Abstract
Almost 65% of the human protein supply in the world originates from plants, with legumes being one of the highest contributors, comprising between 20 and 40% of the protein supply. Bioactive peptides from various food sources including legumes have been reported to show efficacy in modulating starch digestion and glucose absorption. This paper will provide a comprehensive review on recent in vitro studies that have been performed on leguminous antidiabetic peptides, focusing on the α-amylase inhibitor, α-glucosidase inhibitor, and dipeptidyl peptidase-IV (DPP-IV) inhibitor. Variations in legume cultivars and methods affect the release of peptides. Different methods have been used, such as in sample preparation, including fermentation (t, T), germination (t), and pre-cooking; in protein extraction, alkaline extraction, isoelectric precipitation, phosphate buffer extraction, and water extraction; in protein hydrolysis enzyme types and combination, enzyme substrate ratio, pH, and time; and in enzyme inhibitory assays, positive control type and concentration, inhibitor or peptide concentration, and the unit of inhibitory activity. The categorization of the relative scale of inhibitory activities among legume samples becomes difficult because of these method differences. Peptide sequences in samples were identified by means of HPLC/MS. Software and online tools were used in bioactivity prediction and computational modelling. The identification of the types and locations of chemical interactions between the inhibitor peptides and enzymes and the type of enzyme inhibition were achieved through computational modelling and enzyme kinetic studies.
1. Introduction
Diabetes is a global health problem and the world’s fastest growing chronic disease. Diabetes is one of the top ten causes of death in the world [1]. Over the course of 19 years (2000–2019), diabetes-related mortality increased by 3% [2]. Approximately 537 million adults worldwide between the ages of 20 and 79 years had diabetes mellitus in 2021. This number is estimated to increase to 643 million by 2030 and 783 million by 2045 [3].
There are three main types of diabetes: diabetes mellitus type 1 (T1DM), diabetes mellitus type 2 (T2DM), and gestational diabetes [3]. Among the three, T2DM is the most common, and affects 85–95% of the diabetic population worldwide [4].
T2DM is a chronic condition characterized by insulin deficiency and peripheral insulin resistance which causes high blood-glucose levels. Diets high in readily digestible carbohydrates (starch) and highly processed foods, as well as a sedentary life, play a crucial role in the epidemy of T2DM [5].
A healthy lifestyle is the foundation of T2DM management. This includes a healthy diet, regular physical exercise, not smoking, and maintaining a healthy body weight [3]. For some people with T2DM, practising a healthy lifestyle is difficult. For some others, over the long run of the course of illness, blood-glucose levels become difficult to maintain control of solely by following a healthy lifestyle. These difficulties cause T2DM patients to use hypoglycaemic drugs. Available oral hypoglycaemic drugs include metformin, sulfonylureas, rosiglitazone, acarbose, voglibose, miglitol, and gliptins. Oral hypoglycaemic drugs help control blood-glucose levels by reducing insulin resistance (metformin), stimulating insulin production (sulfonylureas), increasing insulin sensitivity (rosiglitazone), acting as an α-amylase inhibitor (acarbose), acting as α-glucosidase inhibitors (acarbose, voglibose, miglitol), and acting as a DPP-IV inhibitor (gliptins) [6,7,8,9,10,11,12,13,14]. Further, if treatment with oral hypoglycaemic medications no longer reflect in the desired blood-glucose level, people with T2DM may need insulin injections.
This paper focuses on in vitro studies that have been performed on leguminous antidiabetic peptides, i.e., an α-amylase inhibitor, an α-glucosidase inhibitor, and a DPP-IV inhibitor, in an attempt to understand the different methods used and their interpretations.
The inhibition of salivary and pancreatic α-amylases will cause some fractions of the starch and polysaccharides in the food bolus to remain undigested when reaching the small intestine. This will reduce the number of substrates for α-glucosidase. Further, the inhibition of α-glucosidase will cause the digested amylose and amylopectin of starch to remain as maltose, maltotriose, branching oligosaccharides, and isomaltose. These intermediate starch digesta fractions are hence not absorbable by intestinal cells for transfer into the blood stream. The inhibition of α-amylase and α-glucosidase will result in a decreased serum glucose level, which is beneficial in controlling the postprandial blood-glucose level [10,12].
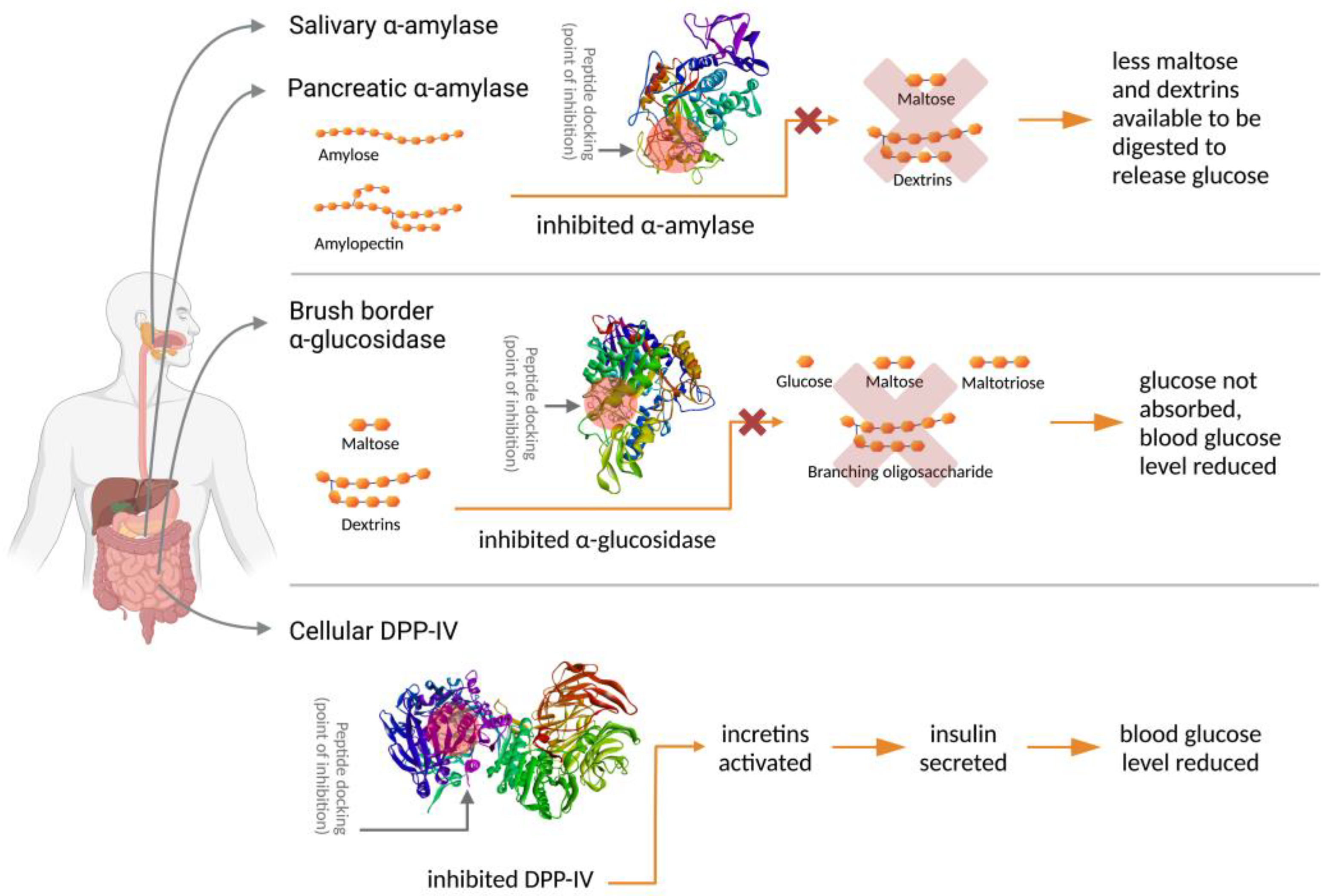
The inhibition of DPP-IV enzymes will prolong the biological activity of incretins. Incretins consist of two hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) [12,15]. These hormones are insulin secretagogues. When incretins are active, or when DPP-IV is inhibited, incretins enhance insulin secretion, which will keep the blood-glucose level under control. The inhibition mechanisms of α-amylase, α-glucosidase, and DPP-IV by peptides in controlling diabetes are presented in Figure 1.
Figure 1.
Inhibition mechanisms of α-amylase, α-glucosidase, and DPP-IV by peptides in controlling diabetes (Created with BioRender.com).
Long-term use of hypoglycaemic synthetic drugs, however, can cause side effects such as bloating, diarrhoea, abdominal pain, fluid retention, osteoporosis, hypoglycaemia, improper function of the liver, risk of kidney injury, and heart failure [16,17]. This causes a demand for alternative treatments, which are safer while having a similar effect. As explained below, natural dietary compounds can offer viable options.
Researchers worldwide have attempted to investigate the use of natural dietary compounds to reduce starch digestibility and glucose absorption by means of increasing resistant starch, either through processing or through its addition into products [18]: the inclusion of soluble fibre [19]; the inclusion of lipids [20,21]; the inclusion of phenolic compounds [22]; the inclusion of proteins [23,24]; and the inclusion of protein hydrolysates and bioactive peptides [25,26,27]. The mechanisms by which these compounds act include: a reduction in the amount of digestible starch (resistant starch); an increased viscosity of the lumen fluid to hinder the binding between the starch-digestion enzymes and their substrate (soluble fibre and protein); a layering at the starch granule surface to hinder the binding between starch digestion enzymes and their substrate (lipid and protein); the inhibition of α-amylase, α-glucosidase, and dipeptidyl peptidase-IV/DPP-IV activity, the delay of glucose absorption in the small intestine, and a delay in glucose uptake by cells (phenolics, protein hydrolysates, bioactive peptides, and amino acids) [18,19,20,21,22,24,25,26,27,28].
The main objective of this review is to provide a comprehensive review of recent in vitro studies that have been performed on leguminous antidiabetic peptides, focusing on the α-amylase inhibitor, α-glucosidase inhibitor, and dipeptidyl peptidase-IV (DPP-IV) inhibitor in an attempt to understand the methods used and their interpretations.
2. Starch to Blood Glucose
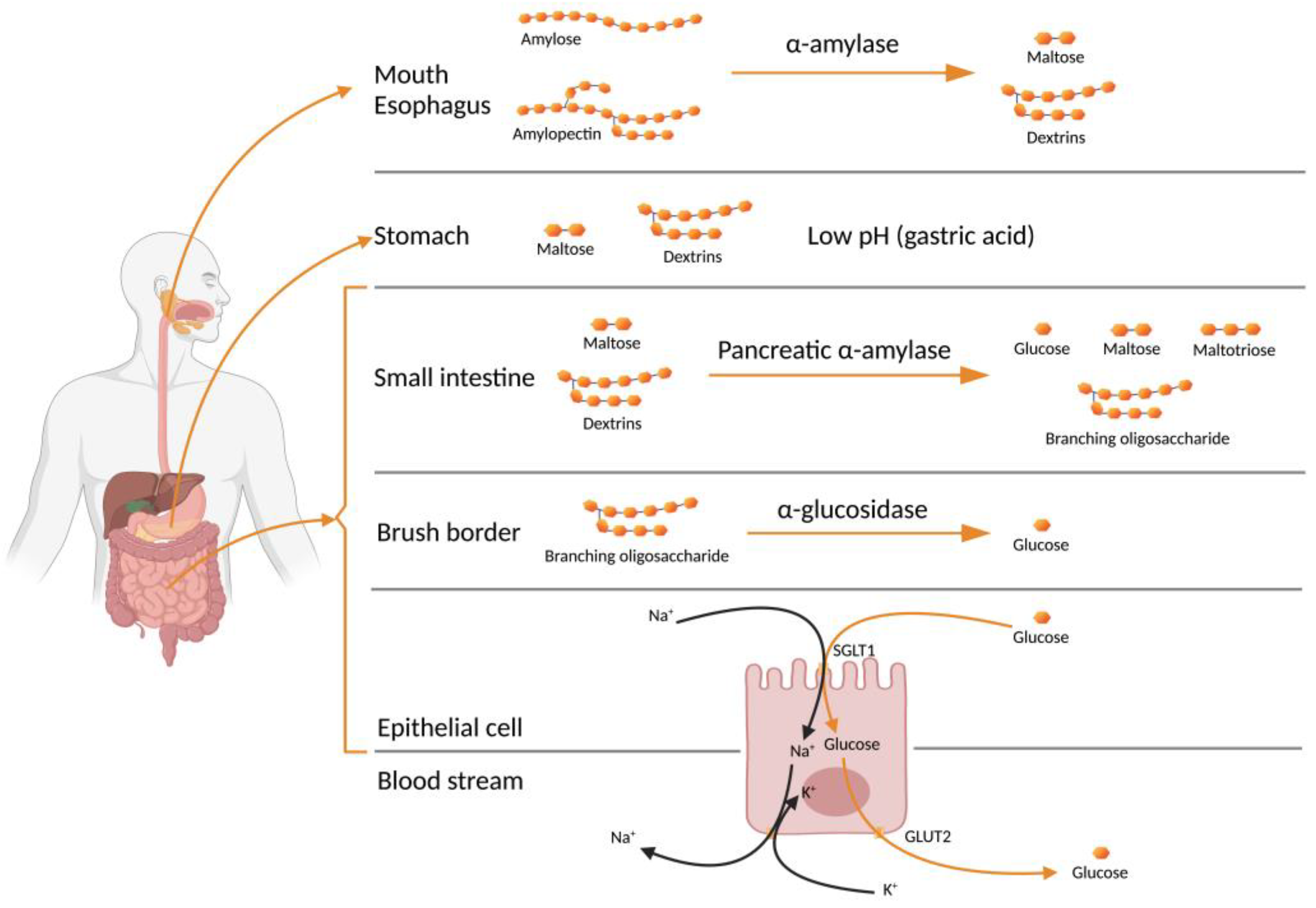
Starch (polysaccharide) digestion begins in the mouth, where α-1,4 glycosidic linkages are cleaved by salivary α-amylase. Figure 2 shows the starch digestion process, from the salivary α-amylase to its absorption into the blood stream as glucose. Digestion by α-amylase in the oral cavity will result in small amounts of monosaccharides as the food is rapidly swallowed and transferred to the stomach. The α-amylase activity continues after food is swallowed down into the stomach. It persists until the gastric acid penetrates the food bolus, and is then deactivated by the low-pH conditions. Dextrins and maltose predominate the digesta as it enters the small intestine. Pancreatic juice creates a neutral-pH buffering; this allows digestion to resume. The presence of α-amylase in the pancreatic juice hydrolyses the amylose chains into glucose, maltose and maltotriose [29]. The amylopectin chains are hydrolysed into glucose, maltose, branching oligosaccharides, and isomaltose. Both branching oligosaccharides and isomaltose have α-1,6 glycosidic linkages. Disaccharides such as α-glucosidase are further digested by enzymes at the brush borders (microvilli of mucosal cells); α-glucosidase is an important, membrane-bound enzyme in the epithelium of the small intestine that produces glucose [30]. The inhibition of α-amylase and α-glucosidase will result in a reduction in the number of glucose units available for absorption [9].
