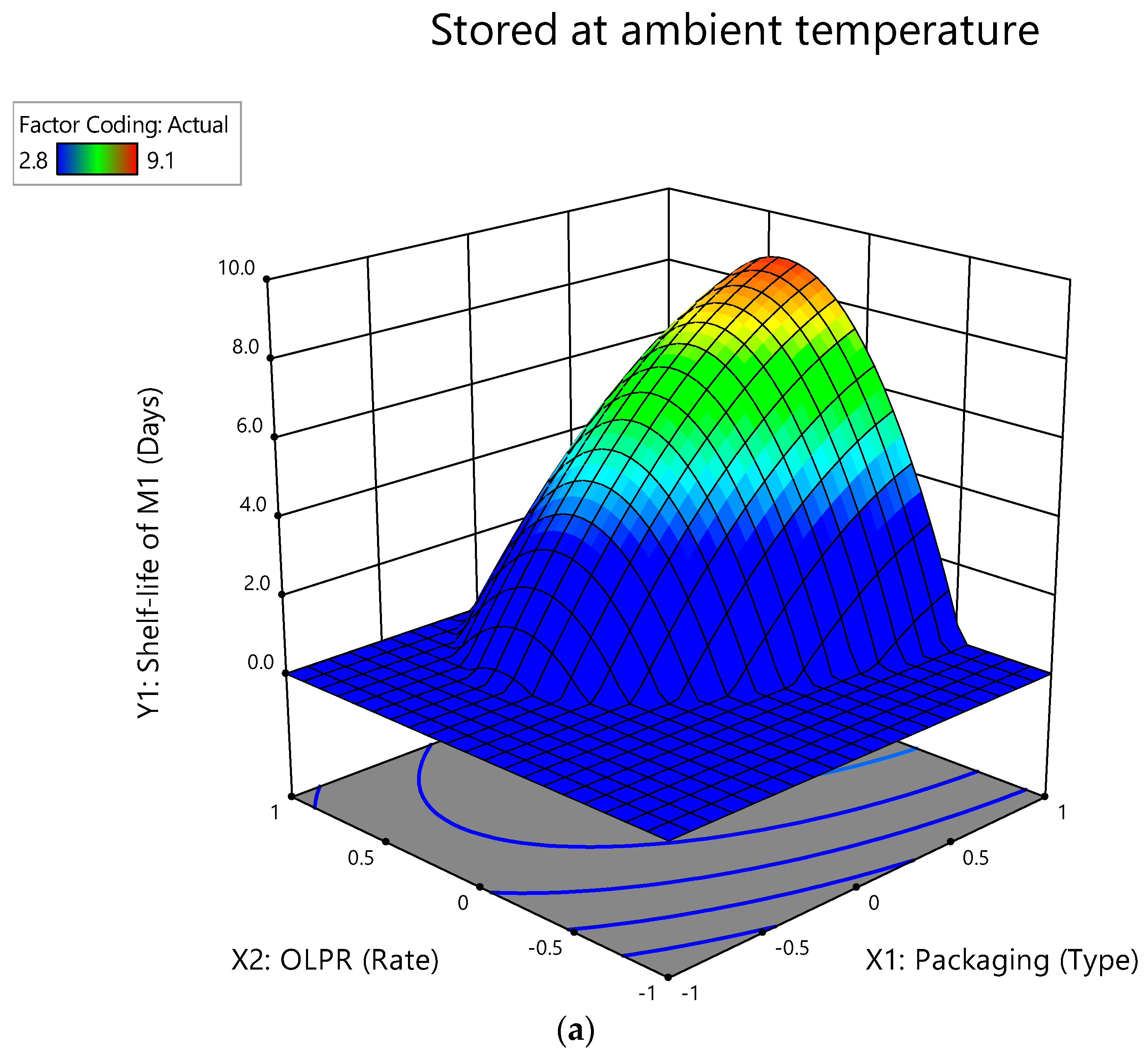
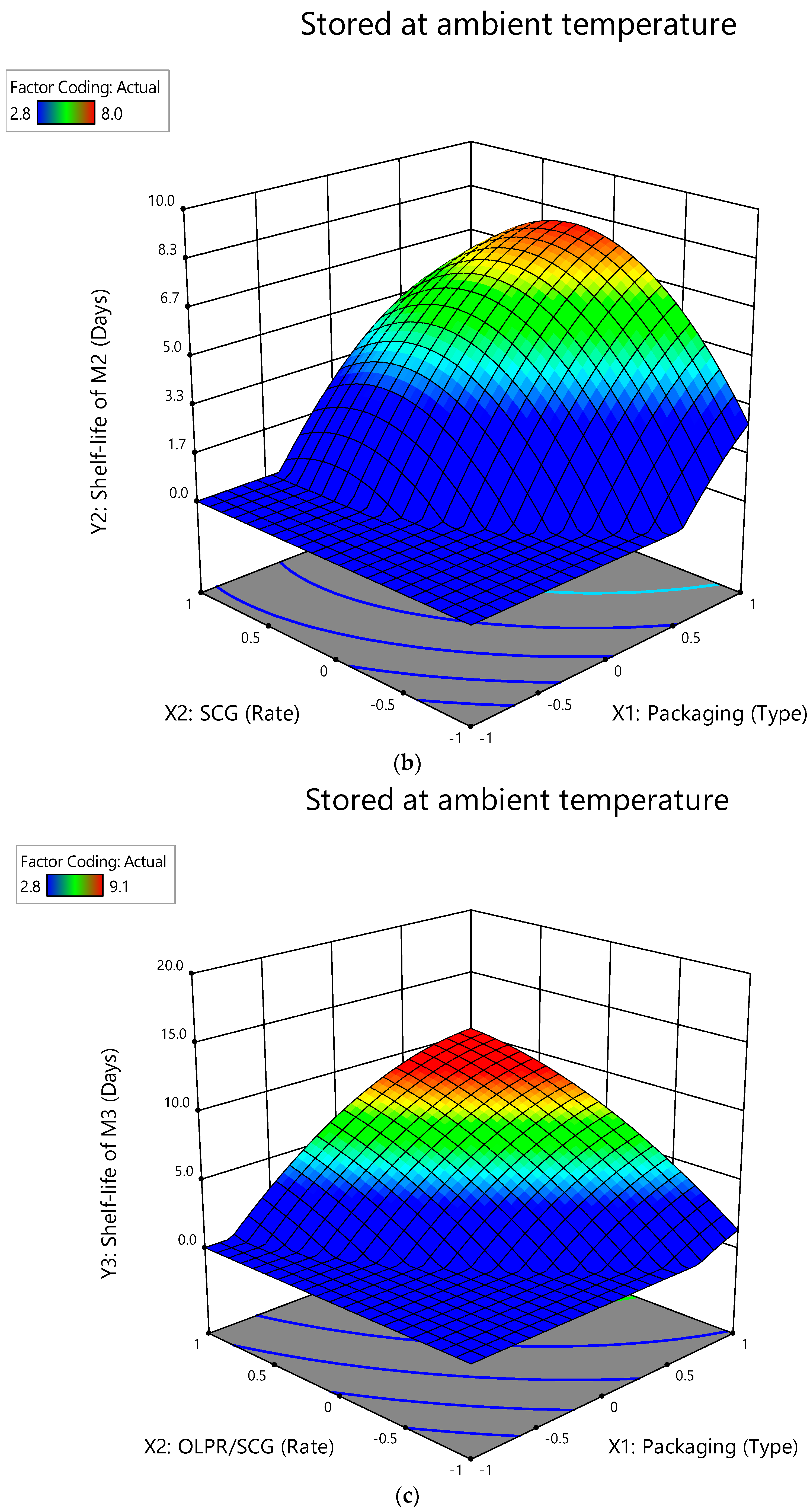
3.2. Interactive Effect of Factors on Shelf-Life of Mushrooms Stored at Ambient Temperature
3.3. Optimization of Factors for Extended Shelf-Life of Mushrooms Stored at Ambient Temperature
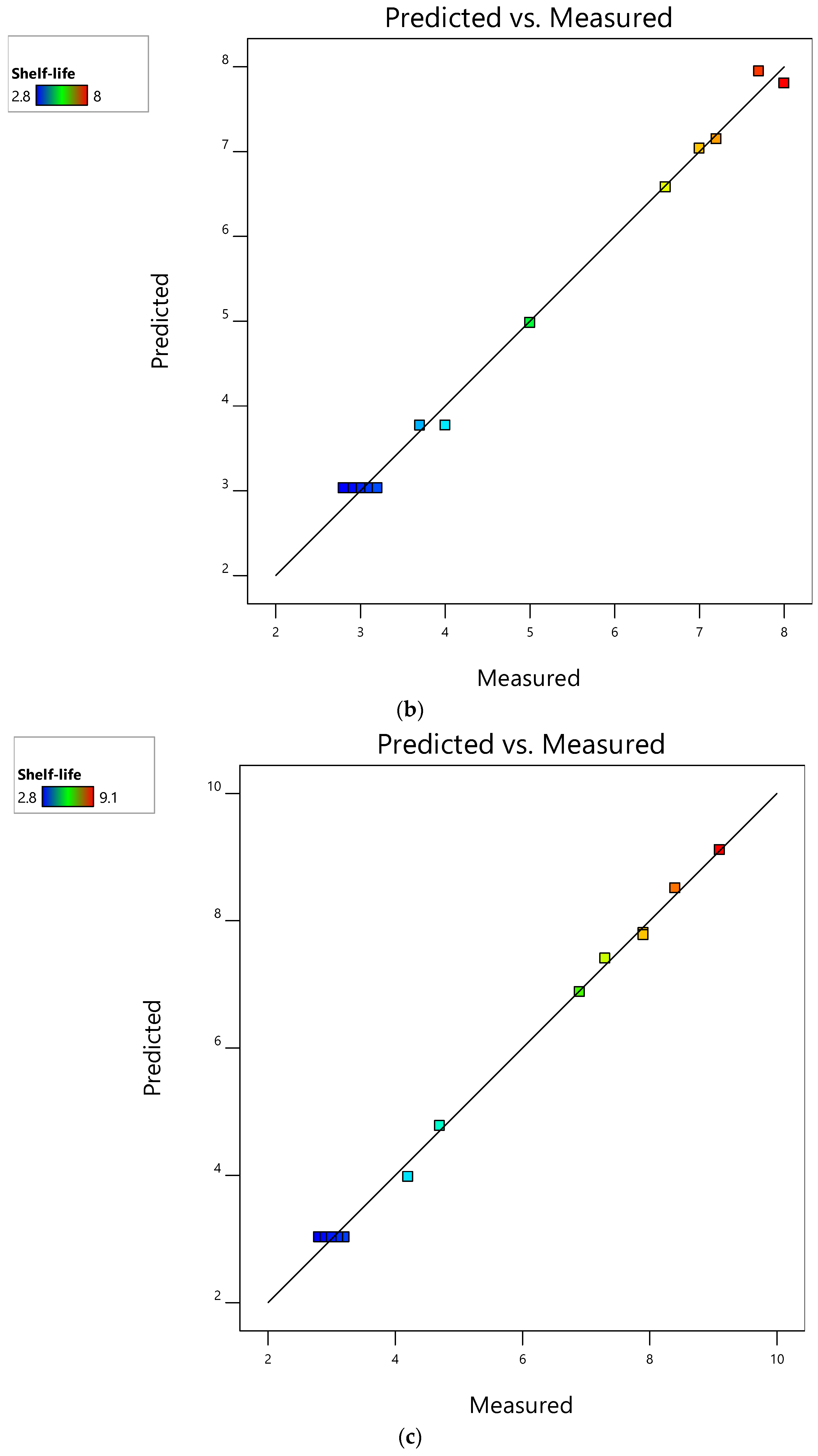
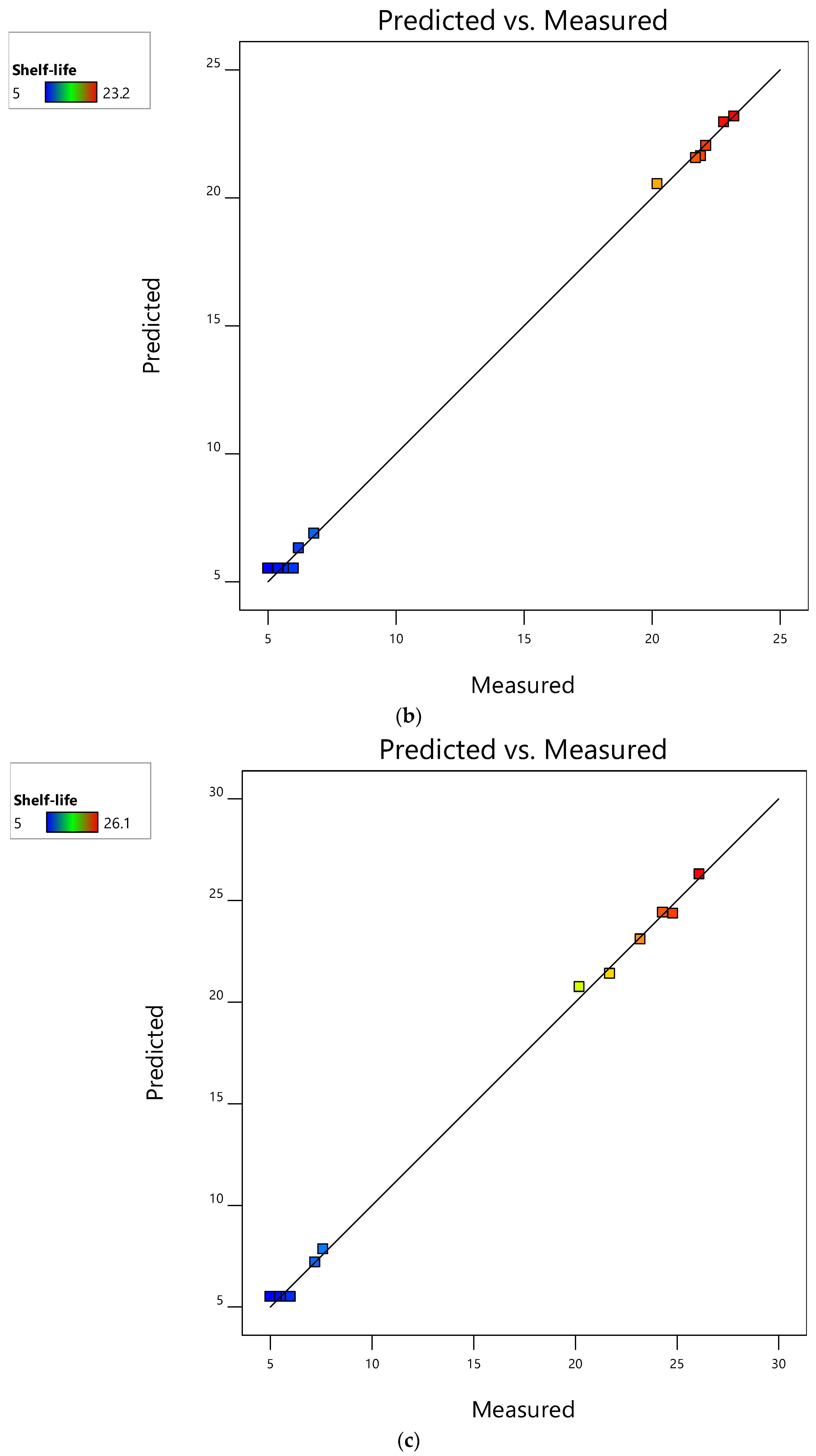
3.4. Evaluation of RSM Models and Their Performance for Mushroom Shelf-Life Extension during Storage at 4 °C
3.5. Interactive Effects of Factors on Shelf-Life of Mushrooms Stored at 4 °C
3.6. Optimization of Factors for Extended Shelf-Life of Mushrooms Stored at 4 °C
3.7. Total Microbial Count (TMC)
3.8. Physicochemical Properties and Nutritional Composition Variation in Mushrooms during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, V.N.; Yadav, N.; Gaur, M. Mushroom Production and Processing Technology; Agrobios: Rajasthan, India, 1998. [Google Scholar]
- Jiang, T.; Feng, L.; Li, J. Changes in microbial and postharvest quality of shiitake mushroom (Lentinus edodes) treated with chitosan–glucose complex coating under cold storage. Food Chem. 2012, 131, 780–786. [Google Scholar] [CrossRef]
- Jiang, T. Effect of alginate coating on physicochemical and sensory qualities of button mushrooms (Agaricus bisporus) under a high oxygen modified atmosphere. Postharvest Biol. Technol. 2013, 76, 91–97. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Abou Fayssal, S.; Alsanad, M.A.; El Sebaaly, Z.; Ismail, A.I.H.; Sassine, Y.N. Valorization of olive pruning residues through bioconversion into edible mushroom Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) of improved nutritional value. Scientifica 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; Alsanad, M.A.; Yordanova, M.H.; El Sebaaly, Z.; Najjar, R.; Sassine, Y.N. Effect of olive pruning residues on substrate temperature and production of oyster mushroom (Pleurotus ostreatus). Acta Hortic. 2021, 1327, 245–252. [Google Scholar] [CrossRef]
