Effect of Flaxseed Oil Cake Extract on the Microbial Quality, Texture and Shelf Life of Gluten-Free Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of Experimental Gluten-Free Bread
2.2. Preparation of Experimental Gluten-Free Bread
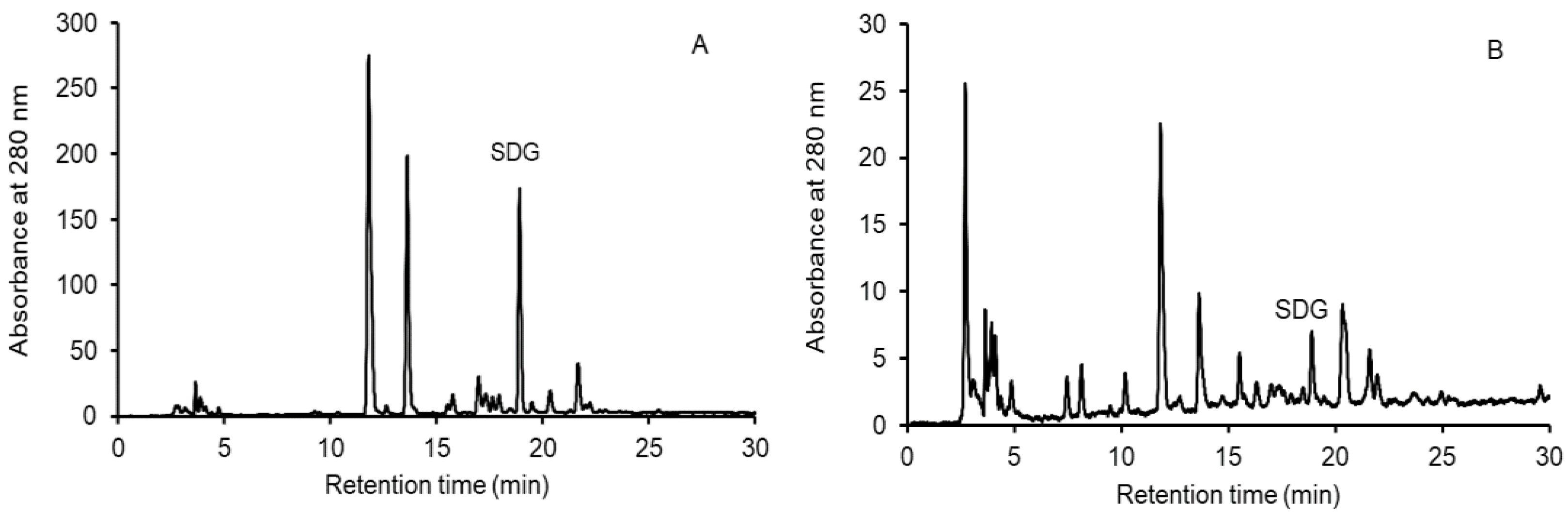
2.3. Analysis of Secoisolariciresinol Diglucoside
2.3.1. Extraction of SDG from the FOCE and GFB
2.3.2. High-Performance Liquid Chromatography (HPLC) Analysis of SDG
2.4. Characteristics of Experimental GFB with FOCE
2.4.1. Microbiological Analysis of Samples and pH Measurements
2.4.2. Moisture Content and Instrumental Evaluation of Texture Profile
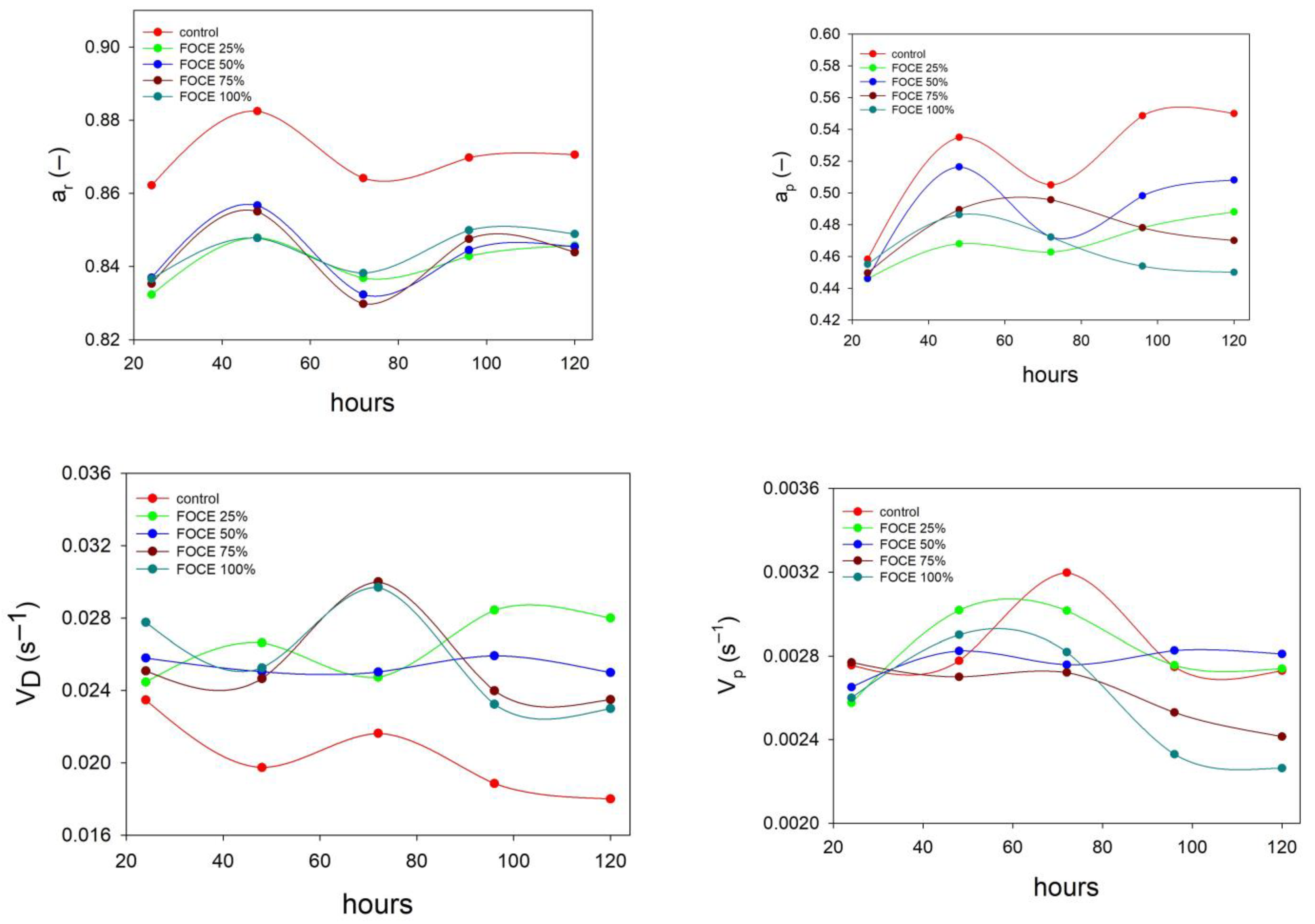
2.4.3. Low-Field NMR Relaxometry
2.4.4. Water Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification and Content of Secoisolariciresinol Diglucoside (SDG) in FOCE and Experimental GFB with FOCE
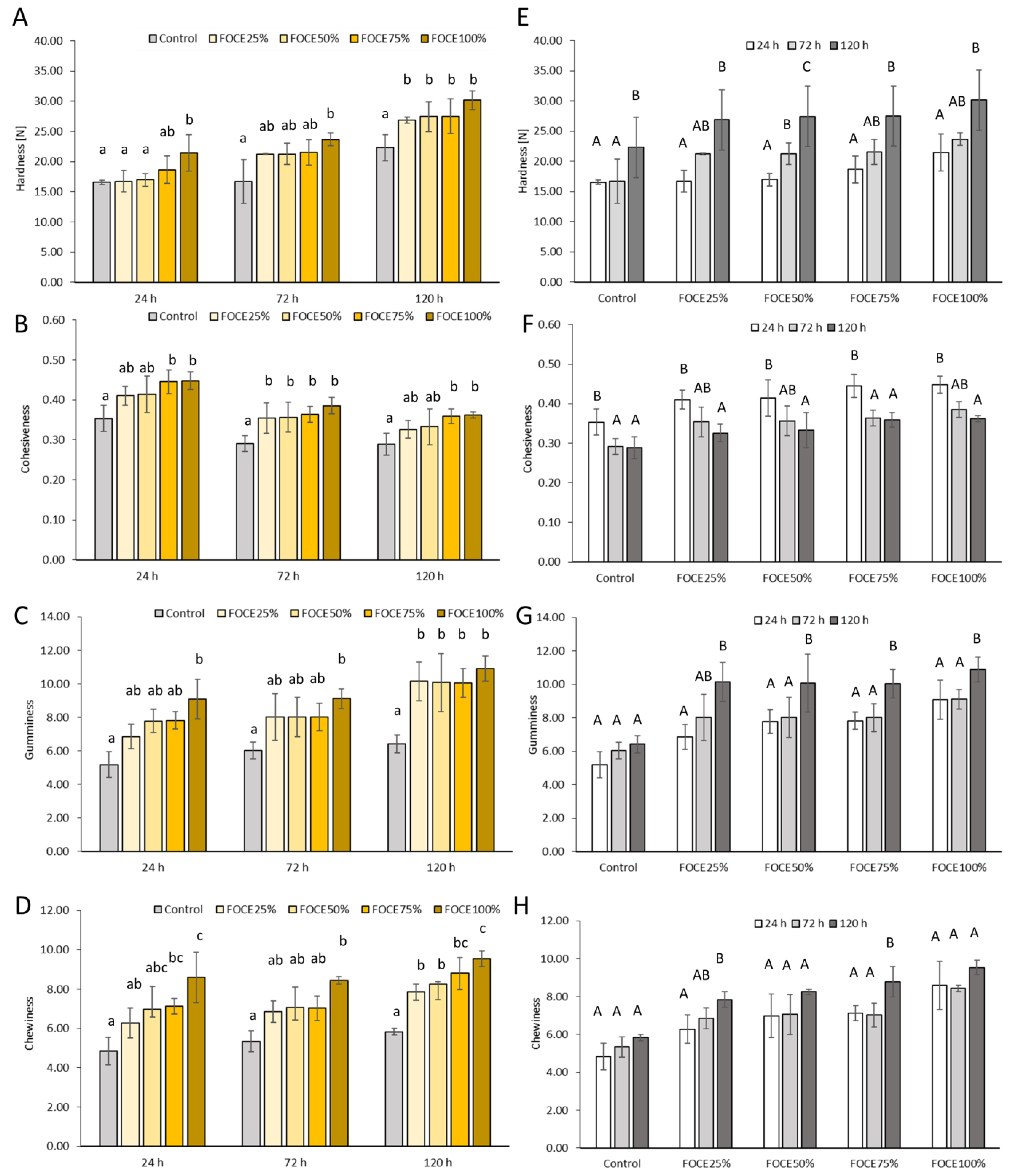
3.2. Characteristics of Experimental GFB with FOCE
3.2.1. Microbial Quality and Acidity Changes of GFB with FOCE during Storage
3.2.2. Moisture and Texture Profile of GFB with FOCE during Storage
3.2.3. Changes in Water Behavior of GFBs with FOCE during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Wójcik, M.; Krajewska, A. Dandelion Flowers as an Additive to Wheat Bread: Physical Properties of Dough and Bread Quality. Appl. Sci. 2023, 13, 477. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Zachariasz, E. Effect of Tomato, Beetroot and Carrot Juice Addition on Physicochemical, Antioxidant and Texture Properties of Wheat Bread. Antioxidants 2022, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and Nutritional Challenges, and Novelty in Gluten-Free Breadmaking—A Review. Pol. J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Bączek, N.; Šimková, K.; Starowicz, M.; Jeliński, T. Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Marciniak-Lukasiak, K.; Lesniewska, P.; Zielińska, D.; Sowinski, M.; Zbikowska, K.; Lukasiak, P.; Zbikowska, A. The Influence of Chestnut Flour on the Quality of Gluten-Free Bread. Appl. Sci. 2022, 12, 8340. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Bączek, N.; Capriles, V.D.; Łopusiewicz, Ł. Novel Gluten-Free Bread with an Extract from Flaxseed By-Product: The Relationship between Water Replacement Level and Nutritional Value, Antioxidant Properties, and Sensory Quality. Molecules 2022, 27, 2690. [Google Scholar] [CrossRef]
