Extraction, Structural Characterization, Biological Functions, and Application of Rice Bran Polysaccharides: A Review
Abstract
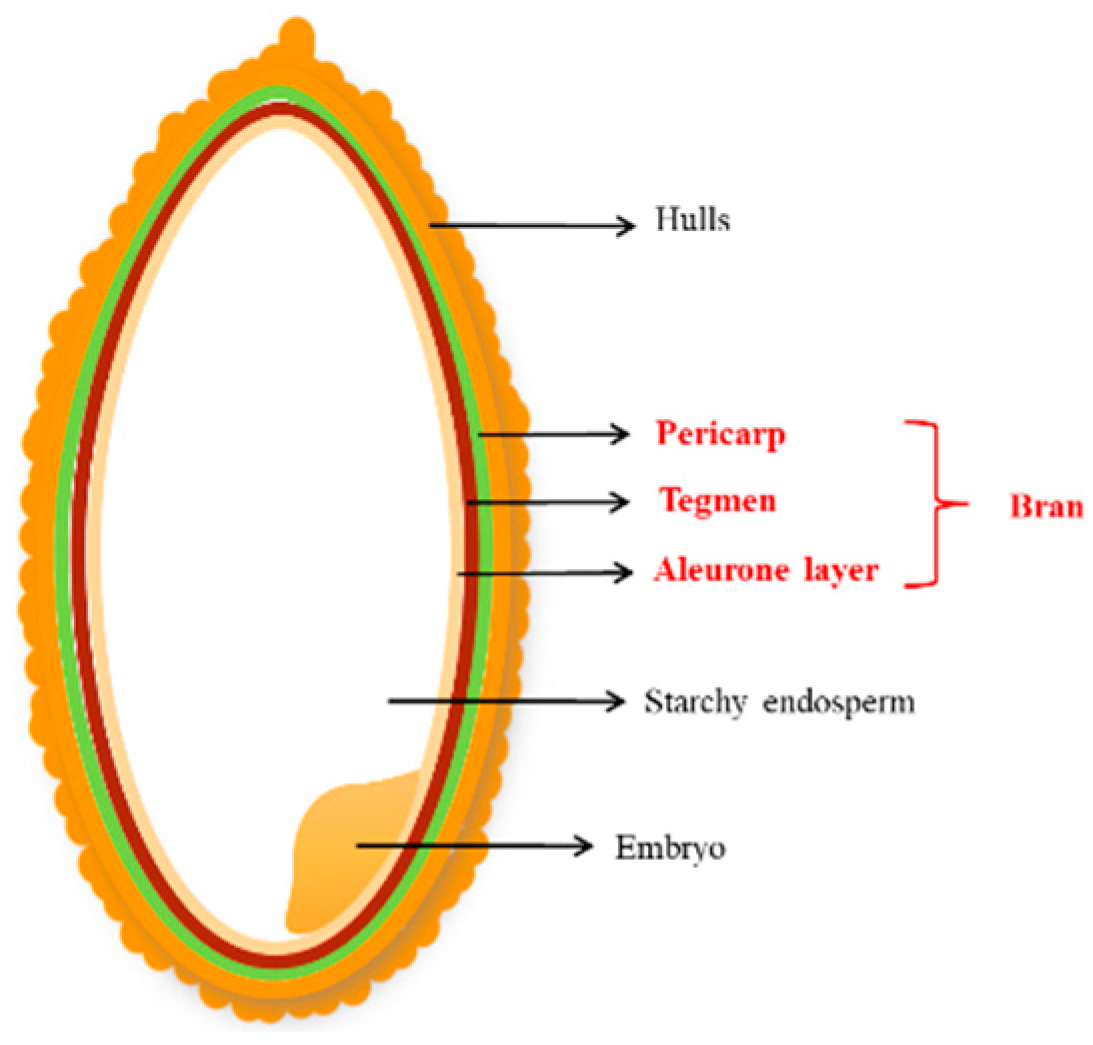
:1. Introduction
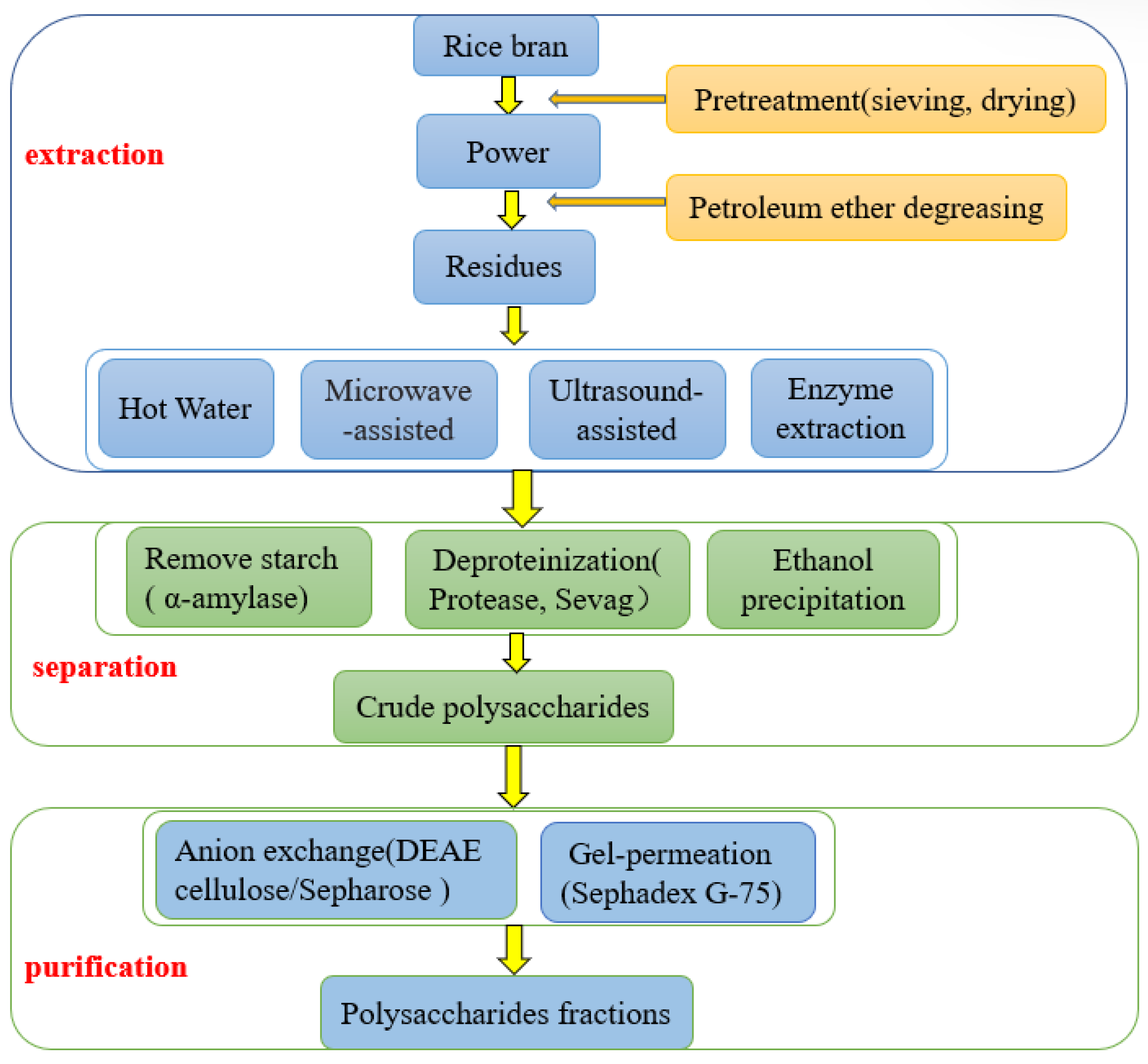
2. Extraction, Isolation, and Purification of Rice Bran Polysaccharides
2.1. Extraction of Polysaccharides
2.2. Isolation and Purification of Polysaccharides
3. Chemical Characterization of Rice Bran Polysaccharides
3.1. Monosaccharide Composition
3.2. Molecular Weight Analysis
3.3. Chemical Structures
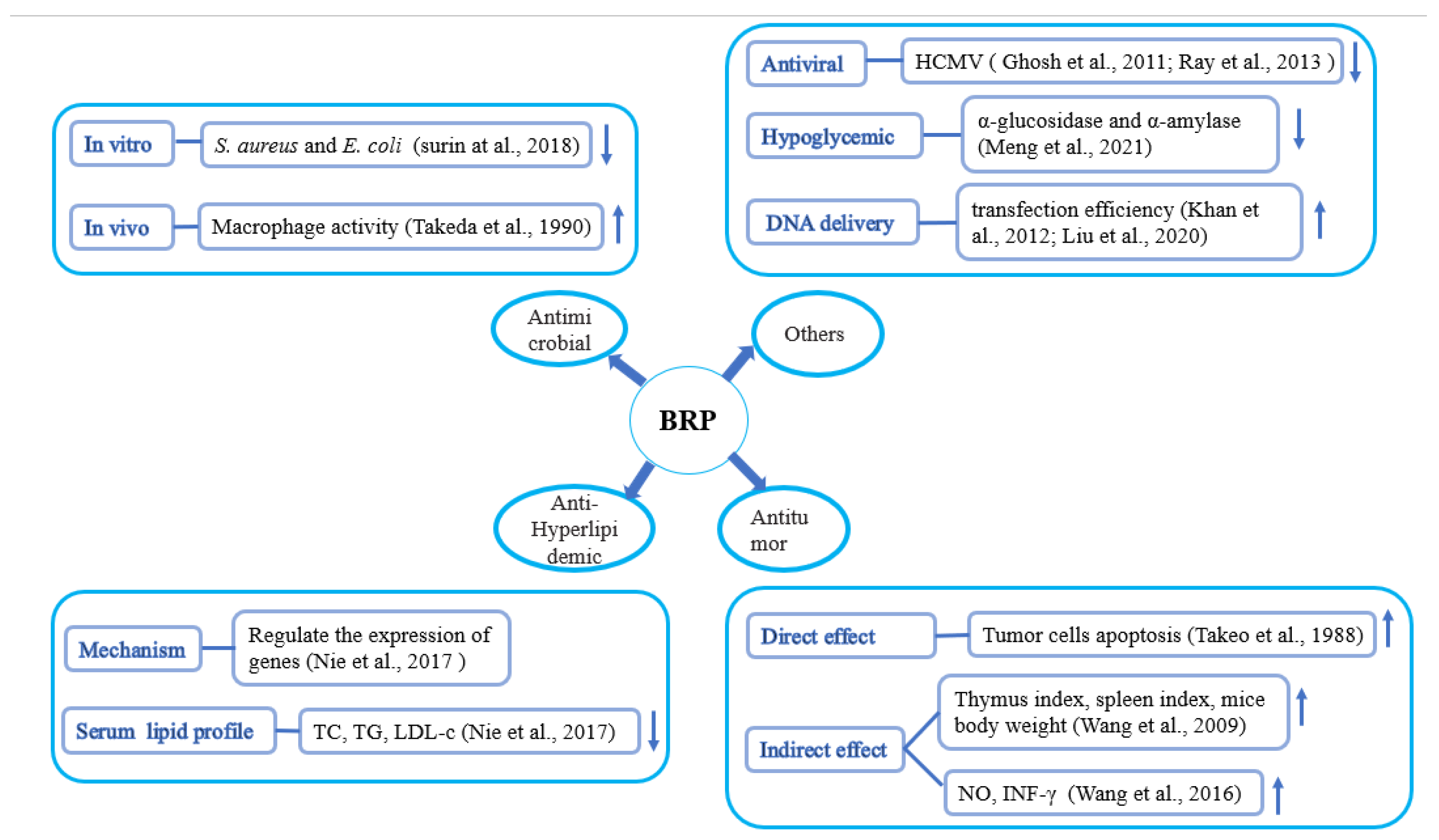
4. Pharmacological Effects of Rice Bran Polysaccharides
4.1. Antioxidant Activity
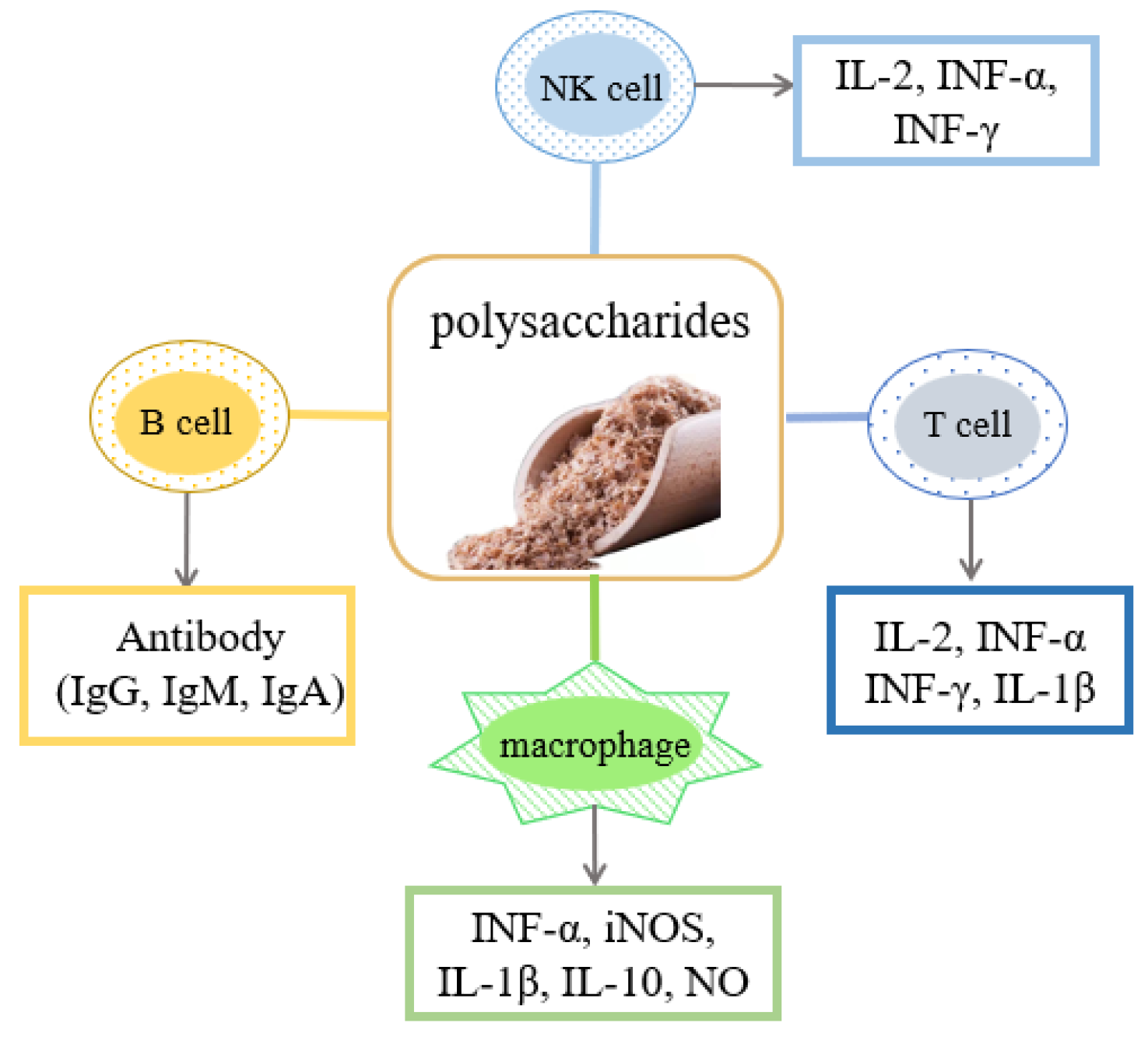
4.2. Immunomodulatory Activity
4.3. Antitumor Activity
4.4. DNA Delivery
4.5. Antibacterial/Antiviral Activity
4.6. Hypoglycemic
4.7. Hypolipidemic
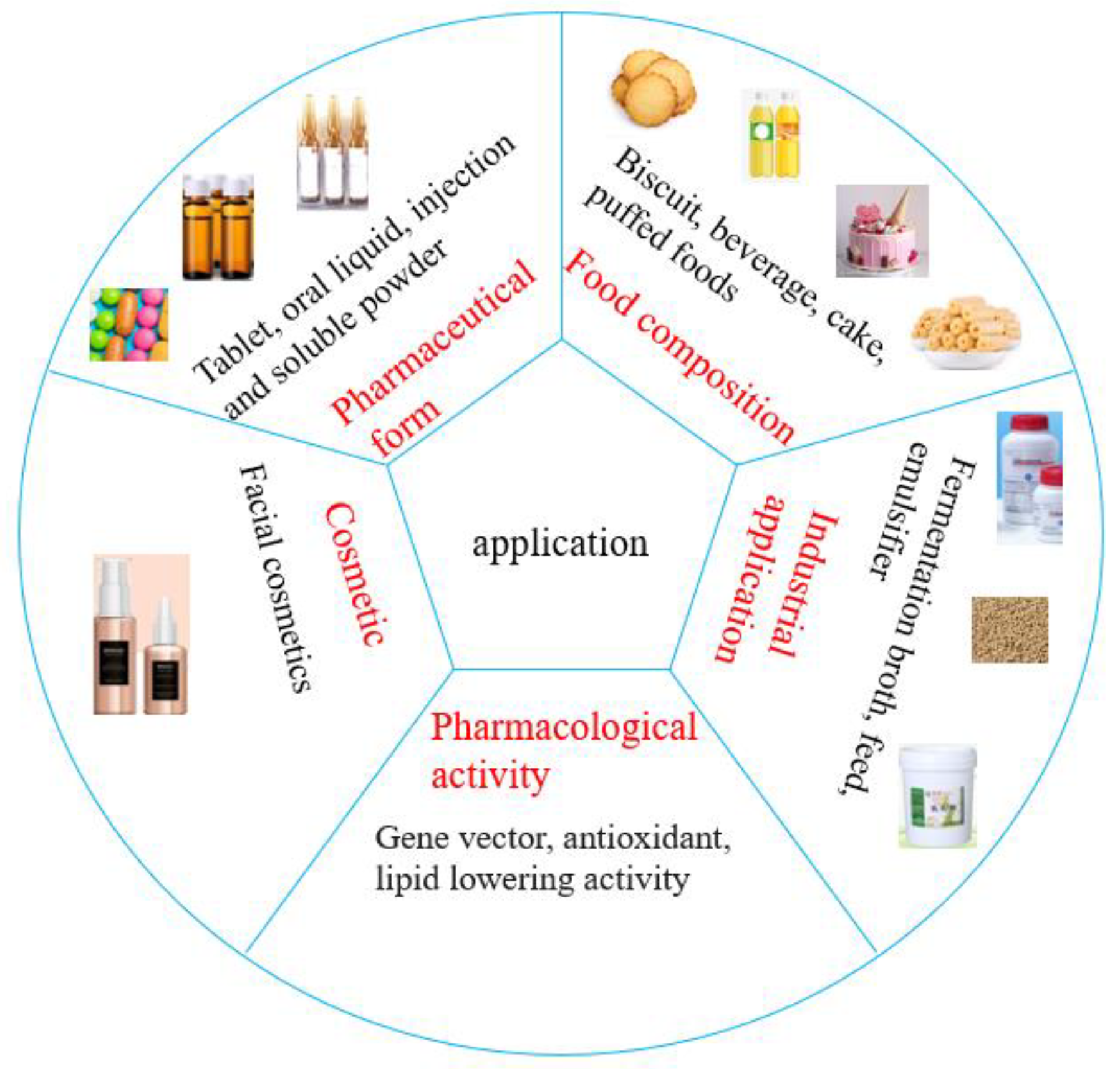
5. Application
5.1. Food Field
5.2. Industry Field
5.3. Medical Field
5.4. Other Fields
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gul, K.; Yousuf, B.; Singha, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for develop-ment of functional food-A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Park, H.