- Alsanad, M.A.; Sassine, Y.N.; El Sebaaly, Z.; Abou Fayssal, S. Spent coffee grounds influence on Pleurotus ostreatus production, composition, fatty acid profile, and lignocellulose biodegradation capacity. CyTA–J. Food 2021, 19, 11–20. [Google Scholar] [CrossRef]
- Naim, L.; Alsanad, M.A.; El Sebaaly, Z.; Shaban, N.; Abou Fayssal, S.; Sassine, Y.N. Variation of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) performance subjected to different doses and timings of nano-urea. Saudi J. Biol. Sci. 2020, 27, 1573–1579. [Google Scholar] [CrossRef]
- Naim, L.; Alsanad, M.A.; Shaban, N.; El Sebaaly, Z.; Abou Fayssal, S.; Sassine, Y.N. Production and composition of Pleurotus ostreatus cultivated on Lithovit ®-Amino25 supplemented spent substrate. AMB Expr. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Naim, L.; El Sebaaly, Z.; Abou Fayssal, S.; Alsanad, M.A.; Najjar, R.; Yordanova, M.H.; Sassine, Y.N. The use of nitrogen-rich nano-supplements affects substrate temperature, delays the production cycle, and increases yield of Pleurotus ostreatus. Acta Hortic. 2021, 1327, 831–840. [Google Scholar] [CrossRef]
- Werghemmi, W.; Abou Fayssal, S.; Mazouz, H.; Hajjaj, H.; Hajji, L. Olive and green tea leaves extract in Pleurotus ostreatus var. florida culture media: Effect on mycelial linear growth rate, diameter and growth induction index. IOP Conf. Ser. Earth Environ. Sci. 2022, 1090, 012020. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; El Sebaaly, Z.; Alsanad, M.A.; Najjar, R.; Bohme, M.; Yordanova, M.H.; Sassine, Y.N. Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition, and nutritional value. PLoS ONE 2021, 16, e0255794. [Google Scholar] [CrossRef]
- Sassine, Y.N.; Naim, L.; El Sebaaly, Z.; Abou Fayssal, S.; Alsanad, M.A.; Yordanova, M.H. Nano urea effects on Pleurotus ostreatus nutritional value depending on the dose and timing of application. Sci. Rep. 2021, 11, 5588. [Google Scholar] [CrossRef] [PubMed]
- Abou Fayssal, S.; Hammoud, M.; El Sebaaly, Z.; Sassine, Y.N. Improvement of compost quality. In Mushrooms: Agaricus bisporus; Sassine, Y.N., Ed.; CAB International: Wallingford, UK, 2021; pp. 136–175. [Google Scholar]
