Effect of Alkali on the Microbial Community and Aroma Profile of Chinese Steamed Bread Prepared with Chinese Traditional Starter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacture of CSB using CTS
2.3. Isolation and Identification of Dominant Yeast and LAB Strain from CTS
2.4. Manufacture of CSB Fermented Using Cultivated Yeast and LAB
2.5. Specific Volume, pH, and Total Titratable Acidity (TTA) of Dough Fermented by CTS
2.6. High-Throughput Sequencing
2.7. Detection of Volatile Compounds in CSB
2.8. Statistical Analysis
3. Results and Discussion
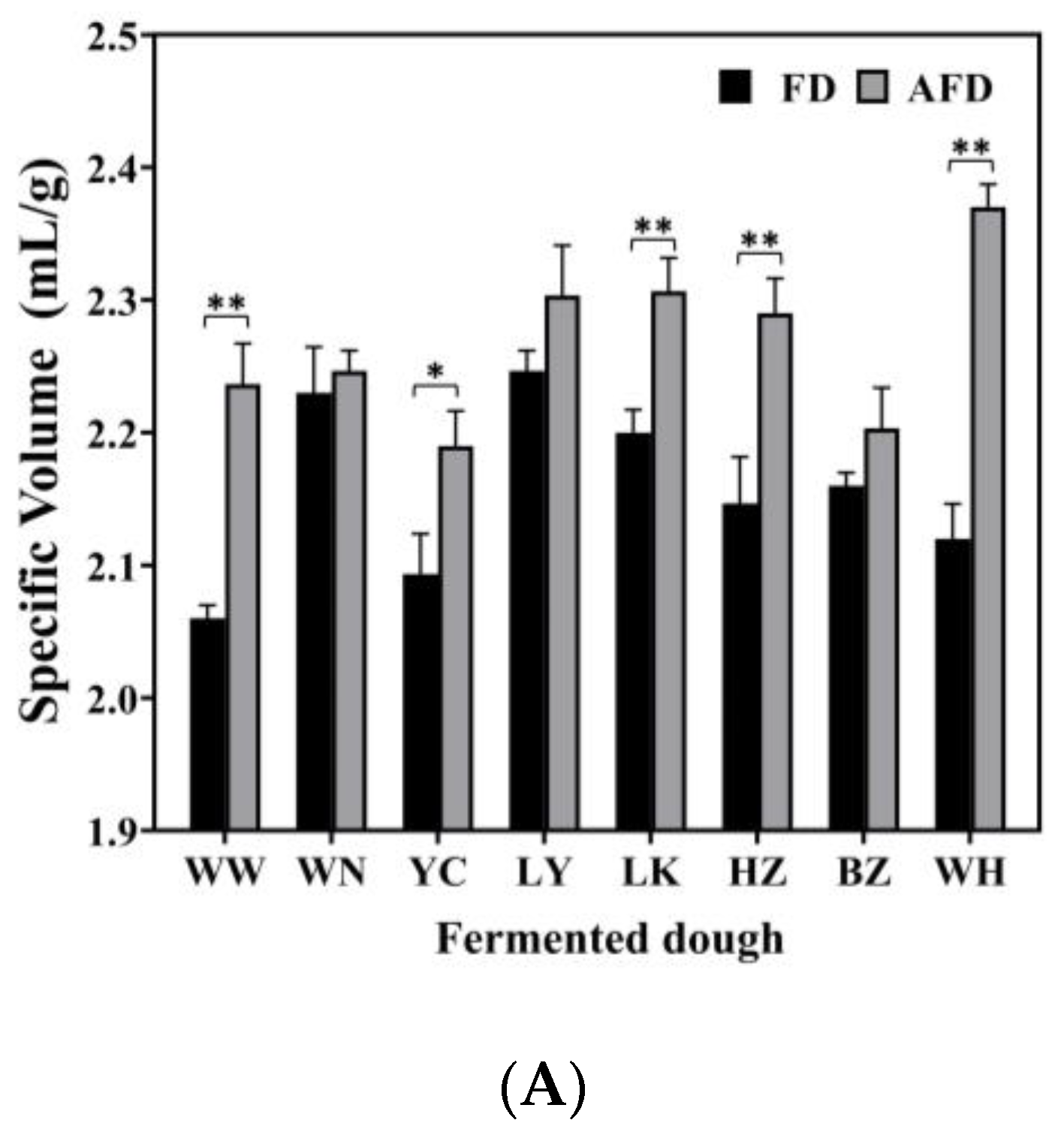
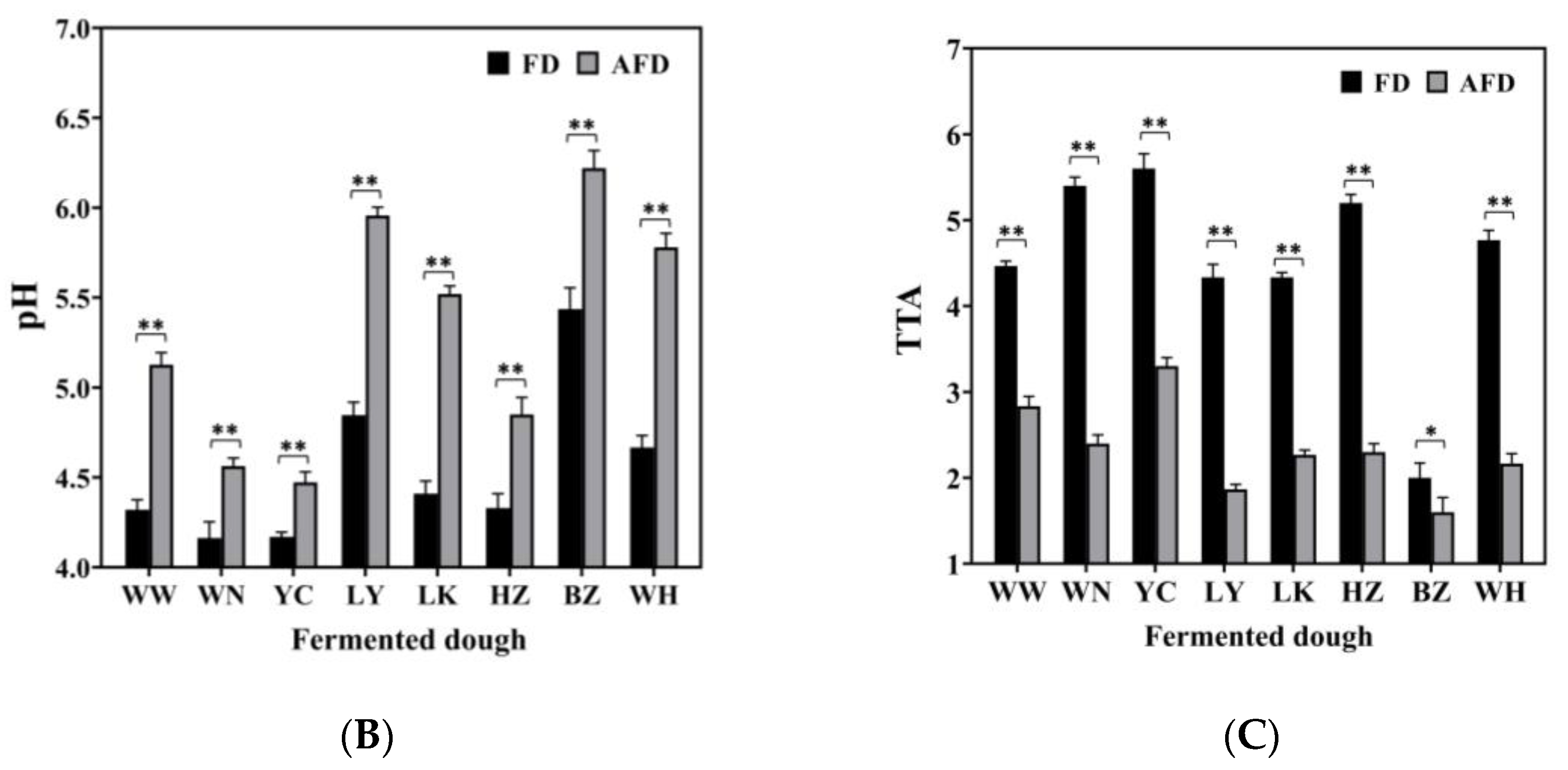
3.1. Specific Volume, pH, and TTA of Fermented Dough
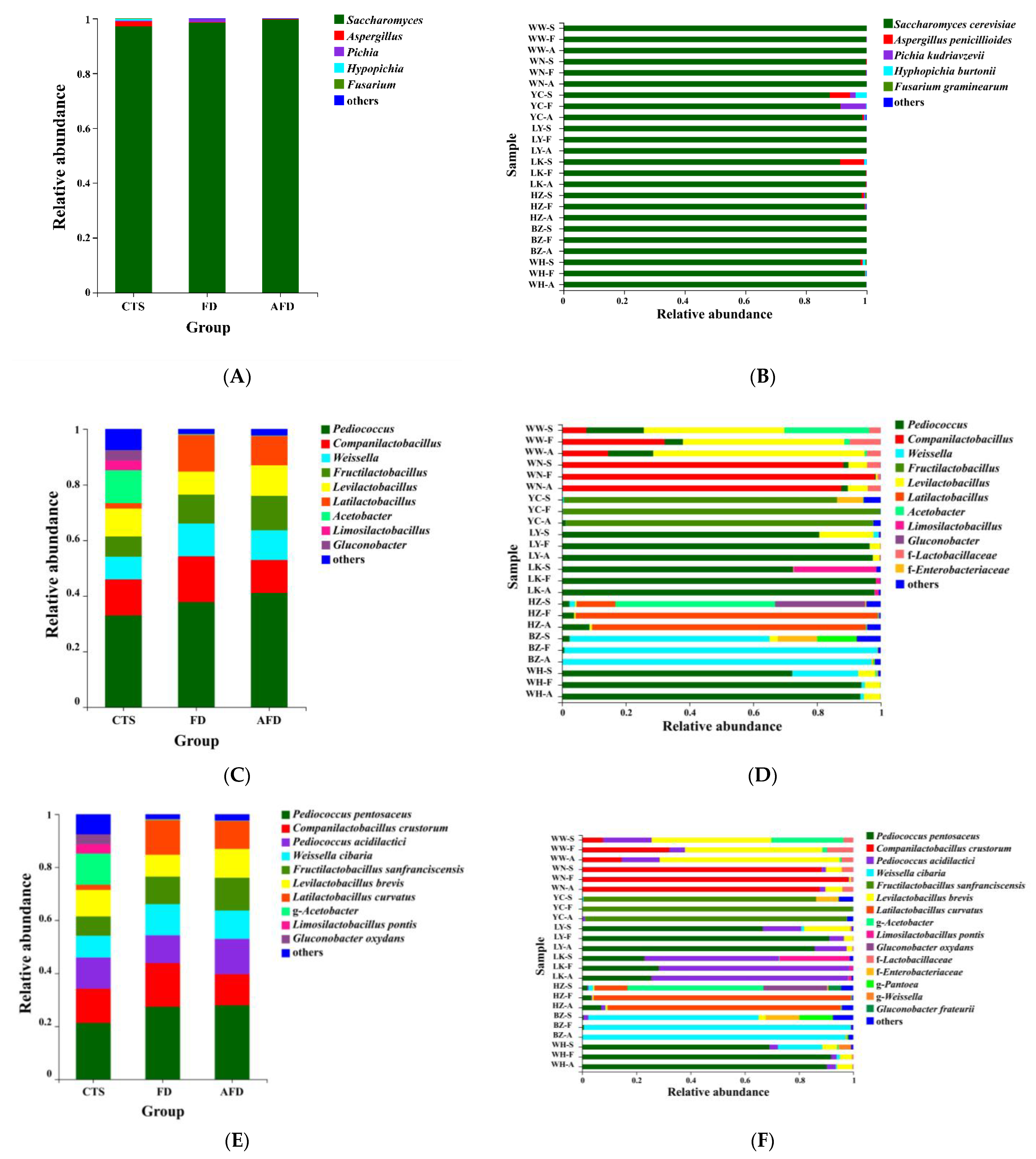
3.2. Fungal Community Analysis in CTS and Fermented Dough
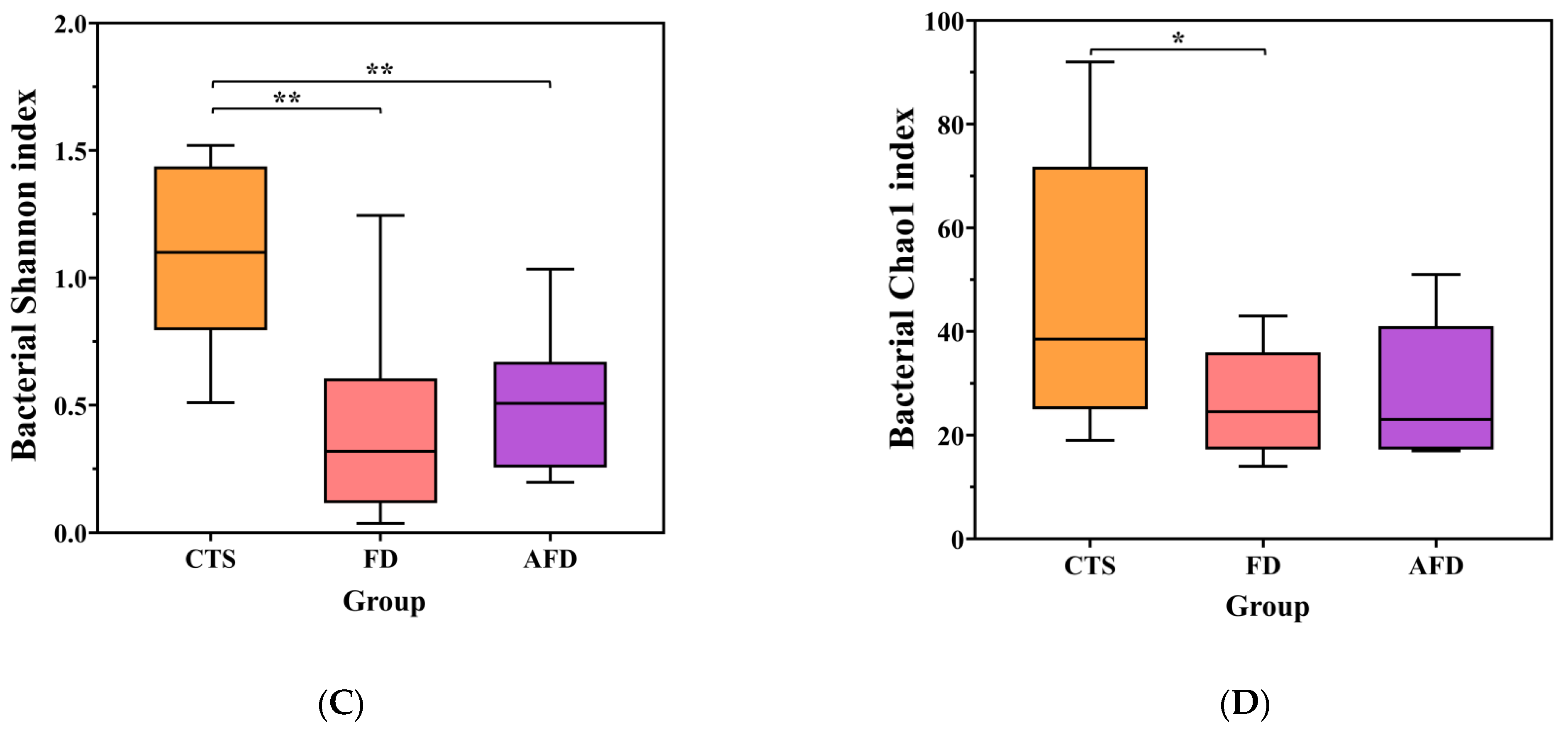
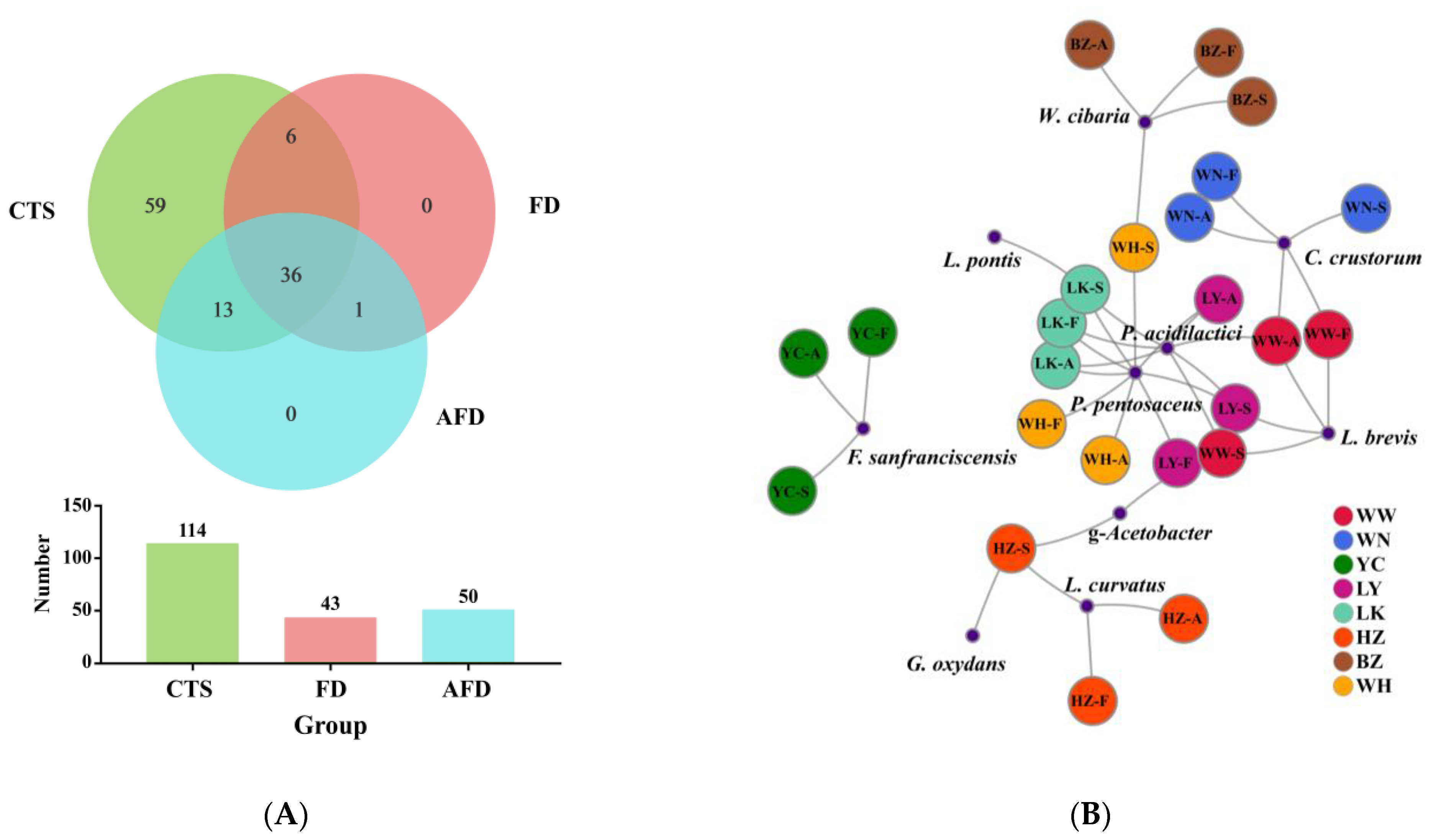
3.3. Bacterial Community Analysis in CTS and Fermented Dough
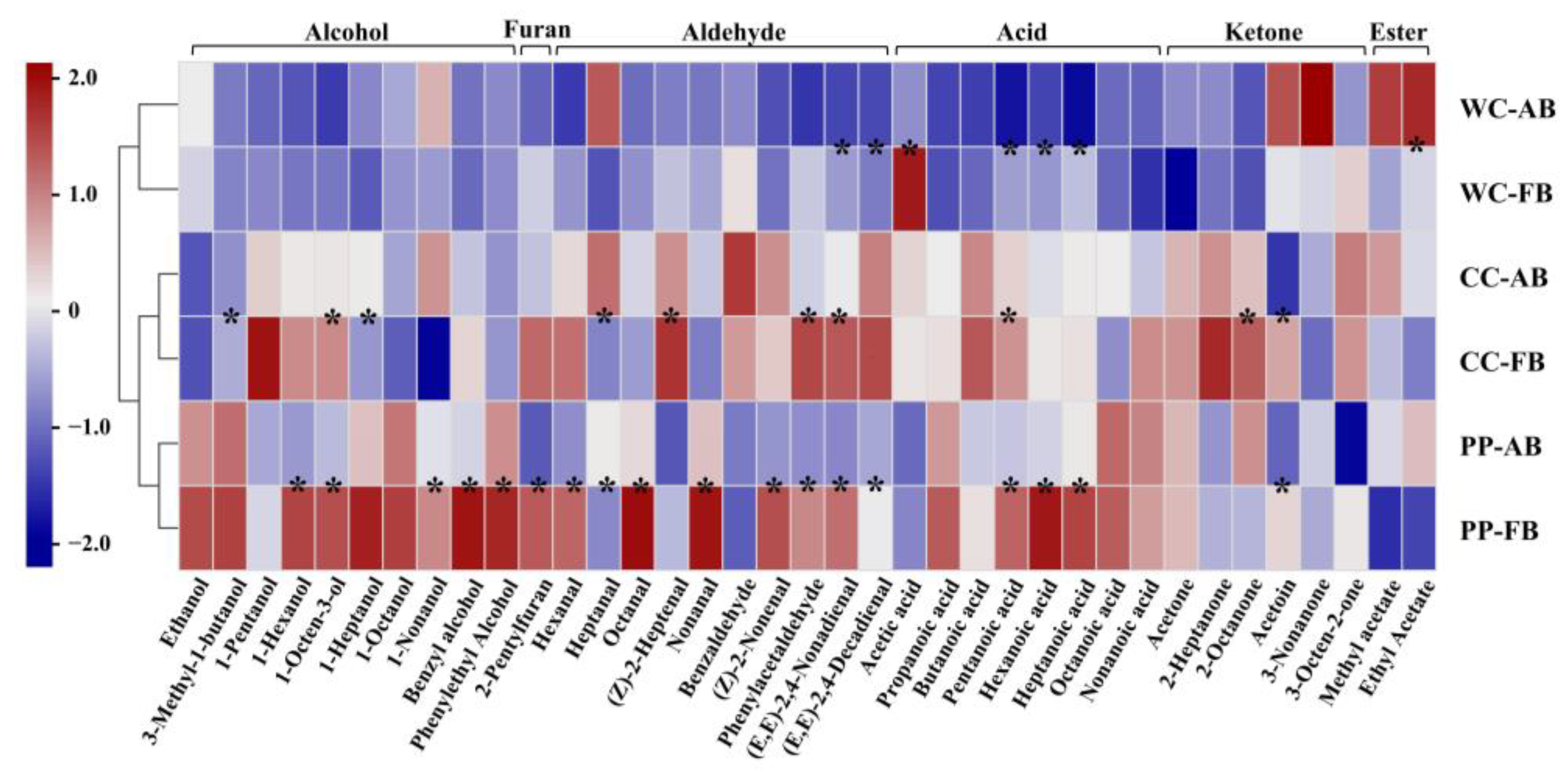
3.4. Analysis of Volatile Compounds in CSB
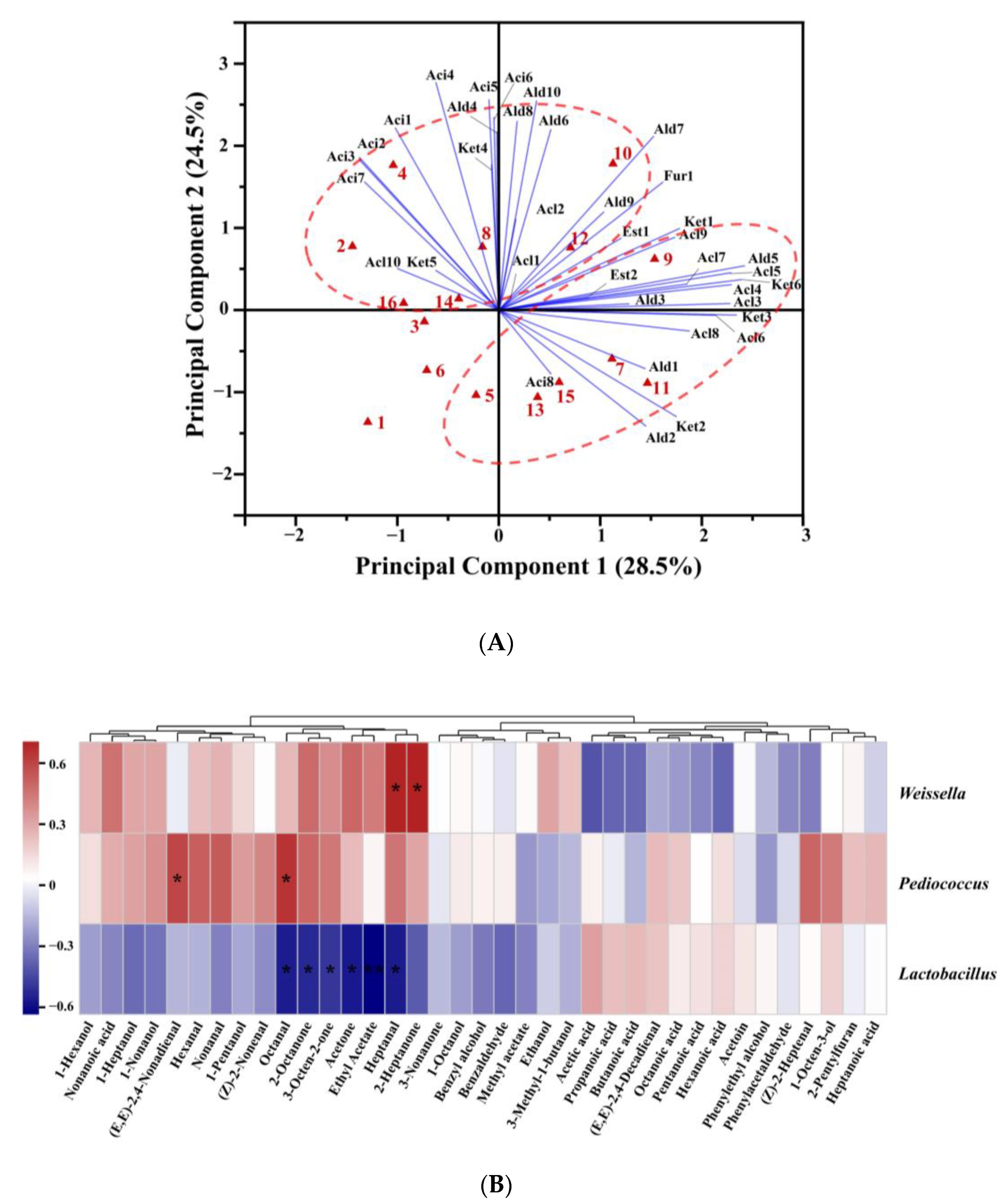
3.5. Correlation Analysis between Aroma Compounds and Bacteria at Genus Level
3.6. Analysis of Aroma Compounds in CSB Prepared with Synthetic Microbial Community Starter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huang, Y.; Wan, J.; Wang, Z.; Sun, M.; Feng, T.; Ho, C.-T.; Song, S. Variation of Volatile Compounds and Corresponding Aroma Profiles in Chinese Steamed Bread by Various Yeast Species Fermented at Different Times. J. Agric. Food Chem. 2022, 70, 3795–3806. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Bi, Y.; Zhao, R.; Nie, Y.; Yuan, W. Dynamics of microbial community and changes of metabolites during production of type Ι sourdough steamed bread made by retarded sponge-dough method. Food Chem. 2020, 330, 127316. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Sadiq, F.A.; Yang, H.; Gu, J.; Yuan, L.; Lee, Y.K.; He, G. Predominant yeasts in Chinese traditional sourdough and their influence on aroma formation in Chinese steamed bread. Food Chem. 2018, 242, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Suo, B.; Nie, W.; Wang, Y.; Ma, J.; Xing, X.; Huang, Z.; Xu, C.; Li, Z.; Ai, Z. Microbial diversity of fermented dough and volatile compounds in steamed bread prepared with traditional Chinese starters. LWT 2020, 126, 109350. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Wang, Y. Influence of Na2CO3 on the quality of dough with rice wine sourdough and steamed bread. Int. J. Food Sci. Technol. 2020, 55, 2261–2270. [Google Scholar] [CrossRef]
- Han, C.; Ma, M.; Li, M.; Sun, Q. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocoll. 2020, 103, 105661. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O. History of corn and wheat tortillas. In Tortillas; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–28. [Google Scholar]
- Yao, N.; Owusu-Apenten, R.; Zhu, L.; Seetharaman, K. Effect of alkali dipping on dough and final product quality. J. Food Sci. 2006, 71, C209–C215. [Google Scholar] [CrossRef]
- Guo, X.-N.; Yang, S.; Zhu, K.-X. Influences of alkali on the quality and protein polymerization of buckwheat Chinese steamed bread. Food Chem. 2019, 283, 52–58. [Google Scholar] [CrossRef]