-Y.; Lee, K.-W.; Choi, H.-D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L. Extraction and characterization of rice bran polysaccharides and their derivative preparation and antioxidant ac tivity. bioRxiv 2020. [CrossRef]
- Huang, S.Y.; Benchamas, G.; Huang, G.L. Whole processing and use of rice polishings. Innov. Food Sci. Emerg. Technol. 2020, 63, 102373. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.X.; Chen, Z.X. Sulfated modification of the polysaccharides obtained from defatted rice bran and their anti-tumor activities. Int. J. Biol. Macromol. 2009, 44, 211–214. [Google Scholar] [CrossRef]
- Zha, X.Q.; Luo, J.P.; Zhang, L.; Hao, J. Antioxidant properties of different polysaccharides extracted with water and sodium hydroxide from rice bran. Food Sci. Biotechnol. 2010, 18, 449–455. [Google Scholar]
- Surin, S.; Surayot, U.; Seesuriyachan, P.; You, S.G.; Phimolsiripol, Y. Antioxidant and immunomodulatory activities of sul-phated polysaccharides from purple glutinous rice bran (Oryza sativa L.). Int. J. Food Sci. Technol. 2018, 53, 994–1004. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.J.; Ni, D.; Wu, S.-H.; Chen, Y.-R.; Chen, X.; Xiong, X.M.; Liu, G. TAT-functionalized PEI-grafting rice bran poly saccharides for safe and efficient gene delivery. Int. J. Biol. Macromol. 2020, 146, 1076–1086. [Google Scholar] [CrossRef]
- Ooi, S.L.; Pak, S.C.; Micalos, P.S.; Schupfer, E.; Lockley, C.; Park, M.H.; Hwang, S.-J. The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme—A Narrative Review. Molecules 2021, 26, 2539. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.K.; Ming, K.; Wang, D.-Y.; Hu, Y.L.; Liu, J.G. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Ji, Y.-F.; Peng, L.-X.; Liu, Q.; Cao, H.-J.; Yang, Y.; He, X.-R.; Zeng, N. Extraction, purification, structural character-istics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, S.Y.; Liu, M.; Chen, F.X.; Yang, W.J.; Chang, X.N.; Liu, N.; Zhao, Y.Y.; Wang, J.; Chen, X.F. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohydr. Res. 2020, 494, 108037. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, J.X.; Diao, W.C.; Wang, C.G. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccha rides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef]