- Aguiar, E.V.; Santos, F.G.; Krupa-Kozak, U.; Capriles, V.D. Nutritional facts regarding commercially available gluten-free bread worldwide: Recent advances and future challenges. Crit. Rev. Food Sci. Nutr. 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jeliński, T.; Ostaszyk, A. Broccoli leaf powder as an attractive by-product ingredient: Effect on batter behaviour, technological properties and sensory quality of gluten-free mini sponge cake. Int. J. Food Sci. Technol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Man, S.M.; Stan, L.; Păucean, A.; Chiş, M.S.; Mureşan, V.; Socaci, S.A.; Pop, A.; Muste, S. Nutritional, Sensory, Texture Properties and Volatile Compounds Profile of Biscuits with Roasted Flaxseed Flour Partially Substituting for Wheat Flour. Appl. Sci. 2021, 11, 4791. [Google Scholar] [CrossRef]
- Tavarini, S.; De Leo, M.; Matteo, R.; Lazzeri, L.; Braca, A.; Angelini, L.G. Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds. Plants 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Velasquez, M.T. Potential effects of lignan-enriched flaxseed powder on bodyweight, visceral fat, lipid profile, and blood pressure in rats. Fitoterapia 2012, 83, 941–946. [Google Scholar] [CrossRef]
- Mercier, S.; Villeneuve, S.; Moresoli, C.; Mondor, M.; Marcos, B.; Power, K.A. Flaxseed-Enriched Cereal-Based Products: A Review of the Impact of Processing Conditions. Compr. Rev. Food Sci. Food Saf. 2014, 13, 400–412. [Google Scholar] [CrossRef]
- Ramcharitar, A.; Badrie, N.; Mattfeldt-Beman, M.; Matsuo, H.; Ridley, C. Consumer Acceptability of Muffins with Flaxseed (Linum usitatissimum). J. Food Sci. 2005, 70, s504–s507. [Google Scholar] [CrossRef]
- Strandås, C.; Kamal-Eldin, A.; Andersson, R.; Åman, P. Phenolic glucosides in bread containing flaxseed. Food Chem. 2008, 110, 997–999. [Google Scholar] [CrossRef]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr. J. 2015, 14, 71. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Łopusiewicz, Ł. Formulation and Evaluation of Spray-Dried Reconstituted Flaxseed Oil-In-Water Emulsions Based on Flaxseed Oil Cake Extract as Emulsifying and Stabilizing Agent. Foods 2021, 10, 256. [Google Scholar] [CrossRef]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Serra, A.; Conte, G.; Flamini, G.; Angelini, L.G. Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake. Appl. Sci. 2020, 10, 5235. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-Like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of flaxseed oil cake into bioactive camembert-analogue using lactic acid bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseed oil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Bartkowiak, A.; Kwiatkowski, P. The development of novel probiotic fermented plant milk alternative from flaxseed oil cake using Lactobacillus rhamnosus GG acting as a preservative agent against pathogenic bacteria during short-term refrigerated storage. Emir. J. Food Agric. 2021, 33, 266–276. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Bogusławska-Wąs, E.; Drozłowska, E.; Trocer, P.; Dłubała, A.; Mazurkiewicz-Zapałowicz, K.; Bartkowiak, A. The Application of Spray-Dried and Reconstituted Flaxseed Oil Cake Extract as Encapsulating Material and Carrier for Probiotic Lacticaseibacillus rhamnosus GG. Materials 2021, 14, 5324. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Dmytrów, I.; Mituniewicz-Małek, A.; Kwiatkowski, P.; Kowalczyk, E.; Sienkiewicz, M.; Drozłowska, E. Natural Gum from Flaxseed By-Product as a Potential Stabilizing and Thickening Agent for Acid Whey Fermented Beverages. Appl. Sci. 2022, 12, 10281. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of flaxseed oil cake residual from cold-press oil production as a material for preparation of spray-dried functional powders for food applications as emulsion stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Bienkiewicz, G.; Łopusiewicz, Ł. The influence of flaxseed oil cake extract on oxidative stability of microencapsulated flaxseed oil in spray-dried powders. Antioxidants 2021, 10, 211. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of flaxseed oil bimodal emulsions prepared with flaxseed oil cake extract applied as a natural emulsifying agent. Polymers 2020, 12, 2207. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Aman, P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef]
