Bati Butter as a Potential Substrate for Lipase Production by Aspergillus terreus NRRL-255
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bati Butter
2.2. Methods
2.2.1. Determination of the Fatty Acids
2.2.2. Lipase Production
Microorganism
Fermentation
Design of the Experiment
2.2.3. Enzyme Extraction
2.2.4. Determination of Enzyme Activity
2.2.5. Spore Count
2.2.6. Lipase Characterization
Effects of Temperature and pH
Effects of Inhibitory and Activating Agents
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Determination of Fatty Acids of Bati Butter
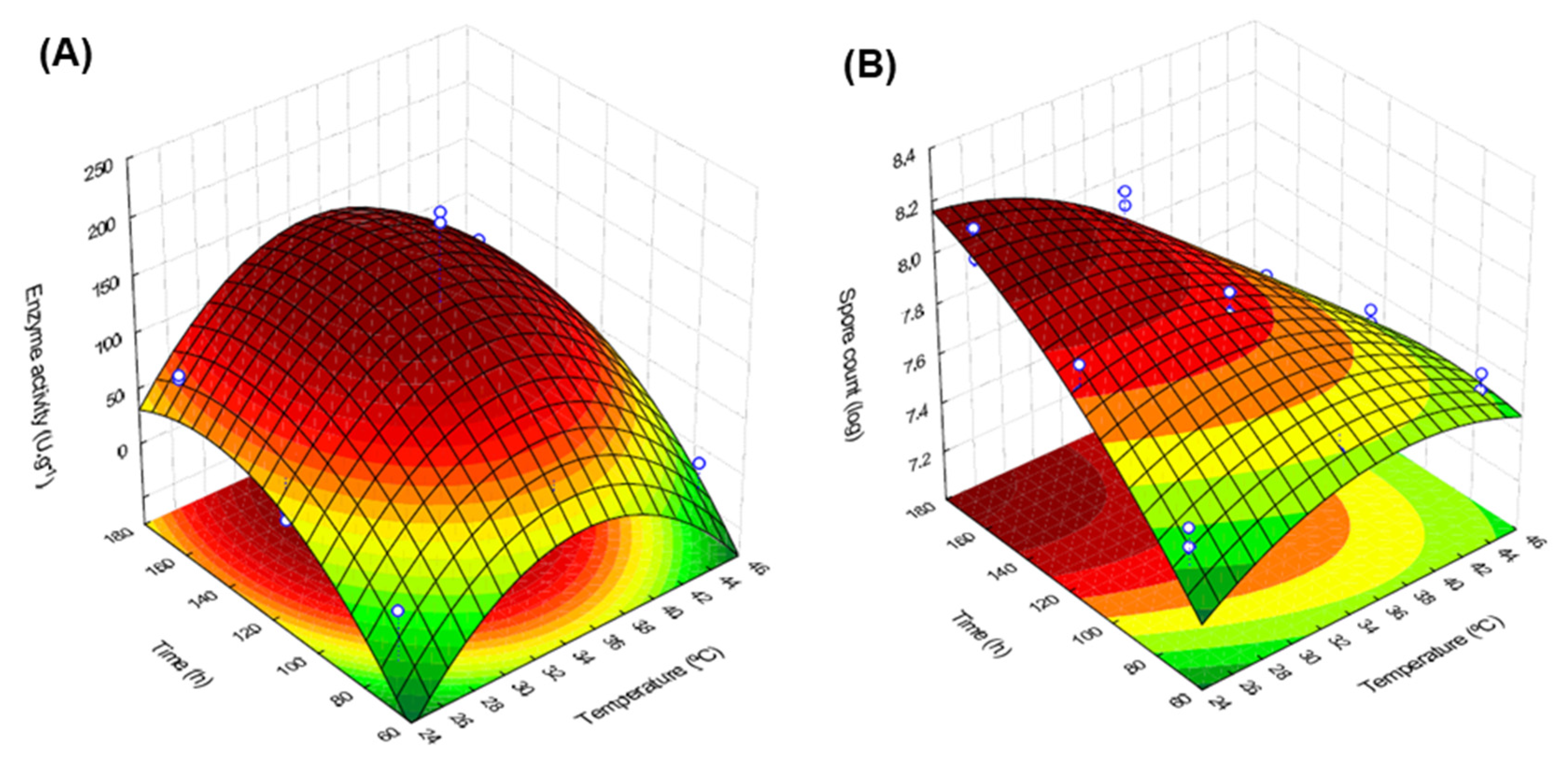
3.2. Lipase Production
3.3. Lipase Characterization
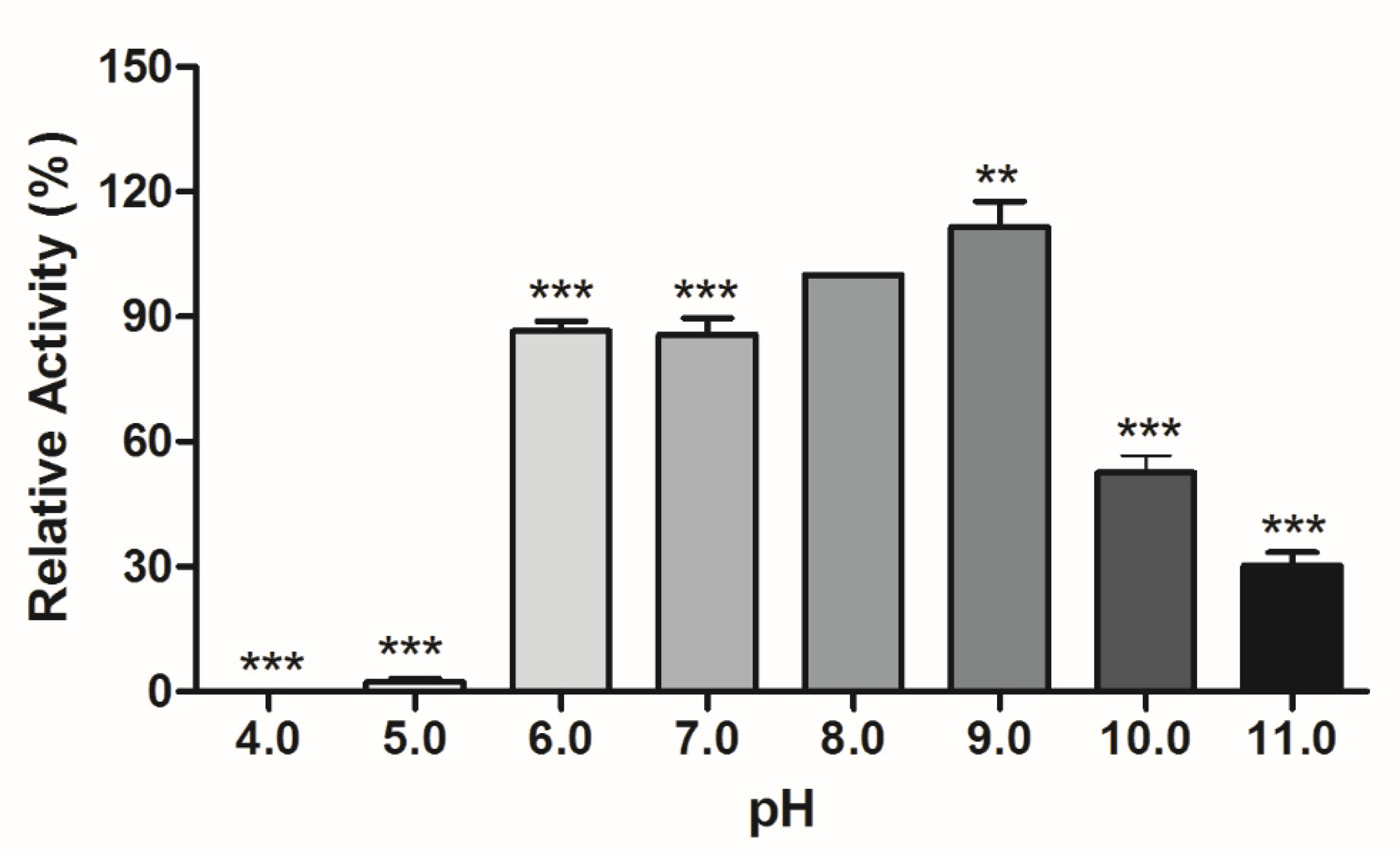
3.3.1. Temperature and pH Effect on Lipolytic Activity
3.3.2. Effect of Inhibitors and Activating Agents on Lipolytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- do Nascimento, J.E.T.; de Morais, S.M.; de Lisboa, D.S.; de Oliveira Sousa, M.; Santos, S.A.A.R.; Magalhães, F.E.A.; Campos, A.R. The Orofacial Antinociceptive Effect of Kaempferol-3-O-Rutinoside, Isolated from the Plant Ouratea Fieldingiana, on Adult Zebrafish (Danio Rerio). Biomed. Pharmacother. 2018, 107, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- de Castro, H.F.; Mendes, A.A.; dos Santos, J.C.; de Aguiar, C.L. Modificação de Óleos e Gorduras Por Biotransformação. Quim. Nova 2004, 27, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.T.; Shimada, Y. Lipases. Encycl. Microbiol. Third edition 2009, 385–392. [Google Scholar] [CrossRef]
- Saisubramanian, N.; Edwinoliver, N.G.; Nandakumar, N.; Kamini, N.R.; Puvanakrishnan, R. Efficacy of Lipase from Aspergillus niger as an Additive in Detergent Formulations: A Statistical Approach. J. Ind. Microbiol. Biotechnol. 2006, 33, 669–676. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial Applications of Microbial Lipases. Enzyme Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, Purification, Characterization, and Applications of Lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Mukhopadhyay, M. Overview of Fungal Lipase: A Review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef]
- Silva, W.O.B.; Mitidieri, S.; Schrank, A.; Vainstein, M.H. Production and Extraction of an Extracellular Lipase from the Entomopathogenic Fungus Metarhizium anisopliae. Process Biochem. 2005, 40, 321–326. [Google Scholar] [CrossRef]