- Han, W.F.; Li, J.T.; Ding, Y.Q.; Xiong, S.B.; Zhao, S.M. Structural Features, Antitumor and Antioxidant Activities of Rice Bran Polysaccharides Using Different Extraction Methods. J. Food Sci. 2017, 82, 2403–2410. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Muthusamy, S.; Udayakumar, G.P.; Narala, V.R. Recent advances in the extraction and characterization of seed polysaccharides, and their bioactivities: A review. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100276. [Google Scholar] [CrossRef]
- Chen, X.X.; Yang, J.; Shen, M.Y.; Chen, Y.; Yu, Q.; Xie, J.H. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Surin, S.; You, S.G.; Seesuriyachan, P.; Muangrat, R.; Wangtueai, S.; Jambrak, A.R.; Phongthai, S.; Jantanasakulwong, K.; Chaiyaso, T.; Phimolsiripol, Y. Optimization of ultrasonic-assisted extraction of polysaccharides from purple glutinous rice bran (Oryza sativa L.) and their antioxidant activities. Sci. Rep. 2020, 10, 10410. [Google Scholar] [CrossRef]
- Optimization of extraction technology of rice bran polysaccharide by extrusion in conjunction with ultrasound. In Proceedings of the 2013 IEEE 11th International Symposium on Applied Machine Intelligence and Informatics (SAMI), Košice, Slovakia, 31 January–2 February 2013.