- Lorenc-Kukuła, K.; Kosińska, A.; Szopa, J.; Amarowicz, R. RP-HPLC-DAD Finger Print Analysis of Phenolic Extracts from Transcgenic Flax. Pol. J. Food Nutr. Sci. 2009, 59, 135–140. [Google Scholar]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg Md.: Rockville, MD, USA, 2000. [Google Scholar]
- Bourne, M.C.; Kenny, J.F.; Barnard, J. Computer-Assisted Readout of Data from Texture Profile Analysis Curves. J. Texture Stud. 1978, 9, 481–494. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Smarzyński, K.; Le Thanh-Blicharz, J.; Kačániová, M.; Baranowska, H.M. LF NMR spectroscopy analysis of water dynamics and texture of Gluten-Free bread with cricket powder during storage. Food Sci. Technol. Int. 2021, 27, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Brosio, E.; Gianferri, R.R. An analytical tool in foods characterization and traceability. In Basic NMR in Foods Characterization; Brosio, E., Ed.; Research Signpost: Kerala, India, 2009; pp. 9–37. [Google Scholar]
- Masewicz, Ł.; Lewandowicz, J.; Le Thanh-Blicharz, J.; Kempka, M.; Baranowska, H. Diffusion of water in potato starch pastes. In Proceedings of the 12th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 19–21 October 2016; Rapkova, R.R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2016; pp. 193–195. [Google Scholar]
- Xu, Y.; Hall, C., 3rd; Wolf-Hall, C. Antifungal activity stability of flaxseed protein extract using response surface methodology. J. Food Sci. 2008, 73, M9–M14. [Google Scholar] [CrossRef]
- Xu, Y.; Hall, C., 3rd; Wolf-Hall, C.; Manthey, F. Fungistatic activity of flaxseed in potato dextrose agar and a fresh noodle system. Int. J. Food Microbiol. 2008, 121, 262–267. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Fernandes, Â.; Heleno, S.A.; Rodrigues, P.; Gonzaléz-Paramás, A.M.; Barros, L.; Ferreira, I.C.F.R. Physicochemical characterization and microbiology of wheat and rye flours. Food Chem. 2019, 280, 123–129. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology. Fermentation 2018, 4, 9. [Google Scholar] [CrossRef]
- Moore, M.M.; Schober, T.J.; Dockery, P.; Arendt, E.K. Textural Comparisons of Gluten-Free and Wheat-Based Doughs, Batters, and Breads. Cereal Chem. 2004, 81, 567–575. [Google Scholar] [CrossRef]