- Putri, D.N.; Khootama, A.; Perdani, M.S.; Utami, T.S.; Hermansyah, H. Optimization of Aspergillus niger Lipase Production by Solid State Fermentation of Agro-Industrial Waste. Energy Rep. 2020, 6, 331–335. [Google Scholar] [CrossRef]
- Utami, T.S.; Hariyani, I.; Alamsyah, G.; Hermansyah, H. Production of Dry Extract Extracellular Lipase from Aspergillus niger by Solid State Fermentation Method to Catalyze Biodiesel Synthesis. Energy Procedia 2017, 136, 41–46. [Google Scholar] [CrossRef]
- de Azevedo, W.; de Oliveira, L.; Alcântara, M.; Cordeiro, A.T.; Damasceno, K.; de Assis, C.; de Sousa Junior, F. Turning Cacay Butter and Wheat Bran into Substrate for Lipase Production by Aspergillus terreus NRRL-255. Prep. Biochem. Biotechnol. 2020, 50, 689–696. [Google Scholar] [CrossRef]
- Galvão, J.G.; Trindade, G.G.G.; Santos, A.J.; Santos, R.L.; Chaves Filho, A.B.; Lira, A.A.M.; Miyamoto, S.; Nunes, R.S. Effect of Ouratea Sp. Butter in the Crystallinity of Solid Lipids Used in Nanostructured Lipid Carriers (NLCs). J. Therm. Anal. Calorim. 2016, 123, 941–948. [Google Scholar] [CrossRef]
- Jain, R.; Naik, S.N. Adding Value to the Oil Cake as a Waste from Oil Processing Industry: Production of Lipase in Solid State Fermentation. Biocatal. Agric. Biotechnol. 2018, 15, 181–184. [Google Scholar] [CrossRef]
- Kamal, M.Z.; Barrow, C.J.; Rao, N.M. A Computational Search for Lipases That Can Preferentially Hydrolyze Long-Chain Omega-3 Fatty Acids from Fish Oil Triacylglycerols. Food Chem. 2015, 173, 1030–1036. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New Developments in Solid State Fermentation: I-Bioprocesses and Products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Joshi, C.; Khare, S.K. Purification and Characterization of Pseudomonas aeruginosa Lipase Produced by SSF of Deoiled Jatropha Seed Cake. Biocatal. Agric. Biotechnol. 2013, 2, 32–37. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Dimou, A.; Fakas, S.; Diamantopoulou, P.; Philippoussis, A.; Galiotou-Panayotou, M.; Aggelis, G. Biotechnological Conversion of Waste Cooking Olive Oil into Lipid-Rich Biomass Using Aspergillus and Penicillium Strains. J. Appl. Microbiol. 2011, 110, 1138–1150. [Google Scholar] [CrossRef]