- Zhan, Y.F.; An, X.X.; Wang, S.; Sun, M.J.; Zhou, H.L. Basil polysaccharides: A review on extraction, bioactivities and pharmacological applications. Bioorg. Med. Chem. 2019, 28, 115179. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zheng, J.; Ye, X. Ultrasonic-assisted citrus pectin modification in the bicarbonate-activated hy-drogen peroxide system: Chemical and microstructural analysis. Ultrason. Sonochem. 2019, 58, 104576. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Wang, Y.-Y.; Qiu, W.-Y.; Ma, H.; Wang, Z.-B.; Wu, J.-Y. Three-phase partitioning as an elegant and versatile platform applied to nonchromatographic bioseparation processes. Crit. Rev. Food Sci. Nutr. 2017, 58, 2416–2431. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Geng, H.Y.; Chen, J.; Wang, X.; Lia, D.; Wang, T.; Yu, D.Y.; Wang, L.Q. Three phase partitioning for simultaneous extraction of oil, protein and polysaccharide from rice bran. Innov. Food Sci. Emerg. Technol. 2020, 65, 102447. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.P.; Zhang, Z.D.; Cai, W.L.; Flores, A.D.R. The preparation and structure analysis methods of natural polysac charides of plants and fungi: A review of recent development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Fabian, C.; Ayucitra, A.; Ismadji, S.; Ju, Y.-H. Isolation and characterization of starch from defatted rice bran. J. Taiwan Inst. Chem. Eng. 2011, 42, 86–91. [Google Scholar] [CrossRef]
- Surin, S.; Seesuriyachan, P.; Thakeow, P.; You, S.G.; Phimolsiripol, Y. Antioxidant and antimicrobial properties of polysaccha rides from rice brans. Chiang Mai J. Sci. 2018, 45, 1372–1382. [Google Scholar]
- Xun, Y.; Zhang, Y.Q. Removing protein and optimizing condition from rice bran polysaccharide. Hubei Agric. Sci. 2013, 20, 5014–5017. [Google Scholar]
- Wu, S.H.; Ni, D.N.; Yan, Y.J.; Pan, X.H.; Chen, X.; Guan, J.T.; Xiong, X.M.; Liu, L. Safe and efficient gene delivery based on rice bran polysaccharide. Int. J. Biol. Macromol. 2019, 137, 1041–1049. [Google Scholar] [CrossRef]
- Yang, L.-C.; Hsieh, C.-C.; Lin, W.-C. Characterization and immunomodulatory activity of rice hull polysaccharides. Carbohydr. Polym. 2015, 124, 150–156. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, X.-J.; Jia, H.-J.; Zhang, Y.; Wang, W.-H. New Polysaccharides from Rice Bran Fermented with Monascus anka and Auricularia auricular. In Medicine and Biopharmaceutical, Proceedings of the 2015 International Conference, Beijing, China, 15–16 August 2016; World Scientific: Singapore. [CrossRef]
- Ji, X.L.; Peng, B.X.; Ding, H.H.; Cui, B.B.; Nie, H.; Yan, Y.Z. Purification, structure and biological activity of pumpkin polysac charides: A Review. Food Rev. Int. 2021, 53, 1–13. [Google Scholar] [CrossRef]
- Hefnawy, H.T.M.; El-shourbagy, G.A. Chemical analysis and antioxidant activity of polysaccharide extracted from rice bran. World J. Dairy Food Sci. 2014, 9, 95–104. [Google Scholar]
- Wang, L.; Zhang, H.B.; Zhang, X.Y.; Chen, Z.X. Purification and identification of a novel heteropolysaccharide RBPS2a with anti-complementary activity from defatted rice bran. Food Chem. 2008, 110, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, S.Y.; Huang, G.L. Preparation, activity, and antioxidant mechanism of rice bran polysaccharide. Food Funct. 2021, 12, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.L.; Zhu, L.D.; Yin, R.; Wang, R.; Luo, X.H.; Li, Y.F.; Li, Y.N.; Chen, Z.X. Antitumor activities and immunomod ulatory of rice bran polysaccharides and its sulfates in vitro. Int. J. Biol. Macromol. 2016, 88, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yi, Y.L.; Guo, S.; Zhang, F.; Yan, H.; Zhan, Z.L.; Zhu, Y.; Duan, J.A. Isolation, structural characterization and bioac tivities of polysaccharides from Laminaria japonica: A review. Food Chem. 2022, 370, 131010. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Wang, Y.; Zhang, X.; Cao, L.Y.; Ji, J.J.; Zheng, Q.H.; Gao, J.P. Isolation and structural identification of a novel fructan from Radix Codonopsis. J. Carbohydr. Chem. 2020, 39, 163–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.F.; Liu, C.X.; Wang, X.; Chen, B.J.; Yao, L.M.; Qiao, Y.J.; Zheng, H.J. Recent developments in Stigma maydis polysaccharides: Isolation, structural characteristics, biological activities and industrial application. Int. J. Biol. Macromol. 2020, 150, 246–252. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, D.D.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. 2019, 59, 96–115. [Google Scholar] [CrossRef]