- Collar, C.; Santos, E.; Rosell, C.M. Assessment of the rheological profile of fibre-enriched bread doughs by response surface methodology. J. Food Eng. 2007, 78, 820–826. [Google Scholar] [CrossRef]
- Giménez, A.; Ares, F.; Ares, G. Sensory shelf-life estimation: A review of current methodological approaches. Food Res. Int. 2012, 49, 311–325. [Google Scholar] [CrossRef]
- Baryłko-Pikielna, N.; Matuszewska, I. Metody sensorycznej analizy opisowej. In Sensoryczne Badania Żywności: Podstawy, Metody, Zastosowania; Wydawnictwo Naukowe “Akapit”: Kraków, Poland, 2009; pp. 181–222. (In Polish) [Google Scholar]
- Fadda, C.; Sanguinetti, A.M.; Del Caro, A.; Collar, C.; Piga, A. Bread Staling: Updating the View. Compr. Rev. Food Sci. Food Saf. 2014, 13, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.; Bemiller, J.N. Bread Staling: Molecular Basis and Control. Compr. Rev. Food Sci. Food Saf. 2003, 2, 1–21. [Google Scholar] [CrossRef]
- Blümich, B. Low-field and benchtop NMR. J. Magn. Reson. 2019, 306, 27–35. [Google Scholar] [CrossRef]
- Smarzyński, K.; Sarbak, P.; Kowalczewski, P.Ł.; Różańska, M.B.; Rybicka, I.; Polanowska, K.; Fedko, M.; Kmiecik, D.; Masewicz, Ł.; Nowicki, M.; et al. Low-Field NMR Study of Shortcake Biscuits with Cricket Powder, and Their Nutritional and Physical Characteristics. Molecules 2021, 26, 5417. [Google Scholar] [CrossRef] [PubMed]
- Makowska, A.; Dwiecki, K.; Kubiak, P.; Baranowska, H.M.; Lewandowicz, G. Polymer-Solvent Interactions in Modified Starches Pastes—Electrokinetic, Dynamic Light Scattering, Rheological and Low Field Nuclear Magnetic Resonance Approach. Polymers 2022, 14, 2977. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, W.; Czerniak, A.; Smarzyński, K.; Jeżowski, P.; Kmiecik, D.; Baranowska, H.M.; Walkowiak, K.; Ostrowska-Ligęza, E.; Różańska, M.B.; Lesiecki, M.; et al. Physicochemical and Morphological Study of the Saccharomyces cerevisiae Cell-Based Microcapsules with Novel Cold-Pressed Oil Blends. Appl. Sci. 2022, 12, 6577. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Baranowska, H.M. Low Field NMR Studies of Wheat Bread Enriched with Potato Juice During Staling. Open Agricul. 2019, 4, 426–430. [Google Scholar] [CrossRef]