- Prabaningtyas, R.K.; Putri, D.N.; Utami, T.S.; Hermansyah, H. Production of Immobilized Extracellular Lipase from Aspergillus niger by Solid State Fermentation Method Using Palm Kernel Cake, Soybean Meal, and Coir Pith as the Substrate. Energy Procedia 2018, 153, 242–247. [Google Scholar] [CrossRef]
- Burkert, J.F.; Maugeri, F.; Rodrigues, M. Optimization of Extracellular Lipase Production by Geotrichum sp. Using Factorial Design. Bioresour. Technol. 2004, 91, 77–84. [Google Scholar] [CrossRef]
- ul-Haq, I.; Idrees, S.; Rajoka, M.I. Production of Lipases by Rhizopus oligosporous by Solid-State Fermentation. Process Biochem. 2002, 37, 637–641. [Google Scholar] [CrossRef]
- Elibol, M.; Ozer, D. Response Surface Analysis of Lipase Production by Freely Suspended Rhizopus arrhizus. Process Biochem. 2002, 38, 367–372. [Google Scholar] [CrossRef]
- Kaushik, R.; Marwah, R.G.; Gupta, P.; Saran, S.; Saso, L.; Parmar, V.S.; Saxena, R.K. Optimization of Lipase Production from Aspergillus terreus by Response Surface Methodology and Its Potential for Synthesis of Partial Glycerides Under Solvent Free Conditions. Indian J. Microbiol. 2010, 50, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, R.; Saxena, R.K.; Gupta, R.; Yadav, R.P.; Davidson, W.S. Parametric Optimisation of Aspergillus terreus Lipase Production and Its Potential in Ester Synthesis. Process Biochem. 1999, 35, 459–464. [Google Scholar] [CrossRef]
- Mahmoud, G.A.; Morsy, F.M.; Bagy, M.K. Mycoflora Isolated from Mazot and Solar Polluted Soils in Upper Egypt. Egypt. J. Soil Sci. 2015, 55, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Sethi, B.K.; Nanda, P.K.; Sahoo, S. Characterization of Biotechnologically Relevant Extracellular Lipase Produced by Aspergillus terreus NCFT 4269.10. Braz. J. Microbiol. 2016, 47, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial Lipases and Their Industrial Applications: A Comprehensive Review; BioMed Central: London, UK, 2020; Volume 19, ISBN 1293402001. [Google Scholar]
- Salgado, C.A.; dos Santos, C.I.A.; Vanetti, M.C.D. Microbial Lipases: Propitious Biocatalysts for the Food Industry. Food Biosci. 2022, 45, 101509. [Google Scholar] [CrossRef]