Figure 2.
Starch digestion (Created with BioRender.com).
Glucose is absorbed through the intestinal lining cells or enterocytes, across the epithelial cell and into the blood stream inside the villus. Glucose enters the epithelium through the apical membrane and leaves it through the basolateral membrane. A sodium-dependent hexose transporter (SGLT1) carries glucose into the enterocyte by first orienting towards the intestinal lumen to bind with sodium. This binding causes the transporter to change its conformation and opens the pocket for glucose to bind. When glucose binds, the transporter reorients and moves glucose and sodium into the cell. The transporter releases sodium and glucose into the cytoplasm and returns to its lumen-facing orientation to pick up more glucose and sodium. The sodium level in the cytoplasm is maintained in balance with potassium by a battery of sodium–potassium pumps on the basolateral membrane. These pumps shuttle out sodium in exchange for potassium. Glucose is transferred into capillary blood in the villus through a facilitated diffusion by another hexose transporter (GLUT2) on the basolateral membrane [31].
The glucose concentration in the blood after a meal is called postprandial glucose. The glycaemic index (GI) is measured by the blood-glucose concentration after a meal. GI is a ranking of the carbohydrates in foods on a scale of 1–100, based on the extent to which they increase blood sugar levels after consumption. The GI is used to classify different sources of carbohydrate-rich foods by their effect on postprandial glycemia [32].
The normal concentration of circulating blood glucose is approximately 3.9–5.4 mmol/L (70–90 mg/dL). It is predominantly controlled by the two pancreatic hormones: insulin and glucagon. These are peptide hormones that lower and raise the blood-glucose concentration, respectively. Insulin stimulates glucose uptake by cells when the blood-glucose level is too high, while glucagon stimulates the conversion of stored glycogen in the liver to glucose when the blood-glucose level is too low. Secretions of both insulin and glucagon are regulated by another hormone, called an incretin. Incretins are a group of metabolic hormones that stimulate the decrease in blood glucose by the secretion of insulin and inhibit the release of glucagon. Incretins can be deactivated by the presence of dipeptidyl peptidase IV (DPP-IV), which can lead to an increase in the blood-glucose level through the secretion of glucagon and the inhibition of insulin release [33]. A DPP-IV inhibitor, used to inhibit the enzymatic activity of DPP-IV, can be used to treat T2DM to maintain the activity of incretins so that a decrease in the blood-glucose level is stimulated.
3. Antidiabetic Activity of Leguminous Protein Hydrolysates and Peptides
Proteins are polymers of amino acids that are linked together by peptide bonds. There are hundreds of amino acids found in nature, but only 20 are found in the human body and other living organisms [34]. Through various combinations of sequence, amino acids are the building blocks of protein. Protein can be hydrolysed by hydrolytic enzymes in the gastrointestinal tract. Hydrolysis cleaves the peptide bonds in protein, and the long protein chains become shorter units called protein hydrolysates. Upon further hydrolysis, these protein hydrolysates are broken down into even smaller units called peptides and amino acids [35].
Short-chain amino acid sequences obtained from protein may have potential physiological functions in addition to their nutritional contribution [36]. The peptides, depending on the original protein–amino acid composition and sequence [37], may have beneficial physiological effects, such as being antihypertensive, acting as immunomodulators and antimicrobials, and having antidiabetic properties [38,39,40].
Specific protein fragments, i.e., peptides, that promote physiological functions and the conditions of human health are defined as bioactive peptides. In the sequence of a parent protein, bioactive peptides are inactive. They can be released through hydrolysis. Enzymatic hydrolysis is the main process for obtaining peptides from their parent proteins and allowing them to actively function biologically [37].
Bioactive peptides have been reported to show efficacy in modulating starch digestion and glucose absorption, such as the inhibition of α-amylase, α-glucosidase, and dipeptidyl peptidase IV (DPP-IV); the stimulation of insulin secretion; the decrease in glucose absorption in the gut; and the improvement of glucose uptake in the peripheral tissues [7,41,42,43,44]. One study found that peptides from the cowpea plant (Vigna unguiculata genotype Epace 10) have a similar amino acid sequence to that of bovine insulin [45]. The amino acid sequence of the bovine insulin α-chain is GIVEQCCASVCSLYQLENYCN, and protein isolated from Vigna unguiculata has the amino acid sequence GIVEQXXASVXSLYQLENYXN. The β-chain of the bovine insulin peptide sequence is FVNQHLCGSHLVEALYLVCGERGFFYTPKA, and protein isolated from Vigna unguiculata has the amino acid sequence FVNQHLXGSHLVEALYLVXGERGFFYTPKA. The X residue in the sequence is probably cysteine. Cysteine residues are not detected because the proteins are not reduced/alkylated [45]. Similarly, one review argued that cowpea peptides exhibited insulin-like properties [46]. They reached this conclusion based on the detection of these peptides in cowpea by Western blotting, and confirmed their role as immunoreactive insulin against anti-human insulin antibodies by an ELISA assay.
Dietary proteins, their protein hydrolysates, and their derived peptides from various food sources are vastly studied for their potential as therapeutic agents and have been proposed as alternative treatments for diabetes prevention and control. Comprehensive reviews on antidiabetic bioactive peptides from various food sources have been published [41,43,47,48,49,50,51]. In general, food-derived antidiabetic hydrolysates and peptides are isolated from two main sources, i.e., animal (dairy, egg, and marine) and plant (cereals and pseudocereals, rice, brewers’ spent grain, amaranth, quinoa, legumes, soybean, fruits, and leafy vegetables) [47].
Nearly 65% of the human protein supply in the world originates from plants [52], with legumes being one of the highest contributors to the protein supply, comprising 20–40% [36]. Legumes contain good-quality protein and are an economical dietary source of protein due to their high protein content, agronomical adaptability, economic value, and availability around the world. Therefore, in countries where animal protein sources are less accessible, legumes are widely consumed as the main source of dietary protein. Legume consumption as the main source of dietary protein indicates that legumes potentially have no side effects [53] and are also potentially safe when consumed as therapeutic agents, such as bioactive peptides. Cytotoxicity assays on cowpea protein hydrolysates and peptide fractions performed against Vero Cells showed very low cytotoxic effects when compared to the control, docetaxel (Taxotere®), even under maximum concentrations of 0.45–0.9 mg protein/mL [54]. Therefore, cowpea protein hydrolysates and peptide fractions are potentially safe. Moreover, peptides are reported to present low toxicity levels and low accumulation in body tissues [36].
Several reports claim that legume consumption is associated with a reduction in the risk of diabetes [13,55,56]. Studies have shown that this property is attributed to the presence of resistant starch, fibre, polyphenols, and bioactive peptides, which are released naturally in the stomach and intestine during digestion [57].
Until now, information on leguminous antidiabetic peptides, i.e., α-amylase, α-glucosidase, and DPP-IV inhibitors, have been limited. Leguminous peptide research is currently focused on other bioactivities, including antioxidant and antihypertension effects. On the other hand, the research on antidiabetic peptides has been using animal and other plant protein sources. This paper will provide a comprehensive review on in vitro studies that have been performed on leguminous antidiabetic peptides in an attempt to understand the different methods used and their interpretations.
Based on their antidiabetic activity analysis, leguminous antidiabetic peptide studies can be divided into four categories: in vitro, cell work, in vivo and ex vivo, and in silico. This paper will cover only the in vitro studies of leguminous antidiabetic peptides obtained through enzymatic hydrolysis. The in vitro studies are listed in Table 1.
4. In Vitro Studies on Leguminous Antidiabetic Peptides
Several common bean (Phaseolus vulgaris L.) cultivars and preparation techniques have been investigated for their antidiabetic peptides using in vitro assays. Soybeans (Glycine max), cowpeas (Vigna unguiculata), and bambara beans (Vigna subterranean) have also been studied. Common beans, soybeans, cowpeas, and bambara beans which have been reported for their α-amylase, α-glucosidase, and DPP-IV inhibitory peptides through in vitro assays are shown in Table 1.
Research on leguminous antidiabetic peptides using in vitro assays generally involves sample preparation, protein extraction/fractionation, protein hydrolysis, inhibitory assays, molecular-mass profiling, peptide sequence identification, potential bioactivity prediction of the peptide, computational modelling, and enzyme kinetics studies. Some studies include the synthesis of selected peptides to reconfirm their inhibitory activities. Similar to this general workflow, Nongonierma and Fitzgerald illustrated the same conventional approach in their research on biologically active peptides derived from food-protein [58].


Table 1.
In vitro studies on antidiabetic leguminous protein hydrolysates and peptides.
Table 1.
In vitro studies on antidiabetic leguminous protein hydrolysates and peptides.