- Xi, J.; Xu, D.; Wu, F.; Jin, Z.; Xu, X. Effect of Na2CO3 on quality and volatile compounds of steamed bread fermented with yeast or sourdough. Food Chem. 2020, 324, 126786. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, T.; Sun, H. Microbial characterization of five Chinese traditional sourdoughs by high-throughput sequencing and their impact on the quality of potato steamed bread. Food Chem. 2019, 274, 710–717. [Google Scholar] [CrossRef]
- Xing, X.; Ma, J.; Fu, Z.; Zhao, Y.; Ai, Z.; Suo, B. Diversity of bacterial communities in traditional sourdough derived from three terrain conditions (mountain, plain and basin) in Henan Province, China. Food Res. Int. 2020, 133, 109139. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough improves the quality of whole-wheat flour products: Mechanisms and challenges—A review. Food Chem. 2021, 360, 130038. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Wang, S.; Liu, Y.; Li, L.; Gao, M. Lactic acid bacteria synergistic fermentation affects the flavor and texture of bread. J. Food Sci. 2022, 87, 1823–1836. [Google Scholar] [CrossRef]
- Yan, B.; Yang, H.; Wu, Y.; Lian, H.; Zhang, H.; Chen, W.; Fan, D.; Zhao, J. Quality Enhancement Mechanism of Alkali-Free Chinese Northern Steamed Bread by Sourdough Acidification. Molecules 2020, 25, 726. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.S.; Chavan, S.R. Sourdough technology—A traditional way for wholesome foods: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Cui, D.; Ke, B.; Liu, C. Effect of Lactobacillus Plantarum DM616 on Dough Fermentation and Chinese Steamed Bread Quality. J. Food Process. Preserv. 2015, 39, 30–37. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1. [Google Scholar] [CrossRef]