- Hartman, L.; Lago, L. Rapid Preparation of Fatty Acid Methyl Esters from Lipids. Lab Pr. 1973, 22, 475–476. [Google Scholar]
- Piaruchi, J.; Duran, N.M.; Ruggieri, G.; Galante, M.; Lombardi, J.; Dib, M.E.; de Sanctis, M.; López, D.N.; Risso, P.H.; Boeris, V.; et al. Peptidase from Aspergillus niger NRRL 3: Optimization of Its Production by Solid-State Fermentation, Purification and Characterization. Lwt 2018, 98, 485–491. [Google Scholar] [CrossRef]
- Nagarajan, S. New Tools for Exploring Old Friends-Microbial Lipases. Appl. Biochem. Biotechnol. 2012, 168, 1163–1196. [Google Scholar] [CrossRef]
- Frota, L.S.; Lopes, F.F.S.; Alves, D.R.; Freitas, L.S.; Franco, G.M.G.; Morais, S.M. de Composição Química e Avaliação Das Atividades Antioxidante e Anticolinesterásica Do Óleo Dos Frutos de Ouratea fieldingiana (Gargner) Engl. Res. Soc. Dev. 2021, 10, e532101019013. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at Interfaces: A Review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef]
- Geoffry, K.; Achur, R.N. Screening and Production of Lipase from Fungal Organisms. Biocatal. Agric. Biotechnol. 2018, 14, 241–253. [Google Scholar] [CrossRef]
- Salgado, J.M.; Abrunhosa, L.; Venâncio, A.; Domínguez, J.M.; Belo, I. Integrated Use of Residues from Olive Mill and Winery for Lipase Production by Solid State Fermentation with Aspergillus sp. Appl. Biochem. Biotechnol. 2013, 172, 1832–1845. [Google Scholar] [CrossRef] [Green Version]
- Fleuri, L.F.; De Oliveira, M.C.; De Lara, M.; Arcuri, C.; Capoville, B.L.; Pereira, M.S.; Hamaio, C.; Delgado, O.; Novelli, P.K. Production of Fungal Lipases Using Wheat Bran and Soybean Bran and Incorporation of Sugarcane Bagasse as a Co-Substrate in Solid-State Fermentation. Food Sci. Biotechnol. 2014, 23, 1199–1205. [Google Scholar] [CrossRef]
- Kim, H.; Choi, N.; Oh, S.W.; Kim, Y.; Hee Kim, B.; Kim, I.H. Synthesis of α-Linolenic Acid-Rich Triacylglycerol Using a Newly Prepared Immobilized Lipase. Food Chem. 2017, 237, 654–658. [Google Scholar] [CrossRef]
- Kamini, N.R.; Mala, J.G.S.; Puvanakrishnan, R. Lipase Production from Aspergillus niger by Solid-State Fermentation Using Gingelly Oil Cake. Process Biochem. 1998, 33, 505–511. [Google Scholar] [CrossRef]