| Legumes | Sample Type | Highest α-Amylase Inhibition * | Highest α-Glucosidase Inhibition * | Highest DPP-IV Inhibition * | References | |||
|---|---|---|---|---|---|---|---|---|
| Value | Sample | Value | Sample | Value | Sample | |||
| Common Bean (Phaseolus vulgaris L.): Black Pinto Red Navy Great Northern | Raw and precooked | 36% inh AC/mg protein [Prot] = not reported [Enz] = 13 U/mL [Ac] = 1 mM | Red beans Raw, whole H: Pepsin-Pancreatin | >40% (~48–67%) inh AC /mg protein (not statistically different) [Prot] = not reported [Enz] = 1 U/mL [Ac] = 1 mM | All beans Raw and precooked, whole H: Pepsin-Pancreatin | 0.093 mg protein/mL [Prot] = 1 mg DW/mL [Enz] = 100 ng/mL [control] = not reported | Navy beans Precooked, whole H: Pepsin-Pancreatin | [59] |
| 0.095 mg protein/mL [Prot] = 1 mg DW/mL [Enz] = 100 ng/mL [control] = not reported | Navy beans Raw, whole H: Pepsin-Pancreatin | |||||||
| Common Bean (Phaseolus vulgaris L.): Mexico, Pinto: Pinto-Bayacora Pinto-Bravo Pinto-Centenario Pinto-Saltillo Mexico, Flores de Mayo and Junio: FMayo-Eugenia FMayo-67 FMayo-199 FMayo-202 FJunio-Leon FJunio-Marcela Mexico, Negros: Negro-Frijozac Negro-Otomi Brazil, Carioca: BRSHorizote BRS-Pontal Perola | Raw | 14.9 ± 1.7% inh AC/mg BPI [Prot] = not reported [Enz] = 13 U/mL [Ac] = 1 mM | Pinto-Bayacora Raw, dehulled H: Pepsin-Pancreatin | [38] | ||||
| 14.9 ± 0.4% inh rel AC/mg BPI [Prot] = not reported [Enz] = 13 U/mL [Ac] = 1 mM | FMayo-67 Raw, dehulled H: Pepsin-Pancreatin | |||||||
| Common Bean (Phaseolus vulgaris L.): Black Otomi BRS-Horizonte BRS-Pontal Perola | Raw | 50.10% inh/mg DW [Prot] = 1 mg DW/mL [Enz] = 1 U/mL [Ac] = 1 mmol/L | BRS-Horizonte Raw, dehulled H: Pepsin-Pancreatin | 0.14 mg DW/mL [Prot] = 1 mg DW/mL [Enz] = 10 ng/mL [control] = not reported | Black Otomi Raw, dehulled H: Pepsin-Pancreatin | [60] | ||
| 49.34% inh/mg DW [Prot] = 1 mg DW/mL [Enz] = 1 U/mL [Ac] = 1 mmol/L | Synthesized peptide: KKSSG | 0.03 mg DW/mL [Prot] = 1 mg DW/mL [Enz] = 10 ng/mL [control] = not reported | Synthesized peptide: KTYGL | |||||
| Black Otomi (Phaseolus vulgaris L.) | Raw | 64.5 ± 2.7% inh/ mg dry matter [Prot] = 1 mg DM/mL [Enz] = 13 U/mL [Ac] = 1 mM | Raw, dehulled H: Flavourzyme, 2 h, 1:20 (E/S) | 75.3 ± 0.7% to 78.4 ± 0.6% inh/mg dry matter (not statistically different) [Prot] = 1 mg DM/mL [Enz] = 1 U/mL [Ac] = 1 mmol/L | Raw, dehulled H: Papain, 2, 3, 4 h, 1:20, 1:30, 1:50 (E/S) | 96.7% inh/ mg dry matter [Prot] = 1 mg DW/mL [Enz] = 10 ng/mL [control] = not reported | Raw, dehulled H: Alcalase, 2 h, 1:20 (E/S) | [61] |
| HTC Common Bean (Phaseolus vulgaris L.): cv Negro 8025 cv. Pinto Durango | Raw | 49.9 ± 1.4% [Prot] = 100 μg/mL [Enz] = 10.8 U/mL [Ac] = 1 mM | Pinto Durango Raw, dehulled H: Bromelain, 2 h F: <1 kDa | 76.4 ± 0.5% [Prot] = not reported [Enz] = 1.0 U/mL [Ac] = 1 mM | Pinto Durango Raw, dehulled H: Alcalase, 2 h F: <1 kDa | 55.3 ± 1.6% [Prot] = 100 μg/mL [Enz] = 100 ng/mL [control] = not reported | Pinto Durango Raw, dehulled H: Alcalase, 2 h F: <1 kDa | [25] |
| Common Bean (Phaseolus vulgaris) | Germinated | 30.88 ± 2.45% AC/mg SP [Prot] = 1 mg/mL [Enz] = 13 U/mL [Ac] = 1 mM | Dehulled, germinated 24 h H: Non-hydrolyzed | 1.2 mg soluble protein/mL [Prot] = 0.1–4.0 mg/mL [Enz] = 100 ng/mL [control] = not reported | Dehulled, non-germinated H: Non-hydrolyzed | [55] | ||
| Pinto Bean (Phaseolus vulgaris cv. Pinto) | Raw | 57.48 ± 2.51% [Prot] = not reported [Enz] = 0.5 mg/mL [Ac] = not used | Raw, whole H: Protamex, pH 6.5, 1 h, 1:10 (E/S) | [42] | ||||
| 62.1 ± 3.49% [Prot] = not reported [Enz] = 0.5 mg/mL [Ac] = not used | Raw, whole H: Protamex, pH 6.5, 1 h, 1:10 (E/S) F: <3 kDa | |||||||
| Pinto Bean (Phaseolus vulgaris cv. Pinto) | Raw | 57.8% inh/100 μg 10.03 ± 0.47 mM [Prot] = 1 mg/mL [Enz] = 0.5 mg/mL [Ac] = not used | Raw, whole H: Protamex, pH 6.5, 1 h, 1:10 (E/S) F: <3 kDa Synthesized pinto bean peptide fraction 5 (PBp5) | [62] | ||||
| Bean (Phaseolus vulgaris L.) | Fermented with L. plantarum 299v | 0.038 µg/mL [Prot] = not reported [Enz] = not reported [Ac] = not used | Dehulled Fermented 22 °C, 3 h H: Amylase-Pepsin-Pancreatin F: Fraction III collected in Sephadex G-10 from 3.5–7 kDa | [63] | ||||
| BRS-Pontal (Phaseolus vulgaris L.) | Raw | 89.1 ± 0.3% [Prot] = 10 mg/mL [Enz] = 10 U/mL [Ac] = 10 mg/mL | Hard-to-cook bean, raw, dehulled H: Non-hydrolyzed F: <3 kDa | 89.2 ± 0.1% [Prot] = 10 mg protein/mL [Enz] = 2 U/mL [Ac] = 10 mg/mL | Easy-to-cook bean, raw, dehulled Non-hydrolyzed F: <3 kDa | [7] | ||
| Carioca Bean (Phaseolus vulgaris L. cv Carioca) | Raw | 101.61 ± 0.78% [Prot] = 1 mg/mL [Enz] = not reported [Ac] = not used | Raw, whole H: Alcalase-Neutrase (1/2:1/2) | 34.73 ± 4.65% [Prot] = 1 mg/mL [Enz] = 0.1 U/mL [Ac] = not used | Raw, whole H: Flavourzyme: Alcalase (1/2:1/2) | [64] | ||
| Cowpea cultivar BRS Novaera (Vigna unguiculata L.) | Germinated | 0.58 mg soluble protein/mL [Prot] = 0.1–4.0 mg/mL [Enz] = 100 ng/mL [control] = not reported | Dehulled, Non-germinated H: Alcalase, 1 h-Pepsin-Pancreatin | [57] | ||||
| Black Cowpea (Vigna unguiculata) | Raw | 96.81% [Prot] = 100 mg/mL [Enz] = 13 U/mL [Ac] = not reported | Raw, whole H: Pepsin-Pancreatin F: <1 kDa | 97.34% [Prot] = 10 mg/mL [Enz] = 2 U/mL [Ac] = not reported | Raw, whole H: Alcalase-Flavourzyme F: >10 kDa | 85% 2.06 mg protein/mL [Prot] = not reported [Enz] = not reported [Stg] = not reported | Raw, whole H: Alcalase-Flavourzyme F: Protein Hydrolysate | [54] |
| Bambara bean (Vigna subterranea) | Raw | 44.253 ± 1.327% [Prot] = 1 mg/mL [Enz] = 0.26 mU/test well [DipA] = not reported | Bambara bean protein isolate H: Alcalase | [13] | ||||
| 29.276 ± 0.878% at 1 mg/mL [Prot] = 1 mg/mL [Enz] = 0.26 mU/test well [DipA] = not reported | Bambara bean protein isolate H: Trypsin-Pepsin-α-chymotrypsin-trypsin-pancreatin | |||||||
| 1.733 mg/mL [Prot] = 1 mg/mL [Enz] = 0.26 mU/test well [DipA] = not reported | Bambara bean protein isolate H: Alcalase H: Thermolysin | |||||||
| Soybean (Glycine max) | Germinated | 1.7 mg/mL [Prot] = 0.2–4 mg/mL [Enz] = 2 U/mL [Ac] = 0.1–1.3 mg/mL | Germinated 6 days, whole H: Pepsin-Pancreatin | As maltase 2.56 mg/mL [Prot] = 1–10 mg/mL [Enz] = 1 U/mL [Ac] = not used | Germinated 6 days, whole H: Pepsin-Pancreatin F: <5 kDa | 0.91 mg/mL [Prot] = 0.08–5 mg/mL [Enz] = 0.26 mU/test well [DipA] = 0.78–50 μM | Germinated 6 days, whole H: Pepsin-Pancreatin F: 5–10 kDa | [16] |
| As sucrase 1.23 mg/mL [Prot] = 1–10 mg/mL [Enz] = 1 U/mL [Ac] = not used | Germinated 6 days, H: Pepsin-Pancreatin F: <5 kDa | |||||||
| ~85% [Prot] = 1 mg/mL [Enz] = 2 U/mL [Ac] = 0.1–1.3 mg/mL | Germinated 6 days, whole H: Pepsin-Pancreatin F1 fraction collected by semi-preparative RP-HPLC from 5–10 kDa | As maltase 32% [Prot] = 1 mg/mL [Enz] = 1 U/mL [Ac] = not used | Germinated 6 days, whole H: Pepsin-Pancreatin F: F4 fraction collected in semi-preparative RP-HPLC from 5–10 kDa | 0.7 mg/mL [Prot] = 0.08–5 mg/mL [Enz] = 0.26 mU/test well [DipA] = 0.78–50 μM | Germinated 6 days, whole H: Pepsin-Pancreatin F: F3 fraction collected by semi-preparative RP-HPLC from 5–10 kDa | |||
| As sucrase 22% [Prot] = 1 mg/mL [Enz] = 1 U/mL [Ac] = not used | Germinated 6 days, whole H: Pepsin-Pancreatin F: F1 fraction collected by semi-preparative RP-HPLC from 5–10 kDa | |||||||
| Soybean | Raw (Isolated soybean protein) | 0.27 mg/mL [Prot] = not reported [Enz] = 0.15 U/mL [Ac] = not used | Protein isolate H: Trypsin F: <5 kDa | [65] | ||||
| 0.049 mg/mL [Prot] = not reported [Enz] = 0.15 U/mL [Ac] = not used | Protein isolate H: Trypsin F: <5 kDa Fraction C-III-2a from RP-HPLC | |||||||
| Soy | Raw (Soy protein powder) | 77.64 ± 1.07% [Prot] = not reported [Enz] = 0.2 U/mL [Ac] = 10 mg/mL | Soy protein isolate H: Alkaline protease-pepsin-pancreatin | 47.94 ± 1.10% [Prot] = not reported [Enz] = 0.02 U/mL [control] = buffer | Soy protein isolate H: Alkaline protease-pepsin-pancreatin | [12] | ||
| 87.10 ± 2.70% [Prot] = not reported [Enz] = 0.2 U/mL [Ac] = 10 mg/mL | Soy protein isolate H: Alkaline protease-pepsin-pancreatin F: H1 fraction from DEAE-52 | |||||||
| 95.35 ± 2.70% [Prot] = not reported [Enz] = 0.2 U/mL [Ac] = 10 mg/mL | Soy protein isolate H: Alkaline protease-pepsin-pancreatin F: H1 fraction from DEAE-52, then H1-2 fraction from Sephadex G-15 | |||||||
| 162.29 ± 0.74 μmol/L [Prot] = not reported [Enz] = 0.2 U/mL [Ac] = 10 mg/mL | Synthesized peptide: WLRL | |||||||
| Yellow field pea (Pisum sativum L.) | Raw (Yellow field pea protein concentrate) | 30.52 ± 0.01% [Prot] = 225 μg/mL [Enz] = 28.57 μg/mL [Ac] = 1.5–3 μg/mL | Yellow field pea protein concentrate H: Chymotrypsin F: 1–3 kDa | 53.35 ± 2.78% [Prot] = 20 mg/mL [Enz] = 8.33 mg/mL [Ac] = 0.00625–0.125 mg/mL | Yellow field pea protein concentrate H: Chymotrypsin F: <1 kDa | [9] | ||
* Units in % indicate the percent inhibition; units in inh AC—relative to acarbose; mg (protein; DW; hydrolysate; soluble protein)/mL, µg/mL, or μmol/L indicate the IC50; BPI—bean protein isolate; SP—soluble protein; Prot—protein, peptide, hydrolysate, inhibitor; Enz—α-amylase, α-glucosidase, DPP-IV; Ac—acarbose; Stg—sitagliptin; DipA—diprotin A; H—hydrolysate; F—hydrolysate fraction.
Several studies have demonstrated factors that affect the release of peptides. These factors have been controlled in studies to show their effects, such as cultivars, sample preparation, hydrolysis optimisation, peptide fractionation, and semi-preparative peptides. Common beans from various cultivars release different peptide sequences that exhibit antidiabetic activities. Sample preparations such as fermentation (t, T), germination (t), and pre-cooking also affect the release of peptides and their antidiabetic activities. By designing experiments on enzyme types and combinations, enzyme substrate ratios (ESR), pH, and time, hydrolysis optimisations using the response surface methodology (RSM) have shown that the length and bioactivity of peptides released are affected. Peptide fractionations by means of ultrafiltration membranes from 1 to 100 kDa, ion-exchange chromatography, gel filtration, or reversed, phase-high performance liquid chromatography (RP-HPLC) were used in the studies. Semi-preparative peptides were obtained using solid-phase methods and standard 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry. The methods used in the studies reflect the influence of these factors. They are discussed in more detail in the following sections.