- Boreczek, J.; Litwinek, D.; Żylińska-Urban, J.; Izak, D.; Buksa, K.; Gawor, J.; Gromadka, R.; Bardowski, J.K.; Kowalczyk, M. Bacterial community dynamics in spontaneous sourdoughs made from wheat, spelt, and rye wholemeal flour. Microbiologyopen 2020, 9, e1009. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Dumba, T.; Sheng, Y.; Li, J.; Lu, Q.; Liu, C.; Cai, C.; Feng, F.; Zhao, M. Microbial diversity and chemical property analyses of sufu products with different producing regions and dressing flavors. LWT 2021, 144, 111245. [Google Scholar] [CrossRef]
- Garcia, M.V.; Bernardi, A.O.; Copetti, M.V. The fungal problem in bread production: Insights of causes, consequences, and control methods. Curr. Opin. Food Sci. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Arata, G.J.; Martínez, M.; Elguezábal, C.; Rojas, D.; Cristos, D.; Dinolfo, M.I.; Arata, A.F. Effects of sowing date, nitrogen fertilization, and Fusarium graminearum in an Argentinean bread wheat: Integrated analysis of disease parameters, mycotoxin contamination, grain quality, and seed deterioration. J. Food Compos. Anal. 2022, 107, 104364. [Google Scholar] [CrossRef]
- Menezes, L.; Sardaro, M.S.; Duarte, R.; Mazzon, R.; Neviani, E.; Gatti, M.; Lindner, J.D.D. Sourdough bacterial dynamics revealed by metagenomic analysis in Brazil. Food Microbiol. 2020, 85, 103302. [Google Scholar] [CrossRef]