- Petrovic, D.; Risso, V.A.; Sanchez-ruiz, J.M.; Kamerlin, S.C.L. Conformational Dynamics and Enzyme Evolution. J. R. Soc. Interface 2018, 15, 20180330. [Google Scholar] [CrossRef]
- Hamdy, H.; Abo-Tahon, M. Extracellular Lipase of Aspergillus terreus Var. africanus (CBS 130.55): Production, Purification and Characterization. Ann. Microbiol. 2012, 62, 1723–1736. [Google Scholar] [CrossRef]
- de Medeiros, W.R.D.B.; de Paiva, W.K.V.; Diniz, D.S.; Padilha, C.E.d.A.; de Azevedo, W.M.; de Assis, C.F.; dos Santos, E.S.; de Sousa Junior, F.C. Low-Cost Approaches to Producing and Concentrating Stable Lipases and the Evaluation of Inductors. Braz. J. Chem. Eng. 2022, 1532–2297. [Google Scholar] [CrossRef]
- Pastore, G.M.; Da Costa, V.D.S.R.; Koblitz, M.G.B. Purificação Parcial e Caracterização Bioquímica de Lipase Extracelular Produzida Por Nova Linhagem de Rhizopus sp. Ciência e Tecnol. Aliment. 2003, 23, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Gururaj, P.; Ramalingam, S.; Nandhini Devi, G.; Gautam, P. Process Optimization for Production and Purification of a Thermostable, Organic Solvent Tolerant Lipase from Acinetobacter sp. AU07. Brazilian J. Microbiol. 2016, 47, 647–657. [Google Scholar] [CrossRef]
- Rahman, R.N.Z.A.; Baharum, S.N.; Salleh, A.B.; Basri, M. S5 Lipase: An Organic Solvent Tolerant Enzyme. J. Microbiol. 2006, 44, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Messias, J.M.; da Costa, B.Z.; de Lima, V.M.G.; Giese, C.; Dekker, R.F.H.; Barbosa, A.d.M. Lipases Microbianas: Produção, Propriedades e Aplicações Biotecnológicas. Semin. Ciências Exatas Tecnológicas 2011, 32, 213–234. [Google Scholar] [CrossRef] [Green Version]
- Rios, N.S.; Pinheiro, B.B.; Pinheiro, M.P.; Bezerra, R.M.; dos Santos, J.C.S.; Gonçalves, L.R.B. Biotechnological Potential of Lipases from Pseudomonas: Sources, Properties and Applications. Process Biochem. 2018, 75, 99–120. [Google Scholar] [CrossRef]

| Fatty Acids | % |
|---|---|
| Capric acid (C10:0) | 0.84 |
| Lauric acid (C12:0) | 2.00 |
| Tridecanoic acid (C13:0) | 0.07 |
| Myristic acid (C14:0) | 14.51 |
| Pentadecanoic acid (C15:0) | 0.16 |
| Palmitic acid (C16:0) | 33.50 |
| Heptadecanoic acid (C17:0) | 0.27 |
| Stearic acid (C18:0) | 0.19 |
| Elaidic acid (C18:1) | 4.37 |
| Oleic acid (C18:1, w-9) | 28.64 |
| Linoleic acid (C18:2, w-6) | 14.56 |
| Linolelaidic acid (C18:2, 6t) | 0.10 |