Sample preparation generally begins with pod separation, sanitisation, rinsing, soaking, germination, fermentation, milling, oven drying, lyophilisation, grinding, and sieving. Protein extraction is performed to isolate protein fractions and ensure no other inhibitory compounds, such as resistant starch, fibre, and phenolic compounds, are present in the samples. Alkaline extraction and the isoelectric precipitation method are commonly used for this purpose. Protein hydrolysis is the focus of several research studies involving optimisation by experimenting on the enzyme types, enzyme mix composition, ESR, pH, and time. Inhibitory assays target in vitro α-amylase, α-glucosidase, and DPP-IV. Samples that show high inhibitory activity is usually investigated further for its peptide’s molecular-mass profile, sequence, potential bioactivity, molecular docking, and enzyme kinetics. The most promising peptide is synthesized using 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry.
Information from molecular-mass profiling will assist the succeeding step: peptide sequence identification. Molecular-mass profiling is mostly performed using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF/TOF MS). Peptide sequence identification makes use of high-performance liquid chromatography/mass spectrometry (HPLC/MS). Online peptide database resources such as BLAST® Tool, PepDraw Tool, and the BIOPEP database are commonly used to confirm the peptide sequence, to predict the peptide structure and physicochemical properties, and to predict the peptide bioactivity, respectively.
Lastly, when used together, computational modelling and an enzyme kinetics study can predict the inhibition mechanism. Information about peptide molecular docking onto the enzymes, such as the types and locations of chemical interactions and the type of inhibition (such as competitive or non-competitive inhibitions), can be investigated using molecular docking software and Lineweaver–Burk plots.
Research that focused on investigating peptides for production as an alternative to the currently available antidiabetic drugs, such as the studies by Jiang et al. in 2018 [65] and Ngoh and Gan in the same year [62], took further steps to synthesise the most potent peptides. The synthesised peptides wee then subjected again to enzyme inhibitory assays for the reconfirmation of activity. Peptide synthesis is usually performed by biotechnology companies using Fmoc chemistry. Fmoc is a chemical synthesis method that produces peptides using an automated solid phase. In this method, a base-sensitive Fmoc (9-fluorenylmethyloxycarbonyl) group or an acid-sensitive Boc (tert-butoxycarbonyl) group are typically used as the α-amino-protecting group. Both the α-amino group and the side-chain protection are immobilized to a resin [66]. Other than chemical synthesis, recombinant DNA (rDNA) technology has been used to express proteins for structural studies [67], but there is no report indicating the use of this method in leguminous antidiabetic peptides research.
Inhibitory activities of leguminous antidiabetic peptides were reported in percent inhibition, or IC50. Acarbose was used by most studies as a positive control in α-amylase and α-glucosidase inhibitory assays. In DPP-IV inhibitory assays, diprotin A and sitagliptin were used as positive controls. The concentration of positive controls varied between studies. Acarbose concentration ranged between 1.5 μg/mL and 10 mg/mL in the α-amylase inhibitory assays and between 6.25 μg/mL and 10 mg/mL in the α-glucosidase inhibitory assays. Diprotin A was used between 0.78 and 50 μM. The majority of the studies used a DPP-IV Inhibitor Kit assay, which provides a pro-luminescent DPP-IV substrate (Gly-Pro-aminoluciferin or H-Gly-Pro-AMC) that is cleaved by DPP-IV to yield a fluorescent product (aminoluciferin or 7-amino-4-methylcoumarin). The fluorescent intensity was measured at 460 nm and was proportional to the DPP-IV activity. Protein or peptide samples containing the inhibitory peptides were prepared in various concentrations, ranging between 0.05 and 50 mg/mL in the α-amylase inhibitory assays, 1 and 200 mg/mL in the α-glucosidase inhibitory assays, and 0.08 and 5 mg/mL in the DPP-IV inhibitory assays [7,9,12,13,16,25,38,42,54,55,57,59,60,61,62,63,64,65]. Discussions on positive control concentrations and their interpretations are provided in their respective sections, i.e., α-amylase inhibitory assays, α-glucosidase inhibitory assays, and DPP-IV inhibitory assays.
The highest activity of α-amylase inhibition ranged between 14.9 and 89.1% relative to acarbose [7,9,16,25,38,55,59,61] and between 57.48 and 101.61% among assays that did not use acarbose [42,54,62,63,64]. IC50 ranged between 0.038 μg/mL and 1.7 mg protein/mL, and one study reported 10.03 mM. The highest activity of α-glucosidase inhibition ranged between 22 and 97.34% inhibition relative to acarbose [7,9,12,16,25,53,54,59,61], and was 34.73% in a non-acarbose assay [64]. IC50 ranged between 0.27 and 162.29 mg protein/mL. The highest activity of DPP-IV inhibition ranged between 47.94 and 96.7% inhibition and an IC50 between 0.03 and 1.2 mg/mL [12,13,16,25,53,54,55,57,59,61]. Discussions on factors affecting inhibitory activities are provided in their respective sections, i.e., α-amylase inhibitory assays, α-glucosidase inhibitory assays, and DPP-IV inhibitory assays.
5. Protein Extraction
Protein extraction is commonly performed using alkaline extraction and isoelectric precipitation methods. Other methods used include water extraction and phosphate buffer extraction. Protein extraction is a crucial step in isolating the protein fraction of the bean and minimising the presence of other potential inhibitors, such as resistant starch, fibres, and phenolic compounds. Some studies skip this step by using a commercially available protein isolate.
In general, prior to protein extraction, samples are prepared by separating the bean from the pod, sanitisation, rinsing, soaking, dehulling, germination, fermentation, blanching, precooking, milling, oven drying, lyophilisation, grinding, and sieving. Details are provided on Table 2. Milling, drying, and sieving are the basic steps in sample preparation. The use of freeze drying is very common. When a conventional oven is used, the drying temperature varies between 40 and 60 °C. The particle size of ready-to-extract samples varies between 20 and 60 mesh.

Table 2.
Protein extraction methods.
Dehulling is a crucial step in all studies. As bean proteins are mainly located in the cotyledons [74] and bean hulls contain fibres and phenolic compounds, the inclusion of hulls in the samples may increase the risk of the co-extraction of fibres and a phenolic load in the final protein isolate. Fibres and phenolic compounds have been proven to show potential antidiabetic properties by many studies. One study argued a higher inhibition potential due to synergistic inhibitory activity between the protein and phenolic compounds during digest because hulls were not removed from their sample [53]. The protein extraction method should be selected based on its specificity to extract the protein fractions. A study on soybeans using alkaline extraction without isoelectric precipitation and directly followed by protein hydrolysis resulted in a digesta containing total identified phenolics of 466.15 ± 21.20 μg/g [75]. In addition to than dehulling and careful method selection, it is also suggested to add an additional alcohol extraction step to remove any co-extracted phenolic compounds. Soy protein isolate can retain notable amounts of isoflavones associated with proteins [16,73]. Total phenolic-content determination can be performed to ensure the removal.
Where alkaline extraction and isoelectric precipitation are used, the ratios between the bean flour sample and water ranged between 1:5 and 1:20. The more water was used, the more protein could be extracted from the legumes. Most studies used a combination of pH 8.0 and 4.3 [7,25,38,53,55,57,59,61]. The pH selection is related to protein solubility (at basic pH) and isoelectric point (at acidic pH). Extraction was carried out at room temperature or 35 °C for 1 h with stirring. The centrifugation speed ranged between 4000× g and 10,000× g at 4, 20, and 25 °C. The effects of extraction temperature and centrifugation speed and temperature have not been discussed in studies in the field.
The protein extraction yield usually reflects the bean protein content; typically around 20%. Studies in the field did not report the protein yield data, except for Ngoh and Gan [42], who reported that protein extraction using a phosphate buffer generally yielded between 33.8 and 57.9 mg protein/g or around 3.38–5.79% from pinto flour [42]. The protein extraction yield from a cooked bean is significantly lower when performed at a pH < 10 [76]. A study on common beans, which included precooking, extracted the protein at a pH of 8 [59] and did not report the protein yield; however, theoretically, the yield should have been low.
The protein content of the isolates were reported between 68.11 and 90.51%, as follows: 68.11–80.17% [55], 71.7–78.7% [57], 78.6–79.7% [25], 86.62% [12], 90.26% [13], and 90.51% [64]. The protein-content determination was performed using the Kjeldahl method [12], the Lowry method [54,63,64], the Protein DC Microplate Assay of Bio-Rad [55,57,59], or the Qubit® Protein Assay Kit [7]. The protein content in isolates is an important indicator of purity and will assist in determining the enzyme–substrate ratio for hydrolysis.
6. Protein Hydrolysis
Enzymatic hydrolysis is used in research on leguminous antidiabetic peptides to obtain protein hydrolysates and peptide fractions. Some studies focus on optimising this step by experimenting on enzyme types, enzyme mix composition, ESR (enzyme–substrate ratio), pH, and time. It can be highlighted that the hydrolysis method by Megías et al. [77] has been used in many studies. Detailed parameters used in protein hydrolysis are provided in Table 3.

Table 3.
Protein hydrolysis methods.
Enzymes used were Alcalase, flavourzyme, protamex, trypsin, neutrase, α-chymotrypsin, bromelain, papain, thermolysin, protease K, alkaline protease, and α-amylase. The use of these enzymes was mostly followed by pepsin and pancreatin. The latter step was taken to simulate human gastro-intestinal digestion; this was a step taken in almost all the studies in the field [7,12,13,16,25,38,53,54,55,57,59,61,63]. Although it is not a protease, α-amylase was used in a study [63] to release sugar-bound proteins, which increases the protein availability to pepsin and pancreatin. Depending on the type of enzymes used in the enzymatic hydrolysis, different chain lengths of peptide sequences can be released, leading to a broad range of peptide bioactivities [51].
The protein hydrolysis yield was 44.03%, 48.18%, 49.56%, and 58.21% using trypsin, chymotrypsin, pepsin, and Alcalase, respectively [9]. The protein hydrolysis yield represents the percent ratio of total protein present in the final hydrolysate to the amount of total protein initially used in the hydrolysis. The protein content of the hydrolysates varied between studies, depending on the method of analysis. For example,0.43–0.78 mg/mL [54], 54.69–87.24% soluble protein [55], 63.5–73.9% of soluble protein [57], and 71.54–87.17% of freeze-dried hydrolysate [9]. The methods used in protein-content determination were the Kjeldahl and Lowry methods or the Protein DC Microplate Assay of Bio-Rad. The degree of hydrolysis was reported with a broad range: between 5.92 and 98.38%. Authors reported that the broad range was contributed to by variations in seed cultivar [9], sample preparation [63], protein extraction methods [9], nature of protein substrates [13], and the enzyme types and specificities [12,13]. The protein hydrolysis yield and the hydrolysate protein content, along with the degree of hydrolysis, can be used as practical references in designing the protein extraction and protein hydrolysis methods.
After hydrolysis, the protein hydrolysates were subjected to clarification, fractionation, separation, purification, and synthesis. Clarification was intended for salt removal using 0.5 kDa, 0.8 kDa, 3 kDa, or 0.45 μm of membrane. Fractionation was used to separate peptide fractions using an ultrafiltration membrane of 1, 3, 3.5, 5, 7, 10, 30, 50, or 100 kDa. Separation and purification were performed to isolate selected peptides that potentially have a high inhibitory activity. The peptides were isolated using ion-exchange chromatography, gel filtration, or RP-HPLC. Some of the studies synthesised the peptides that had the highest inhibitory activity [53,62]. Fmoc chemistry was reported as the method used in peptide synthesis [12,65].
7. α-Amylase Inhibitory Assays
The inhibitory activity of α-amylase ranged from being undetected to an 89.1% inhibition relative to acarbose, and between 0.17 and 101.61% inhibition among assays that did not use acarbose. IC50 ranged between 0.038 μg protein/mL and >10.00 mg protein/mL. The details of the α-amylase inhibitory assays used in the research on leguminous antidiabetic peptides are provided in Table 4.

Table 4.
α-Amylase Inhibitory Assay.
Different enzyme types, enzyme concentrations, sample concentrations, and acarbose concentrations were used. Enzymes were mostly α-amylase from porcine pancreas type VI-B. Some studies used α-amylase from hog pancreas, human saliva, Bacillus licheniformis, and Bacillus subtilis. Different enzyme types or sources may provide different inhibitory assay results [95], as the inhibitory activity of chickpea legumes in an α-amylase inhibitor was compared against α-amylase enzymes from human saliva (7.0 × 10−3 units), Bacillus subtilis (120.9 × 10−3 units), porcine pancreas (89.1 × 10−3 units), maize (5.0 × 10−3 units), and Aspergillus oryzae (148.4 × 10−3 units). The highest inhibitory activity resulted from α-amylase from a porcine pancreas, which was as high as 80.3% [95]. Different concentrations were prepared for each enzyme because enzyme concentration determines the inhibitory activity of the inhibitor. To achieve an inhibitory activity that fell within 0–100% inhibition, the enzyme concentration was adjusted to a suitable range. This is the case in many leguminous antidiabetic peptide studies. The enzyme concentration used varied, including 2 U/mL [16], 10 U/mL [7], 10.8 U/mL [25], 28.57 μg/mL [9], and 0.5 mg/mL [42,62]. An enzyme concentration of 13 U/mL was used in many studies [38,54,55,59,61]. The higher the enzyme concentration used, the higher the inhibitory potential of the sample or the tested inhibitor.