- Yamagishi, T.; Tsuboi, T.; Kikuchi, K. Potent natural immunomodulator, rice water-soluble polysaccharide fractions with an ticomplementary activity. Cereal Chem. 2003, 80, 5–8. [Google Scholar] [CrossRef]
- Shibuya, N.; Iwasaki, T. Structural features of rice bran hemicellulose. Phytochemistry 1985, 24, 285–289. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Zou, H.Y.; Huang, G.L.; Chen, G.Y. Preparations and antioxidant activities of sesamol and it’s derivatives. Bioorg. Med. Chem. Lett. 2021, 31, 127716. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-Q.; Wang, J.-H.; Yang, X.-F.; Liang, H.; Zhao, L.-L.; Bao, S.-H.; Luo, J.-P.; Xu, Y.-Y.; Zhou, B.-B. Antioxidant properties of polysaccharide fractions with different molecular mass extracted with hot-water from rice bran. Carbohydr. Polym. 2009, 78, 570–575. [Google Scholar] [CrossRef]
- Kang, J.; Jia, X.; Wang, N.F.; Xiao, M.; Song, S.; Wu, S.F.; Li, Z.J.; Wang, S.J.; Cui, S.W.; Guo, Q.B. Insights into the struc-ture-bioactivity relationships of marine sulfated polysaccharides: A review. Food Hydrocoll. 2022, 123, 107049. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.; Zhuang, X.; Han, W.; Guo, W.; Xiong, J.; Zhang, X. Rice bran polysaccharides and oligosaccharides modified by Grifola frondosa fermentation: Antioxidant activities and effects on the production of NO. Food Chem. 2017, 223, 49–53. [Google Scholar] [CrossRef]
- Pan, X.; Wu, S.; Yan, Y.; Chen, X.; Guan, J.; Bao, Y.; Xiong, X.; Liu, L. Rice bran polysaccharide-metal complexes showed safe antioxidant activity in vitro. Int. J. Biol. Macromol. 2019, 126, 934–940. [Google Scholar] [CrossRef]
- Pan, X.H.; Wu, S.H.; Yan, Y.J.; Chen, X.; Guan, J.T.; Bao, Y.P.; Xiong, X.M.; Liu, L. Enzymatic modification of rice bran polysac charides by enzymes from Grifola frondosa: Natural Killer Cell Cytotoxicity and Antioxidant Activity. J. Food Sci. 2018, 83, 1948–1955. [Google Scholar]
- He, X.R.; Fang, J.C.; Guo, Q.; Wang, M.; Li, Y.S.; Meng, Y.B.; Huang, L.H. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr. Polym. 2020, 229, 115548. [Google Scholar] [CrossRef]
- Yamagishi, T.; Yamaguchi, A.; Saito, T.; Seguchi, M. Complement-modulating water-soluble polysaccharide fractions in two global staple cereals: Rice and wheat. J. Sci. Food Agric. 2008, 88, 805–812. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.; Wei, Z.; Huang, F. Comparison on structural and immunomodulatory properties between polysaccharides from rice bran and soya bean. J. Chin. Cereals Oils Assoc. 2013, 28, 50–55. [Google Scholar]
- Li, Y.T.; Meng, S.L.; Shi, M.; Hu, X.S.; Yang, Y.N.; Zhang, Z.Y. Bioactivity Evaluation of Crude Polysaccharide from Rice Bran Fermented by Preussia aemulans and the Changes in its Nutritional Contents. J. Food Biochem. 2016, 40, 664–672. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Freitas, M.M.D.S.; Martins, L.; de Carvalho, R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydr. Polym. 2019, 229, 115550. [Google Scholar] [CrossRef] [PubMed]
- Saner, F.A.M.; Herschtal, A.; Nelson, B.H.; deFazio, A.; Goode, E.L.; Ramus, S.J.; Pandey, A.; Beach, J.A.; Fereday, S.; Ber-chuck, A.; et al. Going to extremes: Deter-minants of extraordinary response and survival in patients with cancer. Nat. Rev. Cancer. 2019, 19, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, M.; Nakamura, S.; Makita, F.; Ohwada, S.; Miyamoto, Y.; Morishita, Y. Antitumor effect of RBS (rice bran saccharide) on ENNG-induced carcinogenesis. Biotherapy 1992, 4, 139–145. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; Wei, Y.; Li, X.; Chen, Z. Characterization and anti-tumor activities of sulfated polysaccharide SRBPS2a obtained from defatted rice bran. Int. J. Biol. Macromol. 2009, 45, 427–431. [Google Scholar] [CrossRef]
- Tanigami, Y. Partial degradation and biological activities of an anti-tumor polysaccharide from rice bran. Chem. Pharm. Bull. 1991, 39, 1782–1787. [Google Scholar] [CrossRef]