| Eicosanoic acid (C20:1) | 0.42 |
| Eicosadienoic acid (C20:2) | 0.11 |
| SFA | 51.54 |
| UFA | 48.20 |
| MUFA | 33.43 |
| PUFA | 14.77 |
| Coded Levels (Real Values) | |||||
|---|---|---|---|---|---|
| Assay | Repetition | X1 | X2 | Spore Count * (log) | Enzyme Activity * (U g−1) |
| 1 | 1 | −1 (25) | −1 (72) | 7.50 ± 0.06 | 0.00 ± 0.00 |
| 2 | 1 | 0 (35) | 1 (168) | 7.98 ± 0.04 | 129.08 ± 1.63 |
| 3 | 1 | 1 (45) | 0 (120) | 7.60 ± 0.04 | 28.97 ± 0.81 |
| 4 | 1 | 0 (35) | −1 (72) | 7.51 ± 0.03 | 0.00 ± 0.00 |
| 5 | 1 | 1 (45) | −1 (72) | 7.60 ± 0.05 | 0.00 ± 0.00 |
| 6 | 1 | −1 (25) | 1 (168) | 8.10 ± 0.08 | 68.74 ± 0.81 |
| 7 | 1 | −1 (25) | 0 (120) | 7.80 ± 0.07 | 16.90 ± 1.63 |
| 8 | 1 | 1 (45) | 1 (168) | 7.20 ± 0.17 | 72.76 ± 3.25 |
| 9 | 1 | 0 (35) | 0 (120) | 7.82 ± 0.03 | 216.90 ± 0.001 |
| 10 | 2 | 0 (35) | 1 (168) | 8.04 ± 0.04 | 133.10 ± 0.81 |
| 11 | 2 | 1 (45) | 0 (120) | 7.50 ± 0.04 | 33.56 ± 5.69 |
| 12 | 2 | −1 (25) | −1 (72) | 7.50 ± 0.06 | 0.00 ± 0.00 |
| 13 | 2 | 1 (45) | −1 (72) | 7.50 ± 0.05 | 0.00 ± 0.00 |
| 14 | 2 | 0 (35) | −1 (72) | 7.55 ± 0.03 | 0.00 ± 0.00 |
| 15 | 2 | −1 (25) | 1 (168) | 8.00 ± 0.08 | 72.19 ± 0.81 |
| 16 | 2 | −1 (25) | 0 (120) | 7.90 ± 0.07 | 9.43 ± 0.81 |
| 17 | 2 | 1 (45) | 1 (168) | 7.40 ± 0.17 | 77.36 ± 3.25 |
| 18 | 2 | 0 (35) | 0 (120) | 7.90 ± 0.03 | 208.28 ± 2.44 |
| Independent Variable and Interaction | Enzyme Activity (EA) | Spore Count (LogS) | ||
|---|---|---|---|---|
| Estimated Effect | p-Value | Estimated Effect | p-Value | |
| Mean/intercept | 59.29 * | 0.000000 * | 7.69 * | 0.000000 * |
| X1 (L) | 7.57 * | 0.003473 * | −0.32 * | 0.000053 * |
| X1 (Q) | 82.90 * | 0.000000 * | 0.16 * | 0.002530 * |
| X2 (L) | 92.20 * | 0.000000 * | 0.28 * | 0.000177 * |
| X2 (Q) | 39.57 * | 0.000000 * | 0.08 | 0.071984 |
| X1 (L) × X2(L) | 2.30 | 0.355445 | −0.40 * | 0.000044 * |
| X1 (Q) × X2(L) | 29.17 * | 0.000000 * | 0.15 * | 0.010254 |
| Source of Variation | Square Sum | Degree of Freedom | Mean Square | F-Value |
|---|---|---|---|---|
| Enzyme activity Equation (1) | ||||
| Regression | 59,429.97 | 4 | 14,857.493 | 7.53 |
| Residual | 25,645.99 | 13 | 1972.769 | |
| Lack of fit (p < 0.05) | 25,545.76 | 4 | ||
| Pure error | 100.24 | 9 | ||
| R2 | 0.70 | |||