Similar to enzyme concentration, the concentration of a sample or tested inhibitor also determines the inhibitory activity. To achieve inhibitory activity within the 0–100% range, sample concentrations were adjusted. Different sample concentrations were used for different hydrolysates; for example 50 mg protein/mL and 100 mg protein/mL were used for an Alcalase–Flavourzyme–pepsin–pancreatin hydrolysate and a pepsin–pancreatin hydrolysate, respectively [54]. Sample or inhibitor concentrations used among the studies were between 0.05 and 100 mg protein/mL, and a concentration of 1 mg protein/mL was used in many studies [16,55,61,62,64].
Acarbose was used as a positive control at varying concentrations: 1.5–3 μg/mL [9], 0.1–10 mg/mL [7,16], and 1 mM [25,38,55,59,61]. The latter concentration was the most used. The higher the acarbose concentration used, the higher the inhibitory potential of the sample. All studies used starch solution as a substrate at a concentration of 1%.
A synergistic or additive inhibitory effect among peptides with varying sizes was reported [16]. However, it is evident that, across all studies, shorter peptides are more potent inhibitors. Smaller and narrower peptides have more exposure of terminal groups [42] and can easily bind to the catalytic site [9] to inhibit α-amylase activity. Inhibitory peptides from the food matrix have been reported to have two to three amino acids [63,96,97]. These shorter peptides can be produced from protein hydrolysis with diverse enzyme systems [54] and ultrafiltration [65]. Additionally, acidic hydrolysis conditions are likely to produce peptide inhibitors [42].
A lower inhibitory activity is observed in precooked common beans when they are compared to raw samples [59]. Precooking may have denatured naturally occurring α-amylase inhibitors, which are a part of a common bean-defence response mechanism against insects [28,59]. This is in accordance with the findings on naturally occurring <3 kDa peptides in common beans, which have the highest α-amylase inhibitory effect [7].
8. α-Glucosidase Inhibitory Assays
α-Glucosidase inhibitory activity ranged between 3.6 and 97.34% inhibition relative to acarbose and between 19.23 and 34.73% inhibition among assays that did not use acarbose. IC50 ranged between 0.27 mg protein/mL and >10.00 mg protein/mL. The details of α-glucosidase inhibitory assays used in the research on leguminous antidiabetic peptides are provided in Table 5.

Table 5.
α-Glucosidase Inhibitory Assay.
Different enzyme types, enzyme concentrations, sample concentrations, acarbose concentrations, and substrate concentrations were used. In almost all studies, α-glucosidase from Saccharomyces cerevisiae was used [12,25,53,54,59,61,64], while the rest originated from rat intestines [9,16]. One study used maltase and sucrase [16]. An enzyme concentration of 1 U/mL was used by most studies. Other studies used 0.1, 0.15, 0.2, and 2 U/mL and 8.33 mg/mL. Sample or inhibitor concentrations were between 1 and 20 mg dry matter/mL and 10 and 200 mg protein/mL. A variation of concentrations used in the studies were adjusted to achieve a comparable inhibitory activity within the 100% range. The higher the enzyme concentration and the lower the sample concentration used, the higher the inhibitory potential of the sample.
Acarbose as a positive control was used at varying concentrations. Many studies set acarbose concentration at 1 mM [25,53,59,61], while others set it at 10 mg/mL [12] and between 0.00625 and 0.125 mg/mL [9]. p-nitrophenyl-α-D-glucopyranoside (PNPG) was used across all studies as substrate, except for one study [16], which used maltose and sucrose. PNPG concentration varied; it was mostly 5 mM [9,25,53,59,64], though other studies used 1 mM [54], 50 mM [65], 100 mM [7], and 1 mg/mL [12]. The higher the acarbose concentration and substrate concentration used, the higher the inhibitory potential of the sample.
The highest α-glucosidase inhibitory activities were observed in samples of peptide fractions with a low molecular weight. Large-molecular-weight peptides are sterically hindered from binding to the enzyme, and thus have a weaker inhibitory activity [65]. However, in the work by Castañeda-Perez et al., the highest inhibitory activity (97.34%) was observed in >10 kDa samples [54]. They argued that the hydrophobic amino acids, which were predominantly present in the fraction, are likely to participate in the inhibition.
Hard-to-cook bean peptide fractions demonstrated a lower inhibitory activity when compared to easy-to-cook beans [7]. Interactions between proteins and other molecules in the seed during postharvest hardening [102] may have effect on the steric hindrance of peptides to interact with the active sites of α-glucosidase during inhibition [7].
9. Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Assays
DPP-IV inhibitory activity ranged between 5 and 96.7% inhibition. IC50 ranged between 0.03 and 3 mg protein/mL. DPP-IV inhibitor screening kits were used by all the studies [13,16,25,53,54,55,57,59,61] for leguminous antidiabetic peptides. Details of the DPP-IV inhibitory assays used in the studies are provided in Table 6.

Table 6.
DPP-IV Inhibitory Assay.
Different enzyme types, enzyme concentrations, sample concentrations, positive control types, and substrate concentrations were used. Purified or recombinant human DPP-IV enzymes or porcine kidney enzymes were used. Enzyme concentrations were 10 ng/mL [53,61], 100 ng/mL [25,55,57,59], 0.26 mU/test well [13,16], and 0.02 U/mL [12]. Sample or inhibitor concentrations were between 0.08 and 5 mg/mL. Diprotin A and sitagliptin were used as positive controls. H-Gly-Pro-p-nitroaniline, Gly-Pro-4-nitroanilide, and Gly-Pro-p-nitroanilide were used at concentrations of 100 μM, 500 μM, and 12 mM, respectively. Most studies reported to have followed the manufacturer’s instructions for the use of the DPP-IV inhibitor kit during the assay.
In contrast to the highest inhibitory activities of α-amylase and α-glucosidase, which were observed in peptide fractions with a lower molecular weight, the highest DPP-IV inhibitory activities were observed in unfractionated hydrolysates. Longer peptides could bind to the secondary binding site of the DPP-IV enzyme [54]. An exception was found only in the study on hard-to-cook common beans of <1 kDa peptide fractions, which showed the highest activity [25].
Lower inhibitory activities were observed in samples hydrolysed with Alcalase at longer hydrolysis times, or with Alcalase and thermolysin hydrolysis when followed by simulated gastro-intestinal digestion (using pepsin and pancreatin) [13,55,57]. Alcalase and the simulated gastro-intestinal digestion that followed Alcalase and thermolysin hydrolysis may have extensively degraded the bean protein to the extent that peptide fractions became no longer potent. Another study [55] also reported that germination did not affect the DPP-IV inhibitory capacity of the common bean. It was concluded that non-germinated and non-hydrolyzed samples had a high DPP-IV inhibitory activity [55].
It is important to note that DPP-IV inhibitory peptide activity depends on the ability to pass through the intestinal epithelium intact and to function at the target site after absorption into the enterocyte [13,59,107]. The absorbability and bioavailability of larger peptides are still being studied. However, there are reports to suggest that larger leguminous, bioactive peptides can be absorbed by the human digestive system [59,108]. The transcellular movement of cell-penetrating peptides (CPPs), the paracellular pathway, and specific transporters (PEPT1 and PEPT2) may be involved in the absorption [59].
10. Molecular Mass Profiling
Molecular-mass profiling in the literature for leguminous antidiabetic peptides involves the use of SDS-PAGE, MALDI-TOF/TOF MS, or EASY-MS/MS. The distribution of the protein molecular mass can be described by SDS-PAGE. The molecular mass of each peptide within the protein in the samples and the intensities can be measured using MALDI-TOF/TOF MS or EASY-MS/MS. The results from the latter will further assist in peptide sequencing. MALDI-TOF/TOF MS results are reported only for <1 kDa peptides. One study used EASY-MS/MS [12].
Gel concentrations used in SDS-PAGE were between 4 and 20%, except for one study, which used 5% concentrated and 12% separated polyacrylamide gel [63], and other studies that used 16.5% Tris-Tricine polypeptide-ready gels [55,57]. The molecular markers used were Pre-stained Precision Plus Protein Standard [55,57], Precision Protein Plus Dual Color Standard [25], 10–250 kDa [59], 14.4–116.0 kDa [63], and Carl Roth GmbH and SERVA electrophoresis GmbH [13].
The identification of each noticeable band on SDS-PAGE requires data from other measurements, the protein database, and the literature references. Twelve Mexican and Brazilian common-bean protein identifications [38], observed as SDS-PAGE noticeable bands, were based on SDS-PAGE, tryptic gel LC/MS, Database Uniprot KB20130614, NCBInr 20130614, and the literature [109,110,111,112,113].
The SDS-PAGE of all raw common beans showed noticeable bands at 40–50 kDa, which corresponded to phaseolin. Phaseolin is the main storage protein in Phaseolus vulgaris (40–55 kDa) [55,114]. The main storage proteins in Vigna unguiculata and Vigna subterranea are vicilin (40–70 kDa) [57,115] and glycinine (30–40 kDa).
The phaseolin in raw common beans remained almost unhydrolyzed by pepsin and pancreatin, while other proteins were digested [38,53,55,59]. Phaseolin protein bands remained noticeable for up to 3 h after germination [55] and fermentation [63].
Phaseolin was hydrolyzed by Alcalase and bromelain, followed by pepsin and pancreatin [25,55]. The early stage of germination (24 h) caused phaseolin to degrade [55] as storage protein was hydrolysed by the endogenous endopeptidase to provide amino acids for developing new tissues [55,116,117]. A longer period of fermentation (>3 h) caused phaseolin protein bands to disappear [63]. Precooking also caused phaseolin protein bands to disappear [59], as protein denatured during thermal and pressure cooking [59,118,119,120].
The SDS-PAGE profile of raw Vigna unguiculata and Vigna subterranea showed noticeable bands at 50 and 46 kDa, respectively, which corresponded to vicilin. The vicilin fraction remained intact in cowpea beans until 60 h of germination; however, hydrolysis with Alcalase, pepsin, and pancreatin rapidly degraded the fraction [57].
11. Peptide Sequence Identification and Bioactivity Prediction
In leguminous antidiabetic peptide studies, peptide sequences in samples were identified by means of HPLC in tandem with MS, such as RP-HPLC-MS/MS, LC-MS-MS/MS, IT-MS-ESI, HPLC-ESI-MS, MS/MS, LCMS, and LC-MS/MS. Data were then processed using Data AnalysisTM, Mascot Distiller, Xcalibur software v 2.0, MassLynx 4.1 V, and FlexAnalysis 3.4. Further, the data were analysed using data analyser software such as BioTools v 3.1 and v 3.2, Mascot Search, and PEAKS studio v 6.0.
Peptide sequence confirmation was performed using an online protein database. Many studies used the BLAST® tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 4 August 2020)), except for one study, which used the UniProt database (http://www.uniprot.org/ (accessed on 7 February 2019) [13]. Peptide structure and physicochemical properties predictions were aided by an online generator called PepDraw tool (http://www.tulane.edu/~biochem/WW/PepDraw/ (accessed on 4 August 2020).
The potential biological activity of peptides was predicted using BIOPEP database or BioPep tool (http://www.uwm.edu.pl/biochemia/index.php/pl/biopep (accessed on 20 January 2015), Peptide Ranker (http://distilldeep.ucd.ie/PeptideRanker/ (accessed on 20 January 2015), and PeptideDB (http://www.peptides.be/ (accessed on 20 August 2015). Available online databases for potential bioactivities are provided in the form of a list [121]. A list of online tools commonly used in leguminous antidiabetic peptide studies and their functions is provided in Table 7.

Table 7.
Online tools used in leguminous antidiabetic peptides studies.
Only the selected sample fractions were subjected to peptide sequencing, i.e., samples with the highest inhibitory potential. Moreover, not all peptide fractions detected in the sample were reported as contributing to inhibition. However, in general, many reports indicated that the most important peptides were comprised of amino acids between 3 and 20 residues. The peptides needed to meet several criteria, including a high LC-ESI-MSMS elution profile intensity of ≥50% or even ≥70% [61]; a BioTools Flex score of ≥75 [53]; a PeptideRanker score of >0.80 [42]; a PEAKS Studio Average Local Confidence (ALC) of >60%, and a Pepsite2 p-value of <0.05 [62]. LC-ESI-MSMS elution profile intensity ranged from 0 to 100%: the higher the intensity, the more abundant were the peptides present in the sample relative to all the detected peptides. BioTools is a data analysis software used in mass spectrometry to confirm the presence of the detected peptide sequences in a protein of a reference organism. A BioTools Flex score above 70 indicated that the peptide sequence had a higher probability of being a fraction of a protein from the reference organism. PeptideRanker is an online database used to predict peptide bioactivity. A PeptideRanker score of >0.80 meant that the peptide sequence had a higher probability of having the predicted potential bioactivity and having a lower rate of false-positive predictions. PEAKS Studio is a data analysing software used in mass spectrometry to detect the presence of de novo peptide sequences. The confidence levels of peptide novelty based on ALC are classified as very high (>90%), high (80–90%), medium (60–80%), and low (<60%). Pepsite2 is a web server that is used to predict binding sites and analyse the binding mechanisms of bioactive peptides by providing the p-value. The optimal p-value cut-off is 0.04, and a value above this indicates that the binding prediction is more likely to be accurate.