- Takeo, S.; Kado, H.; Yamamoto, H.; Kamimura, M.; Watanabe, N.; Uchida, K.; Mori, Y. Studies on an antitumor polysac-charide RBS derived from rice bran. II. Preparation and general properties of RON, an active fraction of RBS. Chem. Pharm. Bull. 1988, 36, 3609–3613. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, G.J.; Wang, Z.R.; Kan, J.Q. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Shang, H.M.; Wu, H.Y.; Dong, X.Q.; Shi, X.Y.; Wang, X.S.; Tian, Y. Effects of different extraction methods on the properties and activities of polysaccharides from Medicago sativa L. and extraction condition optimization using response surface methodology. Process Biochem. 2019, 82, 179–188. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L.C. Sulfated modification, characterization, and potential bioactivities of polysac charide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Fan, S.T.; Huang, D.F.; Yu, Q.; Liu, X.Z.; Li, C.; Wang, S.; Xiong, T.; Nie, S.P.; Xie, M.Y. Effect of Lactobacillus plantarum NCU116 fermentation on Asparagus officinalis polysaccharide: Characterization, antioxidative, and immuno-regulatory activities. J. Agric. Food Chem. 2018, 66, 10703–10711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Fan, S.T.; Huang, D.F.; Xiong, T.; Nie, S.P.; Xie, M.Y. Polysaccharides from fermented Asparagus officinalis with Lactobacillus plantarum NCUll6 alleviated liver injury via modulation of glutathione homeostasis, bile acid metabolism, and SCFA production. Food Funct. 2020, 11, 7681–7695. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chen, H.J.; Zhou, L.; Zou, H.J.; Luo, X.H.; Sun, B.; Zhuang, X.H. Polysaccharides from Ganoderma sinense—Rice bran fermentation products and their anti-tumor activities on non-small-cell lung cancer. BMC Complement. Med. Ther. 2021, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, J.-H.; Yang, S.-B.; Hong, S.-G.; Lee, S.-A.; Hwang, S.-J.; Shin, K.-S.; Suh, H.-J.; Park, M.-H. A Polysaccharide Extracted from Rice Bran Fermented with Lentinus edodes Enhances Natural Killer Cell Activity and Exhibits Anticancer Effects. J. Med. Food 2007, 10, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Halioua-Haubold, C.-L.; Jolly, J.K.; Smith, J.A.; Pinedo-Villanueva, R.; Brindley, D.A.; MacLaren, R.E. Potential lifetime quality of life benefits of choroideremia gene therapy: Projections from a clinically informed decision model. Eye 2019, 33, 1215–1223. [Google Scholar] [CrossRef]
- Bansal, R.; Seth, B.; Tiwari, S.K.; Jahan, S.; Kumari, M.; Pant, A.B.; Chaturvedi, R.K.; Kumar, P.; Gupta, K.C. Hexadecylated linear PEI self-assembled nanostructures as efficient vectors for neuronal gene delivery. Drug Deliv. Transl. Res. 2018, 8, 1436–1449. [Google Scholar] [CrossRef]
- Rong, Y.; Yang, R.; Yang, Y.; Wen, Y.; Liu, S.; Li, C.; Hu, Z.; Cheng, X.; Li, W. Structural characterization of an active polysac charide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef]
- Huang, Y.J.; Hu, H.; Li, R.Q.; Yu, B.R.; Xu, F.J. Versatile types of MRI-Visible cationic nanoparticles involving pullulan poly saccharides for multifunctional gene carriers. ACS Appl. Mater. Inter. 2016, 83, 919–3927. [Google Scholar]
- Khan, W.; Hosseinkhani, H.; Ickowicz, D.; Hong, P.-D.; Yu, D.-S.; Domb, A.J. Polysaccharide gene transfection agents. Acta Biomater. 2012, 8, 4224–4232. [Google Scholar] [CrossRef]
- Liu, L.; Ni, D.N.; Yan, Y.J.; Wu, S.H.; Chen, X.; Guan, J.T.; Xiong, X.M.; Liu, G. Development of a novel DNA delivery system based on rice bran polysaccharide-Fe(III) complexes. Int. J. Biol. Macromol. 2020, 142, 600–608. [Google Scholar] [CrossRef]
- Takeda, Y.; Yoshikai, Y.; Ohga, S.; Nomoto, K. Augmentation of host defense against infection pretreated intraperitoneally with an α-glucan RBS in mice. Immunopharmacol. Immunotoxicol. 1990, 12, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Auerochs, S.; Saha, S.; Ray, B.; Marschall, M. Anti-Cytomegalovirus Activity of Sulfated Glucans Generated from a Commercial Preparation of Rice Bran. Antivir. Chem. Chemother. 2011, 21, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Hutterer, C.; Bandyopadhyay, S.S.; Ghosh, K.; Chatterjee, U.R.; Ray, S.; Zeitträger, I.; Wagner, S.; Marschall, M. Chemically Engineered Sulfated Glucans from Rice Bran Exert Strong Antiviral Activity at the Stage of Viral Entry. J. Nat. Prod. 2013, 76, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Takahashi, M.; Oshima, Y.; Konno, C. Isolation and Hypoglycemic Activity of Oryzabrans A, B, C and D, Glycans of Oryza sativa Bran. Planta Med. 1988, 54, 1–3. [Google Scholar] [CrossRef]
- Meng, J.Q.; Xu, P.P.; Gu, W.T.; Wang, Q.; Sun, H.Y.; Xue, Y.T. Impacts of extraction methods on physicochemical charac-teristics and bioactivities of polysaccharides from rice bran. J. Food Meas. Charact. 2021, 16, 1137–1145. [Google Scholar] [CrossRef]