- Robert, H.; Gabriel, V.; Fontagné-Faucher, C. Biodiversity of lactic acid bacteria in French wheat sourdough as determined by molecular characterization using species-specific PCR. Int. J. Food Microbiol. 2009, 135, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. The influence of different lactic acid bacteria on sourdough flavor and a deep insight into sourdough fermentation through RNA sequencing. Food Chem. 2020, 307, 125529. [Google Scholar] [CrossRef]
- Plessas, S.; Mantzourani, I.; Bekatorou, A. Evaluation of Pediococcus pentosaceus SP2 as starter culture on sourdough bread making. Foods 2020, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Comasio, A.; Harth, H.; Weckx, S.; De Vuyst, L. The addition of citrate stimulates the production of acetoin and diacetyl by a citrate-positive Lactobacillus crustorum strain during wheat sourdough fermentation. Int. J. Food Microbiol. 2019, 289, 88–105. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Pini, N.; Guerrini, S.; Granchi, L.; Vincenzini, M. Liquid and firm sourdough fermentation: Microbial robustness and interactions during consecutive backsloppings. LWT 2019, 105, 9–15. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhao, J.W.; Xu, F.; Zhang, Q.D.; Ai, Z.L.; Li, B.Y. GC-MS analyses of volatile compounds of steamed breads fermented by Chinese traditional starter “Jiaozi” from different regions. J. Food Process. Preserv. 2021, 45, e15267. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef]
- Wu, S.; Peng, Y.; Xi, J.; Zhao, Q.; Xu, D.; Jin, Z.; Xu, X. Effect of sourdough fermented with corn oil and lactic acid bacteria on bread flavor. LWT 2022, 155, 112935. [Google Scholar] [CrossRef]