- Sajyan, T.K.; Abou Fayssal, S.; Bejjani, R.; El Sebaaly, Z.; Sassine, Y.N. Casing and cropping. Harvest and postharvest technologies. In Mushrooms: Agaricus bisporus; Sassine, Y.N., Ed.; CAB International: Wallingford, UK, 2021; pp. 240–278. [Google Scholar]
- Elbagory, M.; El-Nahrawy, S.; Omara, A.E.-D.; Eid, E.M.; Bachheti, A.; Kumar, P.; Abou Fayssal, S.; Adelodun, B.; Bachheti, R.K.; Kumar, P.; et al. Sustainable Bioconversion of Wetland Plant Biomass for Pleurotus ostreatus var. florida Cultivation: Studies on Proximate and Biochemical Characterization. Agriculture 2022, 12, 2095. [Google Scholar] [CrossRef]
- Ranjbar, M.E.; Olfati, J.A.; Amani, M. Influence of enriched soaking water on shiitake (Lentinus edodes (Berk.) Singer) mushroom yield and properties. Acta Agric. Slov. 2017, 1093, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Hassan, M.K.; Talukder, F.U. Effect of low temperature on postharvest behaviors of oyster mushroom (Pleurotus spp.). Int. J. Hortic. Sci. Technol. 2020, 7, 213–225. [Google Scholar] [CrossRef]
- El Hage, R.; Khalaf, Y.; Abou Fayssal, S.; Hammoud, M.; El Sebaaly, Z.; Sassine, Y.N. Harvest and postharvest technologies. In Mushrooms: Agaricus bisporus; Sassine, Y.N., Ed.; CAB International: Wallingford, UK, 2021; pp. 357–426. [Google Scholar]
- Kumar, P.; Kumar, V.; Eid, E.M.; Al-Huqail, A.A.; Adelodun, B.; Abou Fayssal, S.; Goala, M.; Arya, A.K.; Bachheti, A.; Andabaka, Ž.; et al. Spatial assessment of potentially toxic elements (PTE) concentration in Agaricus bisporus mushroom collected from local vegetable markets of Uttarakhand state, India. J. Fungi 2022, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Širić, I.; Kumar, P.; Adelodun, B.; Abou Fayssal, S.; Bachheti, R.K.; Bachheti, A.; Ajibade, F.O.; Kumar, V.; Taher, M.A.; Eid, E.M. Risk Assessment of Heavy Metals Occurrence in Two Wild Edible Oyster Mushrooms (Pleurotus spp.) Collected from Rajaji National Park. J. Fungi 2022, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Selina Wamucii. Lebanon Mushroom Prices. Available online: https://www.selinawamucii.com/insights/prices/lebanon/mushrooms/ (accessed on 10 November 2022).