Peptide sequence identification is crucial in inhibitory peptide studies. Amino acid residues that have an antidiabetic enzyme inhibitory peptide play an important role in how the peptide interacts with the enzymes, i.e., α-amylase, α-glucosidase, and DPP-IV. The types of amino acids and their sequences within a peptide determines the type of interactions or bonds formed between the peptide and the enzymes, as well as the location of the interaction. An interaction at the enzyme catalytic site will potentially result in competitive inhibition or non-competitive and uncompetitive inhibition. Detailed types and sequences of amino acid residues within peptides which affect antidiabetic enzyme inhibitory activity are explained in the next section.
12. Molecular Docking and Enzyme Kinetics Study
Software and online tools commonly used in leguminous antidiabetic peptides computational modelling research for molecular docking are ChemBio3D Ultra, Instant MarvinSketch, Maestro, VEGA suites, AutoDock Vina, AutoDock Tools, AutoGrid, Docking Server, PyMol, PLANTS, Discovery Studio (Accelrys Software), Discovery Studio Client (Dasssault Systèmes Biovia Corp ®, San Diego, CA, USA), GRAMM-X protein-protein docking web server (http://vakser.compbio.ku.edu/resources/gramm/grammx/ (accessed on 20 August 2020)), and the Rosetta FlexPepDock web server (http://flexpepdock.furmanlab.cs.huji.ac.il/ (accessed on 20 August 2020). Computer-aided techniques to complement empirical methods are powerful tools that allow for the tentative identification of potential leguminous antidiabetic peptides [16,123,125]. A list of software tools used in leguminous antidiabetic peptide studies and their functions is provided in Table 8.

Table 8.
Software tools used in leguminous antidiabetic peptides studies.
Only selected peptides, which demonstrate good inhibitory potential through empirical evidence, are subjected to computational modelling and enzyme kinetic studies. The selections are based on the results from in vitro, cell work, in vivo and ex vivo experiments, and/or the in silico inhibition activity of the peptides. Additionally, some studies based their selection on the overall occurrence of the peptides in the hydrolysates and from which protein they originated. Peptides from phaseolin and arcelin in common beans and vicilin and lectin in cowpea beans, which were considered main storage proteins, are considered eligible for computational modelling. A list of antidiabetic enzyme inhibitory activities of leguminous protein hydrolysate fractions and peptide sequences is provided in Table 9.

Table 9.
Antidiabetic enzyme inhibitory activity of leguminous protein hydrolysate fractions and peptide sequences.
In computer modelling, the inhibitor peptides are analysed using several parameters which include the inhibition constant, energy score (free energy and interface energy), interaction score, distance of interaction, total amino acid interaction, and number of predicted hydrogen bonds. Control molecules are used, i.e., acarbose for α-amylase, α-glucosidase, and sitagliptin for DPP-IV docking analysis. In general, a lower-than-control value indicates better binding conformation and thus better inhibition, except for total amino acid interaction values and the and number of predicted hydrogen bonds, for which higher values indicate a better interaction.
In an enzyme kinetics study, inhibitor peptides were analysed at a minimum of three levels of inhibitor concentrations using Lineweaver–Burk plots (x-axis = 1/[S]; y-axis = 1/V), in which the Michaelis-Menten constant (Km) and the maximum velocity of enzymatic activity (Vmax) were calculated. The slopes, 1/Km values, and 1/Vmax values of the lines from varying inhibitor concentrations defined the type of inhibition. Competitive inhibition was indicated by increasing Km values and unchanged Vmax values with the increase in inhibitor concentrations. This type of inhibition is typical with intersecting lines at the y-axis at the same point, indicating unaffected Vmax values even when more inhibitors are present. Non-competitive inhibition is indicated by unchanged Km values and reduced Vmax values with increasing inhibitor concentrations. This type of inhibition is typical with intersecting lines at the x-axis at the same point, indicating1/Km values unaffected by various inhibitor concentrations. Uncompetitive inhibition is defined by reduced Km values and reduced Vmax values with an increase in inhibitor concentrations. For this type of inhibition, lines share the same slope.
Through molecular docking and an enzyme kinetics study, researchers were able to identify the types and locations of chemical interactions between inhibitor peptides and enzymes, as well as the type of enzyme inhibition. The types of interactions between inhibitory peptides and enzymes were hydrogen bonds, hydrophobic interactions, polar interactions, electrostatic interactions, cation π bonds, or van der Waals interactions. The location of the interactions can be inside or outside the enzyme catalytic region, and with the amino acid residues at the side chain or the backbone of the enzyme.
Three structural domains comprise α-amylase (Domain A, residues 1–99, 169–404; Domain B, residues 100–168; Domain C, residues 405–496). Domain A consists of an eight-stranded, parallel β-barrel surrounded by a cylinder of α-helical segments. In this domain, the active sites are located at, ASN197, GLU233, and ASP300 [127]. An α-glucosidase active site identified from the PDB Site Records in the cavity surrounded by the amino acid residues of ALA26, ASP30, SER31, ASN32, ASP33, ASP34, GLY35, TRP36, GLY37, ASP38, LYS40, GLY41, THR83, SER461, PRO448, ALA465, LYS466, PRO467, and TRP468. Enzyme DPP-IV has three binding pockets/sites in both chain A and B. The S1 pocket consists of the residues SER630, ASP708 (ASN710), and HIS740; the S2 pocket involves ARG125, GLU205, and GLU206; and the S3 pocket has SER209, ARG358, and PHE357 [128,129].
Molecular docking of Phaseolus vulgaris L. inhibitory peptides on α-amylase were investigated [25,61]. Inhibitory peptides with a good inhibition potential from the first study [61] were AKSPLF, QTPF, and LSKSVL, which interacted with at least two of the three amino acid residues at the catalytic site of the enzyme. The peptides’ interactions with α-amylase occurred through hydrophobic interactions, polar interactions, and hydrogen bonds. Peptides FFL and NEGEAH were reported [25] to have better binding conformations and the lowest-energy interactions with α-amylase when compared to the other peptides detected in their samples. Similar to the first study, all the peptides in this study interacted with at least two amino acid residues of the active site triad of the enzyme. The types of interactions between the peptides and α-amylase reported in the second study were van der Waals interactions, electrostatic interactions, hydrogen bonds, charged interactions, and π interactions. As these peptides interact with the catalytic site of α-amylase, the type of the inhibition is competitive. An enzyme kinetics study of α-amylase by Pisum sativum L. inhibitory protein hydrolysates and peptides also reported a similar type of inhibition [9].
It was argued in at least two reports that hydrophobic (A, L, F, V, P, G, and M) and hydrophilic (C, H, and S) amino acids are responsible for human-salivary α-amylase inhibition through hydrophobic interactions and hydrogen bonds at the active site [16,62]. Inhibitory peptides reported in the aforementioned studies [25,61] were mostly composed of hydrophobic and hydrophilic amino acids. Moreover, a review argued that the amino acid residues of H, W, Y, and R are important in α-amylase inhibition [130]. In the same review, authors also mentioned that the inhibition mechanism is initiated when the inhibitory peptides approach the enzyme at the catalytic site, where they cause the formation of an extensive network of interactions and bonds. Enzyme conformational change resulted from the interaction, which disabled the capability of the enzyme to bind with its substrate.
Molecular docking studies between α-glucosidase and soybean [65], common bean [53], or black bean [61] inhibitory peptides have been reported. In the first study, soybean peptides (GSR, EAK) were bound to α-glucosidase outside its active site. The study on soybean peptides [65] found that the interactions were facilitated through van der Waals, anion-π, and hydrogen bonds. The study on common bean peptides [53] reported that polar interactions, hydrophobic interactions, and hydrogen bonds are found between the common bean inhibitory peptides (KTYGL, KKSSG, CPGNK, and GGGLHK) and α-glucosidase. The study on black beans [61] found that the major interactions between black bean peptides (AKSPLF, QTPF, FEELN, and LSKSVL) and α-glucosidase were hydrogen bonds and polar interactions. In the latter studies, peptides bound at the active site of the enzyme, predominantly with ASP34, THR83, and ASN32 at the catalytic site of α-glucosidase. An enzyme kinetic study by Awosika and Aluko [9], however, found non-competitive type of inhibition by Pisum sativum L. peptides, similar to the results of Jiang’s group [65].
Amino acid residues with hydroxyl groups (S, T, and Y) or basic groups (K and R) on the side chain at N-terminal, P close at C-terminal, and A and M at the C-terminal position play important roles in α-glucosidase inhibition [131]. The inhibitory peptide sequences reported in the aforementioned studies [53,61,65] are yet to reflect these amino acid positions. However, the major interactions between α-glucosidase and the inhibitory peptides are hydrogen bonds and polar interactions [131]. This is reported in the study on black beans [61]. Hydrophobicity and isoelectric point are unlikely to contribute to α-glucosidase inhibition, while net charges between 0 and +1 are found in the most potent α-glucosidase inhibitor peptides [131].
At least six papers have reported molecular docking studies between DPP-IV and leguminous inhibitory peptides [25,53,55,57,61,126] from soybeans, lupins, common beans, black beans, hard-to-cook beans, and cowpeas. Electrostatic interaction, van der Waals interaction, polar interaction, and hydrogen bonds are the typical interactions reported between DPP-IV and the inhibitory peptides. Only two studies [57,61] reported binding at the DPP-IV active site. In the first study [57], peptide TTAGLLE from a cowpea bean (Vigna unguiculata) interacted with the S2 (GLU205 and GLU206) and S3 (SER209, ARG358, and PHE357) active site pockets of DPP-IV. The second study [61] found that inhibitory peptides from black beans (EGLELLLLLLAG, AKSPLF, and FEELN) mainly bind with ASP192, GLU191, and ARG253 at the DPP-IV active site. These amino acid residues of DPP-IV active sites reported by the latter study, however, are different from those reported by Juillerat-Jeanneret [128] and Patel and Ghate [129].
The molecular docking of common-bean inhibitory peptides (KKSSG, CPGNK, GGGLHK, and KTYGL) [53] showed binding with DPP-IV next to the active site (TRP124, GLU237, TYR238, PHE240, PRO249, THR251, VAL252, ARG253, and ALA707). The enzyme kinetic study concluded a competitive type of inhibition. Authors [53] argued that peptides which interacted with the enzyme at several binding sites, and which were able to position in the large cavity of the DPP-IV active structure between the dimer, can cause a blocked access for the substrate to bind with the enzyme, and therefore behave similarly with the inhibition mechanism at active site.
One review concluded that inhibitory peptides IPI, VPL, and LPL are very potent DPP-IV inhibitors [132]. In other reports, dipeptides with P, A, G, S, L, or V at N-terminal are found in DPP-IV inhibitors. Longer peptides—A in the second last position of the N-terminal; P at the first-second-third-fourth N-terminal and flanked by L, V, F, A, and G; hydrophobic amino acids (A, G, I, L, F, P, M, W, and V) at the N-terminal, or approaching the N-terminal or next to the C-terminal; peptides containing W, Y, and F—and the presence of aromatic residue display inhibitory activity against DPP-IV [13,16,54,59,123,126,132,133,134]. The peptides that demonstrated good inhibition potential reported in the six research papers had the hydrophobic amino acid present at a strategic position in their sequence to achieve a good binding conformation with DPP-IV.
13. Conclusions
The common bean (Phaseolus vulgaris), cowpea bean (Vigna unguiculata), Bambara bean (Vigna subterranea), soybean (Glycine max), and yellow field pea bean (Pisum sativum) have been the protein sources in antidiabetic α-Amylase, α-Glucosidase, and DPP-IV inhibitor studies to date. The studies describe sample preparation, protein extraction, hydrolysis, fractionation, inhibitory assays, molecular-mass profiling, peptide sequence identification, the potential bioactivity prediction of the peptide, computational modelling, and enzyme kinetics. Variations in legume cultivars and methods affected the release of peptides. Different methods were used in sample preparation, which include fermentation (t, T), germination (t), and pre-cooking; in protein extraction, including alkaline extraction isoelectric precipitation, phosphate buffer extraction, and water extraction; in protein hydrolysis, enzyme types and combination, enzyme substrate ratio, pH, and time; and in enzyme inhibitory assays’ positive control type and concentration, inhibitor or peptide concentration, and units of inhibitory activity. The categorization of the relative scale of inhibitory activities among legume samples becomes difficult due to these method differences. Peptide fractionations used ultrafiltration membranes from 1 to 100 kDa, ion-exchange chromatography, gel filtration, or reversed-phase high performance liquid chromatography (RP-HPLC). Semi-preparative peptides were obtained using solid-phase methods and standard 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry. Peptide sequences in samples were identified by means of HPLC/MS. Software and online tools were used in bioactivity prediction and computational modelling. The identification of the types and locations of chemical interactions between inhibitor peptides and enzymes and the type of enzyme inhibition were achieved through computational modelling and enzyme kinetic studies.