- Krystyjan, M.; Dobosz-Kobędza, A.; Sikora, M.; Baranowska, H.M. Influence of Xanthan Gum Addition on the Short- and Long-Term Retrogradation of Corn Starches of Various Amylose Content. Polymers 2022, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, H.M.; Sikora, M.; Krystyjan, M.; Dobosz, A.; Tomasik, P.; Walkowiak, K.; Masewicz, Ł.; Borczak, B. Analysis of the Retrogradation Processes in Potato Starches Blended with Non-Starchy Polysaccharide Hydrocolloids by LF NMR. Food Biophys. 2020, 15, 64–71. [Google Scholar] [CrossRef]
- Sikora, M.; Krystyjan, M.; Dobosz, A.; Tomasik, P.; Walkowiak, K.; Masewicz, Ł.; Kowalczewski, P.Ł.; Baranowska, H.M. Molecular Analysis of Retrogradation of Corn Starches. Polymers 2019, 11, 1764. [Google Scholar] [CrossRef] [PubMed]
- Farhat, I.A.; Ottenhof, M.A.; Marie, V.; de Bezenac, E. 1H NMR relaxation study of amylopectin retrogradation. In Magnetic Resonance in Food Science: Latest Developments; Belton, P.S., Gil, A.M., Webb, G.A., Rutledge, D., Eds.; The Royal Society of Chemistry: London, UK, 2003; pp. 172–179. ISBN 978-0-85404-886-1. [Google Scholar]
- Hallberg, L.M.; Chinachoti, P. A Fresh Perspective on Staling: The Significance of Starch Recrystallization on the Firming of Bread. J. Food Sci. 2002, 67, 1092–1096. [Google Scholar] [CrossRef]
- Vodovotz, Y.; Vittadini, E.; Sachleben, J.R. Use of 1H cross-relaxation nuclear magnetic resonance spectroscopy to probe the changes in bread and its components during aging. Carbohydr. Res. 2002, 337, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Vittadini, E.; Vodovotz, Y. Effects of water distribution and transport on food microstructure. In Understanding and Controlling the Microstructure of Complex Foods; Woodhead Publishing: Sawston, UK, 2007; pp. 89–112. ISBN 978-1-84569-151-6. [Google Scholar]
- Wanjuu, C.; Abong, G.; Mbogo, D.; Heck, S.; Low, J.; Muzhingi, T. The physiochemical properties and shelf-life of orange-fleshed sweet potato puree composite bread. Food Sci. Nutr. 2018, 6, 1555–1563. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Marzec, A.; Lewicki, P.P. Relationship Between Water Activity of Crisp Bread And its Mechanical Properties and Structure. Pol. J. Food Nutr. Sci. 2008, 58, 45–51. [Google Scholar]
| Ingredient (%) | Control | FOCE25% | FOCE50% | FOCE75% | FOCE100% |
|---|---|---|---|---|---|
| Corn starch | 36.7 | 36.7 | 36.7 | 36.7 | 36.7 |