- Singh, P.; Langowski, H.C.; Wani, A.A.; Saengerlaub, S. Recent advances in extending the shelf life of fresh Agaricus mushrooms: A review. J. Sci. Food Agric. 2010, 90, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A.E.; Didin, O.; Candir, E.; Kaplankiran, M.; Yildiz, E. Effects of rootstocks on storage performance of Nova mandarins. Turk. J. Agric. For. 2019, 43, 307–317. [Google Scholar] [CrossRef]
- Song, Y.; Hum, Q.; Wu, Y.; Pei, F.; Kimatu, B.M.; Su, A.; Yang, W. Storage time assessment and shelf-life prediction models for postharvest Agaricus bisporus. LWT–Food Sci. Technol. 2019, 101, 360–365. [Google Scholar] [CrossRef]
- Kibar, H.; Kibar, B. Hypobaric storage technique in the mushroom preservation. Int. J. Agric. Wildlife Sci. 2015, 1, 117–125. [Google Scholar]
- Kibar, B. Influence of different drying methods and cold storage treatments on the postharvest quality and nutritional properties of P. ostreatus mushroom. Turk. J. Agric. For. 2021, 45, 565–579. [Google Scholar] [CrossRef]
- Galani, J.H.Y.; Patel, J.S.; Patel, N.J.; Talati, J.G. Storage of fruits and vegetables in refrigerator increases their phenolic acids but decreases the total phenolics, anthocyanins and vitamin C with subsequent loss of their antioxidant capacity. Antioxidants 2017, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh mushroom preservation techniques. Foods 2021, 10, 2126. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Singh, J.; Kumar, P. Use of sugar mill wastewater for Agaricus bisporus cultivation: Prediction models for trace metal uptake and health risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, V.; Goala, M.; Singh, J.; Kumar, P. Integrated use of treated dairy wastewater and agro-residue for Agaricus bisporus mushroom cultivation: Experimental and kinetics studies. Biocatal. Agric. Biotechnol. 2021, 32, 101940. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Adelodun, B.; Bedeković, D.; Kos, I.; Širić, I.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M.; Abou Fayssal, S.; et al. Sustainable use of sewage sludge as a casing material for button mushroom (Agaricus bisporus) cultivation: Experimental and prediction modeling studies for uptake of metal elements. J. Fungi 2022, 8, 112. [Google Scholar] [CrossRef]
- Kumar, P.; Eid, E.M.; Al-Huqail, A.A.; Širić, I.; Adelodun, B.; Abou Fayssal, S.; Valadez-Blanco, R.; Goala, M.; Ajibade, F.O.; Choi, K.S.; et al. Kinetic studies on delignification and heavy metals uptake by shiitake (Lentinula edodes) mushroom cultivated on agro-industrial wastes. Horticulturae 2022, 8, 316. [Google Scholar] [CrossRef]
- Ares, G.; Lareo, C.; Lema, P. Modified atmosphere packaging for postharvest storage of mushrooms. A review. Fresh Prod. 2007, 1, 32–40. [Google Scholar]
- Kumar, V.; Singh, J.; Kumar, P.; Kumar, P. Response surface methodology based electro-kinetic modelling of biological and chemical oxygen demand removal from sugar mill effluent by water hyacinth (Eichhornia crassipes) in a continuous stirred tank reactor (CSTR). Environ. Technol. Innov. 2019, 14, 100327. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Kumar, P.; Singh, J. Anaerobic digestion of Azolla pinnata biomass grown in integrated industrial effluent for enhanced biogas production and COD reduction: Optimization and kinetics studies. Environ. Technol. Innov. 2020, 17, 100627. [Google Scholar] [CrossRef]
- Hwang, I.-W.; Kim, B.-M.; Kim, Y.-C.; Lee, S.-H.; Chung, S.-K. Improvement in β-glucan extraction from Ganoderma lucidum with high-pressure steaming and enzymatic pre-treatment. Appl. Biol. Chem. 2018, 61, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Schill, S.; Stessl, B.; Meier, N.; Tichy, A.; Wagner, M.; Ludewig, M. Microbiological Safety and Sensory Quality of Cultivated Mushrooms (Pleurotus eryngii, Pleurotus ostreatus and Lentinula edodes) at Retail Level and Post-Retail Storage. Foods 2021, 10, 816. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Almushhin, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Fikry, M.; Helal, M. Enhancement in physicochemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep. 2021, 11, 7915. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Philippoussis, A. Cultivated mushrooms: Preservation and processing. In Handbook of Vegetable Preservation and Processing; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Choi, M.H.; Kim, G.H. Quality changes in Pleurotus ostreatus during modified atmosphere storage as affected by temperatures and packaging material. Acta Hortic. 2003, 628, 357–362. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.; Sun, D. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of Tragacanth gum impregnated with Satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int. J. Biol. Macromol. 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Guillaume, C.; Schwab, I.; Gastaldi, E.; Gontard, N. Bio-based packaging for improving preservation of fresh common mushrooms (Agaricus bisporus L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 690–696. [Google Scholar] [CrossRef]