The highest activity of α-amylase inhibition ranged between 14.9 and 89.1% relative to acarbose and between 57.48 and 101.61% in non-acarbose assays. The highest activity of α-glucosidase inhibition ranged between 22 and 97.34% inhibition relative to acarbose and 34.73% in non-acarbose assays. The highest activity of DPP-IV inhibition ranged between 47.94 and 96.7% inhibition. Peptide inhibitory activity against α-amylase, α-glucosidase, and DPP-IV was demonstrated to depend on its ability to dock at the enzyme catalytic sites. Studies, however, did not indicate the mechanism of how antidiabetic peptides are released into the human digestive system, absorbed into the blood stream, and transported to the target sites.
Further research on leguminous antidiabetic peptides is needed to investigate protein sources from other legumes; the allergenicity and safety of the peptides; the actual sequence of events and the molecular determinants dictating the inhibition mechanism; and the stability, efficacy, and the bioavailability of the peptides. In addition, encapsulation and sensory studies are needed to reduce the typically bitter taste of hydrophobic amino acids; feeding studies using human volunteers are also needed. Lastly, when consumed as legumes in which peptides are still in their parent protein sequence, the yield following peptide release in the human digestive system is still unknown, especially when present in different matrices, macronutrient interactions, and dietary factors. This information is crucial when suggesting the incorporation of legumes into other starch-containing foods or as ingredients in normal diets.
14. Future Recommendations
The positive results of the in vitro studies prompt the need for further research on leguminous antidiabetic peptides, as follows:
- As legumes are an economical dietary protein source, research to explore antidiabetic peptides from other legume sources is needed;
- Some proteins may cause allergic reactions, and studies on allergenicity and safety of antidiabetic peptides are suggested;
- The mechanism of how antidiabetic peptides are released into the human digestive system, absorbed into the blood stream, and migrated to the target sites is not fully understood. Moreover, at the molecular level, the actual sequence of events and the molecular determinants dictating the inhibition mechanism are far from being understood, requiring an interdisciplinary approach, such as from nutrition and biomolecular science;
- When consumed as peptides, the harsh environment in the human digestion tract may cause changes in the peptides that will affect their bioactivity and bioavailability. Hence, peptide stability, efficacy, and bioavailability studies would assist in determining the dose of peptide intake;
- Low-molecular-weight peptides composed of hydrophobic amino acids are typically bitter, and the application of these peptides in foods without affecting the sensory profile will require a special process such as micro- or nanoparticle encapsulation. Research in this area may offer solutions not only for elucidating sensory aspects, but also bioavailability, bioactivity, and safety;
- Feeding studies using human volunteers will be required prior to the application of the inhibitory peptides as ingredients in the diets, in functional foods, or as nutraceuticals or pharmaceuticals supplements.
Author Contributions
Conceptualization, A.R. and J.A.; Investigation, A.R.; Writing—Original Draft Preparation, A.R.; Writing—Review & Editing, J.A.: Supervision, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Indonesia Endowment Fund for Education (LPDP) 2019-2023.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. Nat. Res. 2020, 10, 14790. [Google Scholar] [CrossRef]
- World Health Organization. Diabetes: Key Facts. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 26 September 2022).
- International Diabetes Federation. IDF Diabetes Atlas. International Diabetes Federation. 2020. Available online: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 3 December 2020).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Korat, A.V.A.; Willett, W.C.; Hu, F.B. Diet, Lifestyle, and Genetic Risk Factors for Type 2 Diabetes: A Review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow-Up Study. Curr. Nutr. Rep. 2014, 3, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.M.; Chen, Y.H.; Yen, P.L.; Chang, S.T. Antihyperglycemic and antioxidant activities of twig extract from Cinnamomum osmophloeum. J. Tradit. Complement. Med. 2016, 6, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Mejía, E.; Batista, K.A.; Fernández, J.J.A.; Fernandes, K.F. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.). Food Res. Int. 2019, 121, 238–246. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro activities of α-amylase, α-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef]
- Johnson, M.H.; Lucius, A.; Meyer, T.; Gonzalez De Mejia, E. Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-amylase and α-glucosidase by highbush blueberry (Vaccinium corombosum). J. Agric. Food Chem. 2011, 59, 8923–8930. [Google Scholar] [CrossRef]
- Widowati, S.; Astawan, M.; Muchtadi, D.; Wresdiyati, T. Hypoglycemic Activity of Some Indonesian Rice Varieties and Their Physicochemical Properties. Indones. J. Agric. Sci. 2016, 7, 57. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]
- Mune Mune, M.A.; Minka, S.R.; Henle, T. Investigation on antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activity of Bambara bean protein hydrolysates. Food Chem. 2018, 250, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Dipeptidyl Peptidase-4 Inhibition and the Treatment of Type 2 Diabetes. Diabetes Care 2007, 30, 1335–1343. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-glucosidase enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Kumar, P.S.; Saravanan, A.; Sheeba, N.; Uma, S. Structural, functional characterization and physicochemical properties of green banana flour from dessert and plantain bananas (Musa spp.). LWT 2019, 116, 108524. [Google Scholar] [CrossRef]
- Jia, M.; Yu, Q.; Chen, J.; He, Z.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Physical quality and in vitro starch digestibility of biscuits as affected by addition of soluble dietary fiber from defatted rice bran. Food Hydrocoll. 2020, 99, 105349. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef]
- Oates, C.G. Towards an understanding of starch granule structure and hydrolysis. Trends Food Sci. Technol. 1997, 8, 375–382. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glycosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L. Understanding the mechanism of starch digestion mitigation by rice protein and its enzymatic hydrolysates. Food Hydrocoll. 2018, 84, 473–480. [Google Scholar] [CrossRef]
- López-Barón, N.; Sagnelli, D.; Blennow, A.; Holse, M.; Gao, J.; Saaby, L.; Müllertz, A.; Jespersen, B.; Vasanthan, T. Hydrolysed pea proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll. 2018, 79, 117–126. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Oseguera Toledo, M.E.; Gonzalez de Mejia, E.; Sivaguru, M.; Amaya-Llano, S.L. Common bean (Phaseolus vulgaris L.) protein-derived peptides increased insulin secretion, inhibited lipid accumulation, increased glucose uptake and reduced the phosphatase and tensin homologue activation in vitro. J. Funct. Foods 2016, 27, 160–177. [Google Scholar] [CrossRef]
- Claessens, M.; Calame, W.; Siemensma, A.D.; van Baak, M.A.; Saris, W.H.M. The effect of different protein hydrolysate/carbohydrate mixtures on postprandial glucagon and insulin responses in healthy subjects. Eur. J. Clin. Nutr. 2009, 63, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kansal, R.; Kalia, V.; Tripathi, M.; Gupta, V.K. A kidney bean trypsin inhibitor with an insecticidal potential against Helicoverpa armigera and Spodoptera litura. Acta Physiol. Plant. 2014, 36, 525–539. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Human α-amylase and starch digestion: An interesting marriage. Starch Staerke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- Matsui, T.; Yoshimoto, C.; Osajima, K.; Oki, T.; Osajima, Y. In vitro survey of α–glucosidase inhibitory food components. Biosci. Biotechnol. Biochem. 1996, 60, 2019–2022. [Google Scholar] [CrossRef]
- Wright, E.M.; Sala-Rabanal, M.; Loo, D.D.F.; Hirayama, B.A. Sugar Absorption, 5th ed.; Academic Press-Elsevier: San Diego, CA, USA, 2012. [Google Scholar]
- Wolever, T.M.S.; Jenkins, D.J.A.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef]
- Litwack, G. Vitamins and Hormones: Incretins and Insulin Secretion, 1st ed.; Academic Press-Elsevier: Oxford, UK, 2010. [Google Scholar]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls: Tampa, FL, USA, 2021. [Google Scholar]
- Petsko, G.; Ringe, D. Protein Structure and Function; New Science Press Ltd.: Singapore, 2004. [Google Scholar]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive Peptides and Hydrolysates from Pulses and Their Potential Use as Functional Ingredients. J. Food Sci. 2014, 79, R273–R283. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Characterization and Comparison of Protein and Peptide Profiles and their Biological Activities of Improved Common Bean Cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum. Nutr. 2015, 70, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Marya Khan, H.; Nabavi, S.M.; Habtemariam, S. Anti-diabetic potential of peptides: Future prospects as therapeutic agents. Life Sci. 2018, 193, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef]
- Lemes, A.C.; Sala, L.; Ores, J.D.C.; Braga, A.R.C.; Egea, M.B.; Fernandes, K.F. A review of the latest advances in encrypted bioactive peptides from protein-richwaste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef]
- Venâncio, T.M.; Oliveira, A.E.A.; Silva, L.B.; Machado, O.L.T.; Fernandes, K.V.S.; Xavier-Filho, J. A protein with amino acid sequence.pdf. Braz. J. Med. Biol. Res. 2003, 36, 1167–1173. [Google Scholar] [CrossRef]
- Xavier-Filho, J.; Oliveira, A.E.A.; da Silva, L.B.; Azevedo, C.R.; Venâncio, T.M.; Machado, O.L.T.; Oliva, M.L.; Fernandes, K.V.S.; Xavier-Neto, J. Plant insulin or glucokinin: A conflicting issue. Braz. J. Plant Physiol. 2003, 15, 67–78. [Google Scholar] [CrossRef]
- Kehinde, B.A.; Sharma, P. Recently isolated antidiabetic hydrolysates and peptides from multiple food sources: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Oseguera-Toledo, M.E.; González de Mejía, E.; Reynoso-Camacho, R.; Cardador-Martínez, A.; Amaya-Llano, S.L. Proteins and bioactive peptides. Nutrafoods 2014, 13, 147–157. [Google Scholar] [CrossRef]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-Derived Bioactive Peptides: A Treatment to Cure Diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, J.; Yang, R.; Zhao, W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019, 54, 1909–1919. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT-Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; González de Mejía, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Jiménez-Morales, K.; Quintal-Novelo, C.; Moo-Puc, R.; Chel-Guerrero, L.; Betancur-Ancona, D. Enzymatic protein hydrolysates and ultrafiltered peptide fractions from Cowpea Vigna unguiculata L bean with in vitro antidiabetic potential. J. Iran. Chem. Soc. 2019, 16, 1773–1781. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Mojica, L.; Johnson, M.H.; Chang, Y.K.; González de Mejía, E. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 2015, 76, 150–159. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A boon to human nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Chang, Y.K.; de Mejía, E.G. Impact of germination and enzymatic hydrolysis of cowpea bean (Vigna unguiculata) on the generation of peptides capable of inhibiting dipeptidyl peptidase IV. Food Res. Int. 2014, 64, 799–809. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Strategies for the discovery and identification of food protein-derived biologically active peptides. Trends Food Sci. Technol. 2017, 69, 289–305. [Google Scholar] [CrossRef]
- Mojica, L.; Chen, K.; de Mejía, E.G. Impact of Commercial Precooking of Common Bean (Phaseolus vulgaris) on the Generation of Peptides, After Pepsin-Pancreatin Hydrolysis, Capable to Inhibit Dipeptidyl Peptidase-IV. J. Food Sci. 2015, 80, H188–H198. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Gonzalez De Mejia Elvira Menjivar, M.; Granados-Silvestre, M.Á. Antidiabetic Effect of Black Bean Peptides through Reduction of Glucose Absorption and Modulation of SGLT1, GLUT2 and DPP-IV in in vitro and in vivo Models. FASEB J. 2016, 30, 125–126. [Google Scholar]
- Mojica, L.; de Mejía, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Gan, C.Y. Identification of Pinto bean peptides with inhibitory effects on α-amylase and angiotensin converting enzyme (ACE) activities using an integrated bioinformatics-assisted approach. Food Chem. 2018, 267, 124–131. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U. Identification of potential inhibitory peptides of enzymes involved in the metabolic syndrome obtained by simulated gastrointestinal digestion of fermented bean (Phaseolus vulgaris L.) seeds. Food Res. Int. 2017, 100, 489–496. [Google Scholar] [CrossRef]