| Potato starch | 8.9 | 8.9 | 8.9 | 8.9 | 8.9 |
| Pectin | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| Sugar | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 |
| Salt | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Oil | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Fresh yeast | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 |
| FOCE 1 | - | 11.1 | 22.2 | 33.3 | 44.4 |
| Water | 44.4 | 33.3 | 22.2 | 11.1 | - |
| Control | FOCE25% | FOCE50% | FOCE75% | FOCE100% | |
|---|---|---|---|---|---|
| SDG (µg/100 g DM) | Nd | 217 ± 11 a | 287 ± 14 b | 345 ± 10 c | 526 ± 33 d |
| Total Mesophilic Bacteria [CFU/g] | Fungi [CFU/g] | Coliforms [CFU/g] | Bacillus sp. [CFU/g] | pH | |
|---|---|---|---|---|---|
| 24 h | |||||
| Control | <10 | <10 | Nd | Nd | 4.21 ± 0.11 a |
| FOCE25% | <10 | <10 | Nd | Nd | 4.22 ± 0.24 a |
| FOCE50% | <10 | <10 | Nd | Nd | 4.23 ± 0.08 a |
| FOCE75% | <10 | <10 | Nd | Nd | 4.26 ± 0.18 a |
| FOCE100% | <10 | <10 | Nd | Nd | 4.27 ± 0.10 b |
| 72 h | |||||
| Control | 1.81 × 101 ± 0.09 a | 1.78 × 101 ± 0.12 a | Nd | Nd | 4.13 ± 0.05 a |
| FOCE25% | 1.67 × 101 ± 0.23 b | 1.54 × 101 ± 0.52 b | Nd | Nd | 4.15 ± 0.14 a |
| FOCE50% | 1.45 × 101 ± 0.54 c | 0.78 × 101 ± 0.57 c | Nd | Nd | 4.17 ± 0.12 a |
| FOCE75% | 1.99 × 101 ± 0.23 d | 1.22 × 101 ± 0.13 d | Nd | Nd | 4.19 ± 0.14 a |
| FOCE100% | 1.44 × 101 ± 0.19 c | 1.09 × 101 ± 0.85 e | Nd | Nd | 4.28 ± 0.07 b |
| 120 h | |||||
| Control | 2.03 × 101 ± 0.43 a | 5.33 × 103 ± 0.12 a | Nd | Nd | 4.04 ± 0.02 a |
| FOCE25% | 1.88 × 101 ± 0.43 a | 2.08 × 102 ± 0.65 b | Nd | Nd | 4.09 ± 0.08 a |
| FOCE50% | 1.55 × 101 ± 0.57 b | 1.39 × 102 ± 0.25 c | Nd | Nd | 4.16 ± 0.22 a |
| FOCE75% | 2.12 × 101 ± 0.69 c | 1.98 × 102 ± 0.35 d | Nd | Nd | 4.18 ± 0.28 a |
| FOCE100% | 1.28 × 101 ± 0.08 d | 1.88 × 102 ± 0.87 e | Nd | Nd | 4.22 ± 0.11 b |
| T1 (ms) | T21 (ms) | T22 (ms) | |
|---|---|---|---|
| 24 h | |||
| Control | 151.46 ± 0.85 | 0.81 ± 0.19 | 3.49 ± 0.10 |
| FOCE25% | 136.05 ± 2.20 | 0.74 ± 0.15 | 3.14 ± 0.08 |
| FOCE50% | 139.00 ± 2.23 | 0.53 ± 0.13 | 3.09 ± 0.03 |
| FOCE75% | 137.86 ± 2.20 | 0.44 ± 0.11 | 3.33 ± 0.11 |
| FOCE100% | 139.70 ± 1.46 | 0.44 ± 0.07 | 3.47 ± 0.05 |
| 48 h | |||
| Control | 153.39 ± 1.27 | 1.76 ± 0.35 | 4.19 ± 0.54 |