- Jafri, M.; Jha, A.; Bunkar, D.S.; Ram, R.C. Quality retention of oyster mushrooms (Pleurotus florida) by a combination of chemical treatments and modified atmosphere packaging. Postharvest Biol. Technol. 2013, 76, 112–118. [Google Scholar] [CrossRef]
- Ajayi, O.; Obadina, A.; Idowu, M.; Adegunwa, M.; Kajihausa, O.; Sanni, L.; Asagbra, Y.; Ashiru, B.; Tomlins, K. Effect of packaging materials on the chemical composition and microbiological quality of edible mushroom (Pleurotus ostreatus) grown on cassava peels. Food Sci. Nutr. 2015, 3, 284–291. [Google Scholar] [CrossRef]
- Šumić, Z.; Tepić, A.; Vidović, S.; Vladić, J.; Pavlić, B. Drying of shiitake mushrooms in a vacuum dryer and optimization of the process by response surface methodology (RSM). J. Food Meas. Character 2016, 10, 425–433. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, M.; Wang, X.; Wu, W.; Zheng, Z.; Hu, Z.; Ma, B. Modeling and Optimization for Konjac Vacuum Drying Based on Response Surface Methodology (RSM) and Artificial Neural Network (ANN). Processes 2020, 8, 1430. [Google Scholar] [CrossRef]
- Subramaniam, S.; Jiao, S.; Zhang, Z.; Jing, P. Impact of post-harvest processing or thermal dehydration on physiochemical, nutritional and sensory quality of shiitake mushrooms. Comp. Rev. Food Sci. Food Saf. 2021, 20, 2560–2595. [Google Scholar] [CrossRef]
- Xiao, G.N.; Zhang, M. Study of respiration regulation of Pinggu mushroom and strawberry under modified atmosphere packaging. J. Wuxi. Univ. Light Ind. 2003, 4, 115–123. [Google Scholar]
- Sebaaly, Z.; Abou Fayssal, S.; Shaban, N.; Sassine, Y.N. Growing Agaricus bisporus on compost mixtures based on chicken manure and banana residues. In Proceedings of the IX International Scientific Agriculture Symposium, Jahorina, Bosnia & Herzegovina, 4–7 October 2018; pp. 1172–1180. [Google Scholar]
- Villaescusa, R.; Gil, M.I. Quality improvement of Pleurotus mushrooms by modified atmosphere packaging and moisture absorbers. Postharvest Biol. Technol. 2003, 28, 169–179. [Google Scholar] [CrossRef]
- Wakchaure, G.C. Mushrooms-value added products. In Mushrooms Cultivation, Marketing and Consumption; Singh, M., Vijay, B., Kamal, S., Wakchaure, G.C., Eds.; Directorate of Mushroom Research: Solan, India, 2011; pp. 235–238. [Google Scholar]
- Yadav, M.K.; Kumar, S.; Chandra, R.; Biswas, S.K.; Dhakad, P.K.; Siddiqui, M.W. Postharvest management and processing technology of mushrooms. In Postharvest Management of Horticultural Crops; Siddiqui, M.W., Ali., A., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Fu, Z.; Zhao, S.; Zhang, X.; Polovka, M.; Wang, X. Quality Characteristics Analysis and Remaining Shelf-Life Prediction of Fresh Tibetan Tricholoma matsutake under Modified Atmosphere Packaging in Cold Chain. Foods 2019, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Zhang, M.; Shan, L.; You, Y.; Salokhe, V.M. Extension of the shelf-life of fresh oyster mushrooms (Pleurotus ostreatus) by modified atmosphere packaging with chemical treatments. Afr. J. Biotechnol. 2011, 10, 9509–9517. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.S.; Park, W.P.; Lee, D.S. Quality of enoki mushrooms as affected by packaging conditions. J. Sci. Food Agric. 2000, 81, 109–114. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Gao, Z.; Xie, Y.; Wang, H. Computer vision online measurement of shiitake mushroom (Lentinus edodes) surface wrinkling and shrinkage during hot air drying with humidity control. J. Food Eng. 2020, 292, 110253. [Google Scholar] [CrossRef]
- Wang, Q.; Chu, L.; Kou, L. UV-C Treatment maintains quality and delays senescence of oyster mushroom (Pleurotus ostreatus). Sci. Hortic. 2017, 225, 380–385. [Google Scholar] [CrossRef]
- Siyoum, N.A.; Surridge, K.; van der Linde, E.J.; Korsten, L. Microbial succession in white button mushroom production systems from compost and casing to a marketable packed product. Ann. Microbiol. 2016, 66, 151–164. [Google Scholar] [CrossRef]