- Yan, B.; Sadiq, F.A.; Cai, Y.; Fan, D.; Zhang, H.; Zhao, J.; Chen, W. Identification of key aroma compounds in type I sourdough-based Chinese steamed bread: Application of untargeted metabolomics analysisp. Int. J. Mol. Sci. 2019, 20, 818. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Maire, M.; Rega, B.; Cuvelier, M.-E.; Soto, P.; Giampaoli, P. Lipid oxidation in baked products: Impact of formula and process on the generation of volatile compounds. Food Chem. 2013, 141, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Chiş, M.S.; Pop, A.; Păucean, A.; Socaci, S.A.; Alexa, E.; Man, S.M.; Bota, M.; Muste, S. Fatty acids, volatile and sensory profile of multigrain biscuits enriched with spent malt rootles. Molecules 2020, 25, 442. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Karboune, S. A review of bread qualities and current strategies for bread bioprotection: Flavor, sensory, rheological, and textural attributes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1937–1981. [Google Scholar] [CrossRef]
- Xu, X.; Bi, S.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Comprehensive investigation on volatile and non-volatile metabolites in broccoli juices fermented by animal-and plant-derived Pediococcus pentosaceus. Food Chem. 2021, 341, 128118. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. Aroma of wheat bread crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]
- Krause, S.; Keller, S.; Hashemi, A.; Descharles, N.; Bonazzi, C.; Rega, B. From flours to cakes: Reactivity potential of pulse ingredients to generate volatile compounds impacting the quality of processed foods. Food Chem. 2022, 371, 131379. [Google Scholar] [CrossRef]