| Spore Count Equation (2) | ||||
| Regression | 0.97 | 4 | 0.242 | 22.00 |
| Residual | 0.14 | 13 | 0.011 | |
| Lack of fit | 0.09 | 4 | ||
| Pure error (p = 0.06) | 0.05 | 9 | ||
| R2 | 0.89 | |||
| Listed F-value (95%) | F4.13 = 3.18 | |||
| Activators/Inhibitors | Relative Activity (%) Mean (SD) |
|---|---|
| NH4Cl | 94.17 ± 0.81 |
| (NH4)2SO4 | 95.00 ± 7.09 |
| CaCl2 | 75.02 * ± 6.10 |
| CuSO4 | 48.18 * ± 2.29 |
| FeCl2 | 16.75 * ± 2.19 |
| FeSO4 | 32.53 * ± 1.74 |
| MgSO4 | 48.11 * ± 1.70 |
| K2SO4 | 82.28 * ± 3.26 |
| KI | 91.56 * ± 1.52 |
| ZnSO4 | 48.67 * ± 0.93 |
| Na2SO4 | 94.77 ± 2.76 |
| NaCl | 96.43 ± 2.14 |
| Triton X-100 | 52.06 * ± 1.79 |
| β-mercaptoethanol | 113.80 * ± 3.24 |
| Urea | 80.26 * ± 8.12 |
| EDTA | 40.53 * ± 0.70 |
| SDS | 0.00 * |
| Glycerol | 50.42 * ± 2.23 |
| Control | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, K.d.S.; Assis, C.F.d.; Jácome, M.C.d.M.B.; Azevedo, W.M.d.; Ramalho, A.M.Z.; Santos, E.S.d.; Passos, T.S.; Sousa Junior, F.C.d.; Damasceno, K.S.F.d.S.C. Bati Butter as a Potential Substrate for Lipase Production by Aspergillus terreus NRRL-255. Foods 2023, 12, 564. https://doi.org/10.3390/foods12030564
Barros KdS, Assis CFd, Jácome MCdMB, Azevedo WMd, Ramalho AMZ, Santos ESd, Passos TS, Sousa Junior FCd, Damasceno KSFdSC. Bati Butter as a Potential Substrate for Lipase Production by Aspergillus terreus NRRL-255. Foods. 2023; 12(3):564. https://doi.org/10.3390/foods12030564
Chicago/Turabian StyleBarros, Karen dos Santos, Cristiane Fernandes de Assis, Millena Cristiane de Medeiros Bezerra Jácome, Wendell Medeiros de Azevedo, Adriana M. Zanbotto Ramalho, Everaldo Silvino dos Santos, Thaís Souza Passos, Francisco Canindé de Sousa Junior, and Karla Suzanne Florentino da Silva Chaves Damasceno. 2023. "Bati Butter as a Potential Substrate for Lipase Production by Aspergillus terreus NRRL-255" Foods 12, no. 3: 564. https://doi.org/10.3390/foods12030564
APA StyleBarros, K. d. S., Assis, C. F. d., Jácome, M. C. d. M. B., Azevedo, W. M. d., Ramalho, A. M. Z., Santos, E. S. d., Passos, T. S., Sousa Junior, F. C. d., & Damasceno, K. S. F. d. S. C. (2023). Bati Butter as a Potential Substrate for Lipase Production by Aspergillus terreus NRRL-255. Foods, 12(3), 564. https://doi.org/10.3390/foods12030564