- Rossouw, W.; Korsten, L. Cultivable microbiome of fresh white button mushrooms. Lett. Appl. Microbiol. 2017, 64, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Reyes, J.E.; Venturini, M.E.; Oria, R.; Blanco, D. Prevalence of Ewingella americana in retail fresh cultivated mushrooms (Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus) in Zaragoza (Spain). FEMS Microbiol. Ecol. 2004, 47, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturini, M.E.; Reyes, J.E.; Rivera, C.S.; Oria, R.; Blanco, D. Microbiological quality and safety of fresh cultivated and wild mushrooms commercialized in Spain. Food Microbiol. 2011, 28, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.V.; Oliveira, F.A.R.; Macedo, I. Effect of temperature and humidity on the transpiration rate of the whole mushrooms. J. Food Eng. 2008, 84, 281–288. [Google Scholar] [CrossRef]
- López-Gómez, A.; Ros-Chumillas, M.; Navarro-Martínez, A.; Barón, M.; Navarro-Segura, L.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Martínez-Hernández, G.B. Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life. Foods 2021, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Jing, J.; Helal, M. Effect of Titanium Dioxide Nanocomposite Material and Antimicrobial Agents on Mushrooms Shelf-Life Preservation. Processes 2020, 8, 1632. [Google Scholar] [CrossRef]



| Coded Symbol | Variable | Levels | ||
|---|---|---|---|---|
| −1 (Low) | 0 (Medium) | +1 (High) | ||
| (X1) | Packaging type | Vacuum bag packaging (VBP) | No packaging (-) | Plastic bag packaging (PBP) |
| (X2) | OLPR rate | 0 | 0.3 | 0.7 |
| SCG rate | 0 | 0.3 | 0.7 | |
| OLPR/SCG rate | 0 | 0.17 | 0.33 | |
| Run | Factor Variables | Response Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Packaging Type (X1) | OLPR, SCG, OLPR/SCG Rates (X2) | d M1 Shelf-Life (Y1: Days) | e M2 Shelf-Life (Y2: Days) | f M3 Shelf-Life (Y3: Days) | ||||
| Measured | Predicted | Measured | Predicted | Measured | Predicted | |||
| 1 | 0 (a-) | −1 (0) | 3.00 | 3.00 | 3.00 | 3.03 | 3.00 | 3.03 |
| 2 | 0 (-) | 0 (0.3 OLPR, 0.3 SCG, 0.17 OLPR/SCG) | 4.30 | 4.32 | 4.00 | 3.77 | 4.20 | 4.00 |
| 3 | 0 (-) | 1 (0.7 OLPR, 0.7 SCG, 0.33 OLPR/SCG) | 3.30 | 3.31 | 3.70 | 3.77 | 4.70 | 4.78 |
| 4 | 1 (b PBP) | −1 (0) | 7.80 | 7.48 | 8.00 | 7.81 | 7.90 | 7.78 |
| 5 | 1 (PBP) | 0 (0.3 OLPR, 0.3 SCG, 0.17 OLPR/SCG) | 8.20 | 8.81 | 7.70 | 7.95 | 8.40 | 8.51 |
| 6 | 1 (PBP) | 1 (0.7 OLPR, 0.7 SCG, 0.33 OLPR/SCG) | 8.10 | 7.81 | 7.20 | 7.15 | 9.10 | 9.11 |
| 7 | −1 (c VBP) | −1 (0) | 6.80 | 7.14 | 7.00 | 7.04 | 6.90 | 6.90 |
| 8 | −1 (VBP) | 0 (0.3 OLPR, 0.3 SCG, 0.17 OLPR/SCG) | 9.10 | 8.47 | 6.60 | 6.58 | 7.30 | 7.39 |
| 9 | −1 (VBP) | 1 (0.7 OLPR, 0.7 SCG, 0.33 OLPR/SCG) | 7.20 | 7.48 | 5.00 | 4.98 | 7.90 | 7.81 |
| 10 | 0 (-) | −1 (0) | 3.10 | 3.00 | 2.90 | 3.03 | 3.10 | 3.03 |
| 11 | 0 (-) | −1 (0) | 2.90 | 3.00 | 3.10 | 3.03 | 2.90 | 3.03 |
| 12 | 0 (-) | −1 (0) | 3.20 | 3.00 | 2.80 | 3.03 | 3.20 | 3.03 |
| 13 | 0 (-) | −1 (0) | 2.80 | 3.00 | 3.20 | 3.03 | 2.80 | 3.03 |
| Parameter | Variable | Sum of Sq. | Mean Sq. | F-Value | p-Value | R2 | Adjusted R2 | Predicted R2 | Adeq. Precision | PRESS |
|---|---|---|---|---|---|---|---|---|---|---|
| Self-life of c M1 (Y1) | Model | 73.04 | 14.61 | 82.15 | <0.0001 | 0.9832 | 0.9713 | 0.8753 | 20.2934 | 9.26 |
| a X1 | 25.30 | 25.30 | 142.30 | <0.0001 | ||||||
| b X2 | 3.32 | 3.32 | 18.69 | 0.0035 | ||||||
| X1X2 | 0.0002 | 0.0002 | 0.0014 | 0.9712 | ||||||
| X12 | 12.31 | 12.31 | 69.24 | <0.0001 | ||||||
| X22 | 3.01 | 3.01 | 16.92 | 0.0045 | ||||||
| Residual | 1.24 | 0.1778 | ||||||||
| Lack of Fit | 1.14 | 0.3816 | 15.26 | 0.1118 | ||||||
| Shelf-life of d M2 (Y2) | Model | 50.17 | 10.03 | 264.28 | <0.0001 | 0.9947 | 0.9910 | 0.9794 | 37.1381 | 1.04 |
| X1 | 30.25 | 30.25 | 796.93 | <0.0001 | ||||||
| X2 | 0.7622 | 0.7622 | 20.08 | 0.0029 | ||||||
| X1X2 | 2.52 | 2.52 | 66.31 | <0.0001 | ||||||
| X12 | 16.23 | 16.23 | 427.54 | <0.0001 | ||||||
| X22 | 0.3838 | 0.3838 | 10.11 | 0.0155 | ||||||
| Residual | 0.2657 | 0.0380 | ||||||||
| Lack of Fit | 0.1657 | 0.0552 | 2.21 | 0.2294 | ||||||
| Shelf-life of e M3 (Y3) | Model | 71.57 | 14.31 | 514.60 | <0.0001 | 0.9973 | 0.9953 | 0.9892 | 53.7123 | 0.7771 |
| X1 | 30.39 | 30.39 | 1092.38 | <0.0001 | ||||||
| X2 | 0.3586 | 0.3586 | 12.89 | 0.0089 | ||||||
| X1X2 | 0.2370 | 0.2370 | 8.52 | 0.0224 | ||||||
| X12 | 16.73 | 16.73 | 601.45 | <0.0001 | ||||||
| X22 | 0.0010 | 0.0010 | 0.0366 | 0.0037 | ||||||
| Residual | 0.1974 | 0.0278 | ||||||||
| Lack of Fit | 0.0947 | 0.0316 | 1.26 | 0.3994 | ||||||
| Variable | Target | Optimum Parameter Range |
|---|---|---|
| X1: Packaging (type) | In-range | a PBP (Y1, Y2, Y3) |
| X2: OLPR, SCG, OLPR/SCG (rates) | In-range | 0.289 (Y1), 0.23 (Y2), 0.303 (Y3) |
| Y1: Shelf-life of b M1 (days) | Maximized | 9.18 |