- Ohara, A.; Cason, V.G.; Nishide, T.G.; Miranda de Matos, F.; de Castro, R.J.S. Improving the antioxidant and antidiabetic properties of common bean proteins by enzymatic hydrolysis using a blend of proteases. Biocatal. Biotransform. 2020, 39, 100–108. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, H.; He, R.; Ma, Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005. [Google Scholar] [CrossRef]
- Nilsson, B.L.; Soellner, M.B.; Raines, R.T. Chemical Synthesis of Proteins. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 91–118. [Google Scholar] [CrossRef]
- Kent, S.B.H. Novel protein science enabled by total chemical synthesis. Protein Sci. 2019, 28, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Oseguera-Toledo, M.E.; De Mejia, E.G.; Dia, V.P.; Amaya-Llano, S.L. Common bean (Phaseolus vulgaris L.) hydrolysates inhibit inflammation in LPS-induced macrophages through suppression of NF-κB pathways. Food Chem. 2011, 127, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.S.; Ying-Yuan, N.; Gan, C.Y. A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chem. 2014, 152, 447–455. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.J.S.; Cason, V.G.; Sato, H.H. Binary mixture of proteases increases the antioxidant properties of white bean (Phaseolus vulgaris L.) protein-derived peptides obtained by enzymatic hydrolysis. Biocatal. Agric. Biotechnol. 2017, 10, 291–297. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Pérez-Flores, V.; Betancur-Ancona, D.; Dávila-Ortiz, G. Functional Properties of Flours and Protein Isolates from Phaseolus lunatus and Canavalia ensiformis Seeds. J. Agric. Food Chem. 2001, 50, 584–591. [Google Scholar] [CrossRef]
- Mune Mune, M.A. Optimizing Functional Properties of Bambara Bean Protein Concentrate by Enzymatic Hydrolysis Using Pancreatin. J. Food Process. Preserv. 2015, 39, 2572–2580. [Google Scholar] [CrossRef]
- Mora-Escobedo, R.; Robles-Ramírez, M.D.C.; Ramón-Gallegos, E.; Reza-Alemán, R. Effect of protein hydrolysates from germinated soybean on cancerous cells of the human cervix: An In Vitro study. Plant Foods Hum. Nutr. 2009, 64, 271–278. [Google Scholar] [CrossRef]
- Los, F.G.B.; Zielinski, A.A.F.; Wojeicchowski, J.P.; Nogueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): Whole seeds with complex chemical composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Bautista-Expósito, S.; Peñas, E.; Dueñas, M.; Silván, J.M.; Frias, J.; Martínez-Villaluenga, C. Individual contributions of Savinase and Lactobacillus plantarum to lentil functionalization during alkaline pH-controlled fermentation. Food Chem. 2018, 257, 341–349. [Google Scholar] [CrossRef]
- Carbonaro, M.; Vecchini, P.; Carnovale, E. Protein Solubility of Raw and Cooked Beans (Phaseolus vulgaris): Role of the Basic Residues. J. Agric. Food Chem. 1993, 41, 1169–1175. [Google Scholar] [CrossRef]
- Megías, C.; Yust, M.D.M.; Pedroche, J.; Lquari, H.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Purification of an ACE Inhibitory Peptide after Hydrolysis of Sunflower (Helianthus annuus L.) Protein Isolates. J. Agric. Food Chem. 2004, 52, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Baraniak, B.; Pietrzak, M. The impact of fermentation and in vitro digestion on formation angiotensin converting enzyme (ACE) inhibitory peptides from pea proteins. Food Chem. 2013, 141, 3774–3780. [Google Scholar] [CrossRef]
- Karaś, M.; Baraniak, B.; Rybczyńska, K.; Gmiński, J.; Gaweł-Bęben, K.; Jakubczyk, A. The influence of heat treatment of chickpea seeds on antioxidant and fibroblast growth-stimulating activity of peptide fractions obtained from proteins digested under simulated gastrointestinal conditions. Int. J. Food Sci. Technol. 2015, 50, 2097–2103. [Google Scholar] [CrossRef]
- Megías, C.; Pedroche, J.; Yust, M.M.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of copper-chelating peptides after hydrolysis of sunflower proteins with pepsin and pancreatin. LWT-Food Sci. Technol. 2008, 41, 1973–1977. [Google Scholar] [CrossRef]
- Campos, M.R.S.; Guerrero, L.A.C.; Ancona, D.A.B. Angiotensin-I converting enzyme inhibitory and antioxidant activities of peptide fractions extracted by ultrafiltration of cowpea Vigna unguiculata hydrolysates. J. Sci. Food Agric. 2010, 90, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Lunow, D.; Kaiser, S.; Brückner, S.; Gotsch, A.; Henle, T. Selective release of ACE-inhibiting tryptophan-containing dipeptides from food proteins by enzymatic hydrolysis. Eur. Food Res. Technol. 2013, 237, 27–37. [Google Scholar] [CrossRef]
- Vaštag, Ž.; Popović, L.; Popović, S.; Peričin-Starčević, I.; Krimer-Malešević, V. In vitro study on digestion of pumpkin oil cake protein hydrolysate: Evaluation of impact on bioactive properties. Int. J. Food Sci. Nutr. 2013, 64, 452–460. [Google Scholar] [CrossRef]
- Marcela, G.M.; Eva, R.G.; del Carmen, R.R.M.; Rosalva, M.E. Evaluation of the Antioxidant and Antiproliferative Effects of Three Peptide Fractions of Germinated Soybeans on Breast and Cervical Cancer Cell Lines. Plant Foods Hum. Nutr. 2016, 71, 368–374. [Google Scholar] [CrossRef]
- Yuan, X.; Gu, X.; Tang, J. Purification and characterisation of a hypoglycemic peptide from Momordica Charantia L. Var. abbreviata Ser. Food Chem. 2008, 111, 415–420. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, J.; Tian, J.; Chen, S.; Ye, X.; Hu, Y.; Chen, J. Inhibitory mechanism of novel allosteric inhibitor, Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins against α-glucosidase. J. Funct. Foods 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Worthington, V. Alpha amylase. In Worthington Enzyme Manual; Worthington Biochemical Corp.: Freehold, NJ, USA, 1993; pp. 36–41. [Google Scholar]
- Świeca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Ghaedian, R.; Shetty, K. Fermentation of Milk and Soymilk by Lactobacillus bulgaricus and Lactobacillus acidophilus Enhances Functionality for Potential Dietary Management of Hyperglycemia and Hypertension. Food Biotechnol. 2007, 21, 217–236. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Siow, H.L.; Lim, T.S.; Gan, C.Y. Development of a workflow for screening and identification of α-amylase inhibitory peptides from food source using an integrated Bioinformatics-phage display approach: Case study—Cumin seed. Food Chem. 2017, 214, 67–76. [Google Scholar] [CrossRef]
- Hao, X.; Li, J.; Shi, Q.; Zhang, J.; He, X.; Ma, H. Characterization of a novel legumin α-amylase inhibitor from chickpea (Cicer arietinum L.) seeds. Biosci. Biotechnol. Biochem. 2009, 73, 1200–1202. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D. Plant food-derived angiotensin i converting enzyme inhibitory peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef]
- Rudolph, S.; Lunow, D.; Kaiser, S.; Henle, T. Identification and quantification of ACE-inhibiting peptides in enzymatic hydrolysates of plant proteins. Food Chem. 2017, 224, 19–25. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.S.; Choi, J.W.; Ra, K.S.; Kim, J.M.; Suh, H.J. Novel tripeptides with α-glucosidase inhibitory activity isolated from silk cocoon hydrolysate. J. Agric. Food Chem. 2011, 59, 11522–11525. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Wang, M.H.; Rhee, H.I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Uraipong, C.; Zhao, J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase, β-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2016, 96, 1101–1110. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.; Martínez-Ayala, A.; Drago, S.; González, R.; Betancur-Ancona, D.; Chel-Guerrero, L. Extrusion of a hard-to-cook bean (Phaseolus vulgaris L.) and quality protein maize (Zea mays L.) flour blend. LWT-Food Sci. Technol. 2008, 41, 1799–1807. [Google Scholar] [CrossRef]
- Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Lara-González, S.; Montero-Morán, G.M.; Díaz-Gois, A.; de Mejia, E.G.; de la Rosa, A.P.B. In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem. 2013, 136, 758–764. [Google Scholar] [CrossRef]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martínez-Maqueda, D.; Recio, I.; Hernández-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; O’Keeffe, M.B.; Fitzgerald, R.J. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Dia, V.P.; Torres, S.; De Lumen, B.O.; Erdman, J.W.; De Mejia, E.G. Presence of lunasin in plasma of men after soy protein consumption. J. Agric. Food Chem. 2009, 57, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Chaves, I.; Carrilho, D.; Veloso, M.; Ricardo, C.P. Detection of novel trypsin inhibitors in the cotyledons of Phaseolus vulgaris seeds. J. Plant Physiol. 2010, 167, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Zhang, Y.; Ng, T.B. Brown kidney bean bowman-birk trypsin inhibitor is heat and pH stable and exhibits anti-proliferative activity. Appl. Biochem. Biotechnol. 2013, 169, 1306–1314. [Google Scholar] [CrossRef]
- Rui, X.; Boye, J.I.; Ribereau, S.; Simpson, B.K.; Prasher, S.O. Comparative study of the composition and thermal properties of protein isolates prepared from nine Phaseolus vulgaris legume varieties. Food Res. Int. 2011, 44, 2497–2504. [Google Scholar] [CrossRef]
- Montoya, C.A.; Lallès, J.P.; Beebe, S.; Leterme, P. Phaseolin diversity as a possible strategy to improve the nutritional value of common beans (Phaseolus vulgaris). Food Res. Int. 2010, 43, 443–449. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Jacinto-Hernández, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012, 131, 1157–1164. [Google Scholar] [CrossRef]
- Montoya, C.A.; Leterme, P.; Victoria, N.F.; Toro, O.; Souffrant, W.B.; Beebe, S.; Lallès, J.-P. Susceptibility of phaseolin to in vitro proteolysis is highly variable across common bean varieties (Phaseolus vulgaris). J. Agric. Food Chem. 2008, 56, 2183–2191. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Maia, F.M.M.; Farias, D.F.; Campello, C.C.; Carvalho, A.F.U.; Moreira, R.D.A.; de Oliveira, J.T.A. Protein fractions, amino acid composition and antinutritional constituents of high-yielding cowpea cultivars. J. Food Compos. Anal. 2010, 23, 54–60. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Alonso, J.; Zapata, C. Evidence for phosphorylation of the major seed storage protein of the common bean and its phosphorylation-dependent degradation during germination. Plant Mol. Biol. 2014, 84, 415–428. [Google Scholar] [CrossRef]
- Sangronis, E.; Rodríguez, M.; Cava, R.; Torres, A. Protein quality of germinated Phaseolus vulgaris. Eur. Food Res. Technol. 2006, 222, 144–148. [Google Scholar] [CrossRef]
- Peñas, E.; Préstamo, G.; Gomez, R. High pressure and the enzymatic hydrolysis of soybean whey proteins. Food Chem. 2004, 85, 641–648. [Google Scholar] [CrossRef]
- Montoya, C.A.; Leterme, P.; Beebe, S.; Souffrant, W.B.; Mollé, D.; Lallès, J.P. Phaseolin type and heat treatment influence the biochemistry of protein digestion in the rat intestine. Br. J. Nutr. 2008, 99, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Unzón, H.Y.; Ortega-Delgado, M.L. Denaturation by heat, sodium dodecyl sulphate and dithiothreitol of globulins and phaseolin from dry bean (Phaseolus vulgaris L.). Plant Foods Hum. Nutr. 1988, 38, 211–223. [Google Scholar] [CrossRef]
- Orona-Tamayo, D.; Valverde, M.E.; Paredes-López, O. Bioactive peptides from selected latin american food crops—A nutraceutical and molecular approach. Crit. Rev. Food Sci. Nutr. 2019, 59, 1949–1975. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Black bean peptides inhibit glucose uptake in Caco-2 adenocarcinoma cells by blocking the expression and translocation pathway of glucose transporters. Toxicol. Rep. 2018, 5, 552–560. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J. Funct. Foods 2012, 4, 403–422. [Google Scholar] [CrossRef]
- Mojica, L.; Gonzalez de Mejia, E.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Liu, R.; Cheng, J.; Wu, H. Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: A review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Peptides Derived from Soy and Lupin Protein as Dipeptidyl-Peptidase IV Inhibitors: In Vitro Biochemical Screening and in Silico Molecular Modeling Study. J. Agric. Food Chem. 2016, 64, 9601–9606. [Google Scholar] [CrossRef] [PubMed]
- Brayer, G.; Luo, Y.; Withers, S. The structure of human pancreatic a-amylase at 1.8 Å resolution and comparisons with related enzymes. Protein Sci. 1995, 4, 1730–1742. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L. Dipeptidyl peptidase IV and its inhibitors: Therapeutics for type 2 diabetes and what else? J. Med. Chem. 2014, 57, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.D.; Ghate, M.D. Recent approaches to medicinal chemistry and therapeutic potential of dipeptidyl peptidase-4 (DPP-4) inhibitors. Eur. J. Med. Chem. 2014, 74, 574–605. [Google Scholar] [CrossRef] [PubMed]
- Obiro, W.C.; Zhang, T.; Jiang, B. The nutraceutical role of the Phaseolus vulgaris α-amylase inhibitor. Br. J. Nutr. 2008, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.H.; Gaspar, A.R.M. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018, 91, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef]
- Power, O.; Nongonierma, A.B.; Jakeman, P.; Fitzgerald, R.J. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc. Nutr. Soc. 2014, 73, 34–46. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Dellafiora, L.; Paolella, S.; Galaverna, G.; Cozzini, P.; FitzGerald, R.J. In silico approaches applied to the study of peptide analogs of Ile-Pro-Ile in relation to their dipeptidyl peptidase IV inhibitory properties. Front. Endocrinol. 2018, 9, 329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).