- Minervini, F.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Ecological parameters influencing microbial diversity and stability of traditional sourdough. Int. J. Food Microbiol. 2014, 171, 136–146. [Google Scholar] [CrossRef]
- Yang, H.; Liu, T.; Zhang, G.; He, G. Intraspecific diversity and fermentative properties of Saccharomyces cerevisiae from Chinese traditional sourdough. LWT 2020, 124, 109195. [Google Scholar] [CrossRef]
- Choińska, R.; Giryn, H.; Piasecka-Jóźwiak, K.; Kotyrba, D.; Bartosiak, E. Glucose Consumption and Lactic Acid Formation in Millet Sourdough Fermented with Different Strains of Lactic Acid Bacteria. J.Microbiol. Biotechnol. Food Sci. 2021, 2021, 921–923. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, C.R.; Chang, H.C. Heterofermentative lactic acid bacteria as a starter culture to control kimchi fermentation. LWT 2018, 88, 181–188. [Google Scholar] [CrossRef]
- Katsi, P.; Kosma, I.S.; Michailidou, S.; Argiriou, A.; Badeka, A.V.; Kontominas, M.G. Characterization of Artisanal Spontaneous Sourdough Wheat Bread from Central Greece: Evaluation of Physico-Chemical, Microbiological, and Sensory Properties in Relation to Conventional Yeast Leavened Wheat Bread. Foods 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]

| CTS | Sampling Site | Location | Raw Materials | Microbial Materials | Season |
|---|---|---|---|---|---|
| WW | Wuwei City, Gansu Province | 37°33′ N, 102°18′ E | Wheat flour | Sourdough | Summer |
| WN | Weinan City, Shaanxi Province | 37°30′ N, 109°36′ E | Wheat flour, Corn flour | Sourdough | Summer |