| Y2: Shelf-life of c M2 (days) | Maximized | 8.15 |
| Y3: Shelf-life of d M3 (days) | Maximized | 9.14 |
| Run | Factor Variables | Response Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Packaging Type (X1) | OLPR, SCG, OLPR/SCG Rates (X2) | d M1 Shelf-Life (Y1: Days) | e M2 Shelf-Life (Y2: Days) | f M3 Shelf-Life (Y3: Days) | ||||
| Measured | Predicted | Measured | Predicted | Measured | Predicted | |||
| 1 | 0 (a-) | −1 (0) | 5.00 | 5.50 | 5.00 | 5.52 | 5.00 | 5.51 |
| 2 | 0 (-) | 0 (0.3 OLPR, 0.3 SCG, 0.17 OLPR/SCG) | 7.10 | 7.46 | 6.80 | 6.89 | 7.20 | 7.20 |
| 3 | 0 (-) | +1 (0.7 OLPR, 0.7 SCG, 0.33 OLPR/SCG) | 6.40 | 6.36 | 6.20 | 6.31 | 7.60 | 7.85 |
| 4 | +1 (b PBP) | −1 (0) | 20.20 | 20.58 | 20.20 | 20.58 | 20.20 | 20.75 |
| 5 | +1 (PBP) | 0 (0.3 OLPR, 0.3 OLPR, 0.17 OLPR/SCG) | 22.90 | 22.64 | 22.10 | 22.03 | 23.20 | 23.09 |
| 6 | +1 (PBP) | +1 (0.7 OLPR, 0.7 SCG, 0.33 OLPR/SCG) | 21.80 | 21.68 | 21.90 | 21.63 | 24.80 | 24.36 |
| 7 | −1 (c VBP) | −1 (0) | 21.70 | 21.64 | 21.70 | 21.56 | 21.70 | 21.40 |
| 8 | −1 (VBP) | 0 (0.3 OLPR, 0.3 OLPR, 0.17 OLPR/SCG) | 23.90 | 23.80 | 23.20 | 23.18 | 24.30 | 24.41 |
| 9 | −1 (VBP) | +1 (0.7 OLPR, 0.7 SCG, 0.33 OLPR/SCG) | 22.80 | 22.96 | 22.80 | 22.96 | 26.10 | 26.29 |
| 10 | 0 (-) | −1 (0) | 5.40 | 5.50 | 5.40 | 5.52 | 5.40 | 5.51 |
| 11 | 0 (-) | −1 (0) | 5.60 | 5.50 | 5.60 | 5.52 | 5.60 | 5.51 |
| 12 | 0 (-) | −1 (0) | 5.80 | 5.50 | 5.80 | 5.52 | 5.80 | 5.51 |
| 13 | 0 (-) | −1 (0) | 6.00 | 5.50 | 6.00 | 5.52 | 6.00 | 5.51 |
| Parameter | Variable | Sum of Sq. | Mean Sq. | F-Value | p-Value | R2 | Adjusted R2 | Predicted R2 | Adeq. Precision | PRESS |
|---|---|---|---|---|---|---|---|---|---|---|
| Self-life of c M1 (Y1) | Model | 870.14 | 174.03 | 1205.59 | <0.0001 | 0.9988 | 0.9980 | 0.9962 | 70.9096 | 3.33 |
| a X1 | 259.56 | 259.56 | 1798.15 | <0.0001 | ||||||
| b X2 | 6.78 | 6.78 | 46.96 | 0.0002 | ||||||
| X1X2 | 0.659 | 0.659 | 0.5563 | 0.5210 | ||||||
| X12 | 103.99 | 103.99 | 720.38 | <0.0001 | ||||||
| X22 | 5.41 | 5.41 | 37.49 | 0.0005 | ||||||
| Residual | 1.01 | 0.1444 | ||||||||
| Lack of Fit | 0.4185 | 0.1395 | 0.9425 | 0.4993 | ||||||
| Shelf-life of d M2 (Y2) | Model | 849.76 | 169.95 | 1392.08 | <0.0001 | 0.9999 | 0.9983 | 0.9961 | 74.4103 | 3.29 |
| X1 | 257.55 | 257.55 | 2109.56 | <0.0001 | ||||||
| X2 | 3.09 | 3.09 | 25.35 | 0.0015 | ||||||
| X1X2 | 0.1174 | 0.1174 | 0.9613 | 0.3595 | ||||||
| X12 | 103.49 | 103.49 | 847.71 | <0.0001 | ||||||
| X22 | 2.24 | 2.24 | 18.38 | 0.0036 | ||||||
| Residual | 0.8546 | 0.1221 | ||||||||
| Lack of Fit | 0.2626 | 0.0875 | 0.5914 | 0.6527 | ||||||
| Shelf-life of e M3 (Y3) | Model | 994.10 | 198.92 | 1065.54 | <0.0001 | 0.9987 | 0.9978 | 0.9920 | 70.8166 | 7.97 |
| X1 | 269.30 | 269.30 | 1443.28 | <0.0001 | ||||||
| X2 | 2.44 | 2.44 | 13.06 | 0.0086 | ||||||
| X1X2 | 2.15 | 2.15 | 11.51 | 0.0115 | ||||||
| X12 | 112.17 | 112.17 | 601.18 | <0.0001 | ||||||
| X22 | 0.4959 | 0.4959 | 2.66 | 0.0147 | ||||||
| Residual | 1.31 | 0.1866 | ||||||||
| Lack of Fit | 0.7141 | 0.2380 | 1.61 | 0.3209 | ||||||
| Variable | Target | Optimum Parameter Range |
|---|---|---|
| X1: Packaging (type) | In-range | a VBP (Y1, Y2, Y3) |
| X2: OLPR, SCG, OLPR/SCG (rates) | In-range | 0.27 (Y1), 0.13(Y2), 0.22(Y3) |
| Y1: Shelf-life of b M1 (days) | Maximized | 24.90 |
| Y2: Shelf-life of c M2 (days) | Maximized | 23.67 |
| Y3: Shelf-life of d M3 (days) | Maximized | 26.26 |
| Packaging Type | Mushrooms | Total Microbial Count (Log CFU/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | ||
| d- | a M1A | 3.3 | 4.8 | 6.5 | 7.8 | 8.6 | 9.2 | 10.4 | 10.8 | 11.6 | 12.2 | 12.8 |
| M1B | 2.7 | 4.1 | 5.7 | 7.4 | 7.8 | 8.3 | 8.8 | 9.9 | 11.4 | 12.5 | 13.1 | |
| M1C | 3.1 | 4.6 | 6.3 | 7.6 | 8.3 | 8.8 | 9.9 | 10.5 | 11.5 | 12.3 | 12.9 | |
| b M2A | 3.3 | 4.8 | 6.5 | 7.8 | 8.6 | 9.2 | 10.3 | 10.9 | 11.8 | 12.7 | 13.4 | |
| M2B | 3.1 | 4.3 | 5.9 | 7.6 | 8.0 | 8.6 | 9.1 | 9.9 | 11.1 | 11.8 | 12.9 | |
| M2C | 3.1 | 4.5 | 6.1 | 7.7 | 8.2 | 8.7 | 9.8 | 10.4 | 11.4 | 12.4 | 13.0 | |
| c M3A | 3.3 | 4.8 | 6.5 | 7.8 | 8.6 | 9.2 | 103 | 10.9 | 11.8 | 12.7 | 13.4 | |
| M3B | 2.9 | 4.2 | 5.8 | 7.5 | 7.9 | 8.4 | 8.9 | 10.1 | 11.6 | 12.7 | 13.3 | |
| M3C | 2.6 | 3.8 | 5.4 | 7.1 | 7.6 | 8.2 | 8.7 | 9.7 | 11.2 | 12.3 | 12.7 | |
| e PBP | M1A | 2.5 | 2.9 | 3.4 | 3.9 | 4.6 | 5.8 | 6.9 | 7.8 | 8.4 | 8.8 | 10.2 |
| M1B | 2.2 | 2.6 | 3.1 | 3.5 | 4.2 | 5.5 | 6.6 | 7.4 | 8.0 | 8.2 | 8.7 | |
| M1C | 2.3 | 2.7 | 3.2 | 3.6 | 4.3 | 5.6 | 6.7 | 7.5 | 8.1 | 8.3 | 8.9 | |
| M2A | 2.7 | 3.1 | 3.6 | 4.1 | 4.8 | 5.7 | 6.9 | 7.5 | 8.0 | 8.5 | 9.1 | |