| FOCE25% | 141.73 ± 1.28 | 1.34 ± 0.26 | 3.67 ± 0.99 |
| FOCE50% | 144.06 ± 1.14 | 1.08 ± 0.13 | 3.39 ± 0.08 |
| FOCE75% | 143.12 ± 0.79 | 0.95 ± 0.05 | 3.64 ± 0.69 |
| FOCE100% | 141.18 ± 1.30 | 0.81 ± 0.08 | 3.68 ± 0.09 |
| 72 h | |||
| Control | 151.87 ± 1.11 | 1.69 ± 0.56 | 3.87 ± 0.59 |
| FOCE25% | 141.00 ± 1.65 | 1.41 ± 0.38 | 3.39 ± 0.40 |
| FOCE50% | 142.85 ± 1.27 | 1.17 ± 0.53 | 3.31 ± 0.94 |
| FOCE75% | 142.35 ± 0.78 | 1.01 ± 0.37 | 2.97 ± 0.62 |
| FOCE100% | 139.86 ± 0.75 | 0.64 ± 0.11 | 3.34 ± 0.06 |
| 96 h | |||
| Control | 152.20 ± 0.91 | 0.92 ± 0.09 | 3.58 ± 0.10 |
| FOCE25% | 142.15 ± 1.37 | 0.60 ± 0.11 | 2.98 ± 0.07 |
| FOCE50% | 139.99± 0.89 | 0.64 ± 0.09 | 3.18 ± 0.94 |
| FOCE75% | 136.84± 1.71 | 0.68 ± 0.06 | 2.84 ± 0.05 |
| FOCE100% | 137.59 ± 1.13 | 0.57 ± 0.05 | 3.28 ± 0.06 |
| 120 h | |||
| Control | 153.66 ± 1.36 | 0.80 ± 0.08 | 3.40 ± 1.03 |
| FOCE25% | 142.88 ± 1.45 | 0.54 ± 0.12 | 2.80 ± 0.70 |
| FOCE50% | 138.17 ± 0.99 | 0.60 ± 0.04 | 3.00± 0.70 |
| FOCE75% | 135.12 ± 2.20 | 0.60 ± 0.08 | 2.78 ± 0.80 |
| FOCE100% | 137.00 ± 0.87 | 0.52 ± 0.09 | 3.20 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Kowalczewski, P.Ł.; Baranowska, H.M.; Masewicz, Ł.; Amarowicz, R.; Krupa-Kozak, U. Effect of Flaxseed Oil Cake Extract on the Microbial Quality, Texture and Shelf Life of Gluten-Free Bread. Foods 2023, 12, 595. https://doi.org/10.3390/foods12030595
Łopusiewicz Ł, Kowalczewski PŁ, Baranowska HM, Masewicz Ł, Amarowicz R, Krupa-Kozak U. Effect of Flaxseed Oil Cake Extract on the Microbial Quality, Texture and Shelf Life of Gluten-Free Bread. Foods. 2023; 12(3):595. https://doi.org/10.3390/foods12030595
Chicago/Turabian StyleŁopusiewicz, Łukasz, Przemysław Łukasz Kowalczewski, Hanna Maria Baranowska, Łukasz Masewicz, Ryszard Amarowicz, and Urszula Krupa-Kozak. 2023. "Effect of Flaxseed Oil Cake Extract on the Microbial Quality, Texture and Shelf Life of Gluten-Free Bread" Foods 12, no. 3: 595. https://doi.org/10.3390/foods12030595
APA StyleŁopusiewicz, Ł., Kowalczewski, P. Ł., Baranowska, H. M., Masewicz, Ł., Amarowicz, R., & Krupa-Kozak, U. (2023). Effect of Flaxseed Oil Cake Extract on the Microbial Quality, Texture and Shelf Life of Gluten-Free Bread. Foods, 12(3), 595. https://doi.org/10.3390/foods12030595