| YC | Yuncheng City, Shanxi Province | 34°50′ N, 111°13′ E | Wheat flour, Corn flour | Sourdough | Summer |
| LY | Luoyang City, Henan Province | 34°31′ N, 112°03′ E | Wheat flour, Corn flour | Cucumis melo, Rice wine | Summer |
| LK | Lankao City, Henan Province | 34°41′ N, 114°48′ E | Wheat flour, Corn flour | Cucumis melo, Rice wine | Summer |
| HZ | Heze City, Shandong Province | 35°14′ N, 115°28′ E | Wheat flour, Corn flour | Cucumis melo, Chinese jute (Abutilon theophrasti) | Summer |
| BZ | Binzhou City, Shandong Province | 37°22′ N, 118°02′ E | Wheat flour | Sourdough | Summer |
| WH | Weihai City, Shandong Province | 37°52′ N, 122°12′ E | Corn flour, Proso millet (Panicum miliaceum) | Sourdough | Summer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, N.; Xing, X.; Li, H.; Jiao, H.; Ji, S.; Ai, Z. Effect of Alkali on the Microbial Community and Aroma Profile of Chinese Steamed Bread Prepared with Chinese Traditional Starter. Foods 2023, 12, 617. https://doi.org/10.3390/foods12030617
Tang N, Xing X, Li H, Jiao H, Ji S, Ai Z. Effect of Alkali on the Microbial Community and Aroma Profile of Chinese Steamed Bread Prepared with Chinese Traditional Starter. Foods. 2023; 12(3):617. https://doi.org/10.3390/foods12030617
Chicago/Turabian StyleTang, Ning, Xiaolong Xing, Huipin Li, Honggang Jiao, Shengxin Ji, and Zhilu Ai. 2023. "Effect of Alkali on the Microbial Community and Aroma Profile of Chinese Steamed Bread Prepared with Chinese Traditional Starter" Foods 12, no. 3: 617. https://doi.org/10.3390/foods12030617