| M2B | 2.8 | 3.3 | 4.0 | 4.5 | 5.5 | 6.1 | 7.4 | 7.9 | 8.2 | 8.8 | 9.4 | |
| M2C | 2.9 | 3.4 | 4.1 | 4.6 | 5.6 | 6.2 | 7.5 | 8.1 | 8.3 | 8.6 | 9.2 | |
| M3A | 2.6 | 3.0 | 3.5 | 4.0 | 4.7 | 5.9 | 7.0 | 7.9 | 8.3 | 8.7 | 9.1 | |
| M3B | 2.1 | 2.7 | 3.3 | 3.9 | 4.2 | 5.1 | 5.9 | 6.7 | 7.9 | 8.2 | 8.6 | |
| M3C | 2.1 | 2.6 | 3.2 | 3.8 | 4.3 | 5.2 | 5.9 | 6.9 | 7.7 | 8.1 | 8.4 | |
| f VBP | M1A | 2.8 | 3.5 | 4.2 | 4.8 | 5.7 | 6.6 | 7.8 | 8.8 | 9.3 | 9.9 | 10.6 |
| M1B | 2.3 | 2.9 | 3.2 | 3.8 | 4.4 | 5.0 | 5.8 | 6.6 | 7.8 | 8.0 | 8.3 | |
| M1C | 2.7 | 3.4 | 4.1 | 4.7 | 5.6 | 6.3 | 7.4 | 8.1 | 8.4 | 8.8 | 9.2 | |
| M2A | 3.0 | 3.7 | 4.4 | 5.0 | 5.9 | 6.8 | 8.0 | 8.8 | 9.2 | 9.6 | 10.7 | |
| M2B | 3.2 | 3.8 | 4.6 | 5.2 | 6.1 | 7.2 | 8.1 | 8.9 | 9.4 | 9.9 | 10.9 | |
| M2C | 3.4 | 4.2 | 4.9 | 5.7 | 6.5 | 7.7 | 8.8 | 9.4 | 9.9 | 10.6 | 11.4 | |
| M3A | 2.7 | 3.4 | 4.1 | 4.6 | 5.5 | 6.4 | 7.6 | 8.6 | 9.1 | 9.7 | 10.5 | |
| M3B | 2.6 | 3.2 | 3.9 | 4.3 | 5.1 | 6.1 | 7.3 | 7.9 | 8.2 | 8.6 | 8.8 | |
| M3C | 2.5 | 3.3 | 4.0 | 4.4 | 5.2 | 6.2 | 7.4 | 8.0 | 8.4 | 8.9 | 9.2 | |
| Packaging Type | Mushrooms | Total Microbial Count (Log CFU/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 5 | Day 6 | Day 7 | Day 15 | Day 20 | Day 21 | Day 22 | Day 23 | Day 24 | Day 27 | ||
| d- | a M1A | 4.1 | 6.5 | 8.4 | 8.6 | 13.1 | 14.2 | 14.5 | 14.8 | 15.4 | 15.8 | 16.5 |
| M1B | 3.6 | 5.6 | 7.3 | 8.1 | 12.3 | 13.5 | 13.9 | 14.4 | 14.9 | 15.7 | 16.2 | |
| M1C | 3.7 | 5.9 | 7.8 | 8.4 | 12.8 | 13.8 | 14.2 | 14.7 | 15.2 | 15.8 | 16.4 | |
| b M2A | 4.1 | 6.5 | 8.4 | 8.8 | 13.4 | 14.8 | 15.2 | 15.8 | 16.7 | 17.4 | 17.9 | |
| M2B | 3.6 | 5.7 | 7.5 | 8.2 | 12.4 | 13.7 | 14.1 | 14.6 | 15.0 | 15.9 | 16.3 | |
| M2C | 3.8 | 5.9 | 7.9 | 8.5 | 13.0 | 14.0 | 14.3 | 14.6 | 15.3 | 15.5 | 16.0 | |
| c M3A | 4.1 | 6.5 | 8.4 | 8.8 | 13.3 | 14.6 | 15.1 | 15.6 | 16.5 | 17.3 | 17.8 | |
| M3B | 3.5 | 5.5 | 7.2 | 8.1 | 11.8 | 12.5 | 13.3 | 13.9 | 14.1 | 14.5 | 15.1 | |
| M3C | 3.4 | 5.3 | 6.9 | 7.8 | 11.6 | 12.3 | 12.8 | 13.4 | 13.9 | 14.2 | 14.9 | |
| e PBP | M1A | 1.4 | 2.5 | 3.7 | 4.2 | 6.5 | 7.8 | 8.4 | 8.8 | 9.4 | 9.8 | 10.2 |
| M1B | 1.2 | 1.9 | 2.6 | 3.5 | 5.8 | 7.7 | 7.9 | 8.0 | 8.2 | 8.6 | 9.8 | |
| M1C | 1.3 | 2.1 | 2.8 | 3.7 | 6.0 | 7.6 | 8.1 | 8.3 | 8.7 | 9.4 | 10.0 | |
| M2A | 1.4 | 2.5 | 3.7 | 4.2 | 6.5 | 7.8 | 8.4 | 8.9 | 9.4 | 10.2 | 10.9 | |
| M2B | 1.2 | 1.9 | 2.6 | 3.5 | 5.8 | 7.4 | 7.9 | 8.2 | 8.7 | 9.2 | 9.9 | |
| M2C | 1.3 | 2.0 | 2.7 | 3.6 | 5.9 | 7.5 | 8.0 | 8.2 | 8.7 | 9.2 | 9.9 | |
| M3A | 1.4 | 2.5 | 3.7 | 4.2 | 6.5 | 7.8 | 8.4 | 8.9 | 9.4 | 10.2 | 10.9 | |
| M3B | 1.1 | 1.7 | 2.4 | 3.3 | 4.9 | 6.2 | 6.7 | 7.3 | 7.9 | 8.2 | 8.6 | |
| M3C | 1.0 | 1.5 | 2.2 | 3.1 | 4.7 | 5.9 | 6.6 | 7.2 | 7.7 | 8.0 | 8.8 | |
| f VBP | M1A | 1.4 | 2.6 | 3.8 | 4.3 | 6.5 | 7.9 | 8.5 | 9.0 | 9.5 | 10.3 | 11.0 |
| M1B | 1.1 | 1.6 | 2.3 | 3.2 | 4.7 | 6.0 | 6.5 | 7.1 | 7.7 | 8.2 | 8.7 | |
| M1C | 1.2 | 2.0 | 2.7 | 3.6 | 5.9 | 7.7 | 7.9 | 8.0 | 8.2 | 8.7 | 9.6 | |
| M2A | 1.4 | 2.6 | 3.8 | 4.3 | 6.5 | 7.9 | 8.5 | 9.0 | 9.5 | 10.3 | 10.9 | |
| M2B | 1.1 | 1.7 | 2.4 | 3.3 | 4.9 | 6.2 | 6.7 | 7.3 | 7.9 | 8.2 | 8.6 | |
| M2C | 1.2 | 2.0 | 2.7 | 3.6 | 5.9 | 7.7 | 7.9 | 8.0 | 8.2 | 8.7 | 9.4 | |
| M3A | 1.4 | 2.6 | 3.8 | 4.3 | 6.5 | 7.9 | 8.5 | 9.0 | 9.5 | 10.3 | 10.9 | |
| M3B | 1.0 | 1.5 | 2.3 | 3.2 | 4.7 | 5.8 | 6.6 | 7.2 | 7.7 | 8.1 | 8.7 | |
| M3C | 1.0 | 1.3 | 1.8 | 2.4 | 4.2 | 5.1 | 5.9 | 6.5 | 6.9 | 7.5 | 8.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Fayssal, S.; El Sebaaly, Z.; Sassine, Y.N. Pleurotus ostreatus Grown on Agro-Industrial Residues: Studies on Microbial Contamination and Shelf-Life Prediction under Different Packaging Types and Storage Temperatures. Foods 2023, 12, 524. https://doi.org/10.3390/foods12030524
Abou Fayssal S, El Sebaaly Z, Sassine YN. Pleurotus ostreatus Grown on Agro-Industrial Residues: Studies on Microbial Contamination and Shelf-Life Prediction under Different Packaging Types and Storage Temperatures. Foods. 2023; 12(3):524. https://doi.org/10.3390/foods12030524
Chicago/Turabian StyleAbou Fayssal, Sami, Zeina El Sebaaly, and Youssef N. Sassine. 2023. "Pleurotus ostreatus Grown on Agro-Industrial Residues: Studies on Microbial Contamination and Shelf-Life Prediction under Different Packaging Types and Storage Temperatures" Foods 12, no. 3: 524. https://doi.org/10.3390/foods12030524
APA StyleAbou Fayssal, S., El Sebaaly, Z., & Sassine, Y. N. (2023). Pleurotus ostreatus Grown on Agro-Industrial Residues: Studies on Microbial Contamination and Shelf-Life Prediction under Different Packaging Types and Storage Temperatures. Foods, 12(3), 524. https://doi.org/10.3390/foods12030524