- Hu, G.H.; Yu, W.J. Binding of cholesterol and bile acid to hemicelluloses from rice bran. Int. J. Food Sci. Nutr. 2013, 64, 461–466. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F.; Wang, L.; Yang, T.; Shi, L.; Li, X.; Shen, J.; Xu, W.; Guo, T.; Lin, Q. Anti-hyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice. Food Funct. 2017, 8, 4028–4041. [Google Scholar] [CrossRef]
- Issara, U.; Rawdkuen, S. Rice bran: A potential of main ingredient in healthy beverage. Int. Food Res. J. 2016, 23, 2306–2318. [Google Scholar]
- Faccin, G.L.; Miotto, L.A.; Vieira, L.D.N.; Barreto, P.L.M.; Amante, E.R. Chemical, Sensorial and Rheological Properties of a New Organic Rice Bran Beverage. Rice Sci. 2009, 16, 226–234. [Google Scholar] [CrossRef]
- Hu, G.H.; Ma, Z.Z.; Wu, B. Health care function of rice bran polysaccharide. In Proceedings of the 3rd National Japonica Rice Industry Conference, Changchun, China, 12–14 August 2008. [Google Scholar]
- Park, K.M.; Kim, Y.N.; Choi, S.J.; Park, J.H.; Chang, P.S. Chemoselective oxidation of C6 primary hydroxyl groups of polysac charides in rice bran for the application as a novel water-soluble dietary fiber. Int. J. Food Prop. 2015, 18, 1664–1676. [Google Scholar] [CrossRef]
- Yang, T.; Liu, S.G.; Lin, Q.L.; Liu, B.; Wu, J.; Lan, C.H.; Zhu, H.; Wu, X.X. Extracting Method of Polysaccharide of Rice Polishing and Biscuit Produced by Same and Method. Br. Patent CN109608560, 12 April 2019. [Google Scholar]
- Zhang, T.; Zhang, T.; Zhang, Y.X. Pomegranate Fruit Vinegar Beverage Containing Rice Bran Polysaccharides and Preparation Method of Beverage. Br. Patent CN106281943, 4 January 2017. [Google Scholar]
- Su, Y.; Huang, L.; Fu, X.K.; Xu, T.H. Rice Bran Dietary-Fiber Cake and Making Method Thereof. Br. Patent CN110250419, 20 January 2019. [Google Scholar]
- Cao, L.S.; Deng, F.; Yao, J.; Fu, T.T.; Yang, J.N.; Lan, X.D.; Chen, Z. Multiple-Grain Puffed Foods and Making Method Thereof. Br. Patent CN109363084, 22 February 2019. [Google Scholar]
- Xing, X.D. Preparation Method of Crayfish Feed with Rice Bran Polysaccharide. Br. Patent CN108041341, 18 May 2018. [Google Scholar]
- Zheng, L.; Duarte, M.E.; Park, I.; Kim, S.W. 159 Supplemental effects of fermented rice bran extracts on growth performance, bone characteristics, and immune response of broiler chickens. J. Anim. Sci. 2017, 95, 75–76. [Google Scholar] [CrossRef]
- Zheng, L.; Duarte, M.E.; Park, I.; Kim, S.W. Supplemental effects of fermented rice bran extracts on gut health and growth of nursery pigs. J. Anim. Sci. 2017, 95, 109. [Google Scholar] [CrossRef]
- Cao, M.; Nie, Y.Y.; Yan, L.; Zhao, J.; Zhu, J.Q. Tricholoma Matsutake Polysaccharose by Utilizing High-Yield Selenium-Rich Function Hybridization Red Rice Bran Self-Fermentation Broth. Br. Patent CN101748153, 6 June 2012. [Google Scholar]
- Fujii, N.; Tobe, J.; Nakamura, A. Water-Soluble Polysaccharides Originating in Rice Bran, Method of Producing the same and Emulsifier Using the same. Br. Patent WO2007086389, 2 August 2007. [Google Scholar]
- Huang, W.J.; Wu, S.; Shen, W.Y.; Hu, Z.Z.; Wang, Z. Acid-Resistant and Salt-Resistant Glycosylated Protein-Rice Bran Polysac-charide Emulsifier and Preparation Method Thereof. Br. Patent CN113951498, 21 January 2022. [Google Scholar]
- Liu, L.; Wu, S.H.; Chen, X.; Xiong, X.M. Preparation Method of Spermine Grafted Rice Bran Polysaccharide and Gene Vector. Br. Patent CN109912727, 21 June 2019. [Google Scholar]
- Liu, L.; Ni, D.N.; Chen, X.; Xiong, X.M. Preparation Method of Polyethylenimine Grafted Rice Bran Polysaccharide Iron and Gene Vector. Br. Patent CN109880108, 14 June 2019. [Google Scholar]
- Liu, L.; Ni, D.N.; Chen, X.; Xiong, X.M. Polyethyleneimine grafted rice bran polysaccharide, and preparation method and gene vector thereof. Br. Patent CN 109897187, 18 June 2019. [Google Scholar]
- Liu, L.; Pan, X.H.; Chen, X.; Liao, R.; Xiong, X.M. Preparation Method for Rice Bran Polysaccharide Metal Complex, and Antiox idant. Br. Patent CN109879980, 14 June 2019. [Google Scholar]
- Xie, M.Y.; Liu, H.; Gao, L.H.; Fu, A.P. Rice Bran Polysaccharide-Peptide Compound with High Oxidation Resistance and Prepa ration Method Thereof. Br. Patent CN 113603799, 5 November 2021. [Google Scholar]
- Liu, L.; Yan, Y.J.; Wu, S.H.; Pan, X.H.; Xiong, X.M. Antioxidant and Preparation Method Thereof. Br. Patent CN 109879979, 14 June 2019. [Google Scholar]
- Liu, Q.; Wei, T.; Fan, Y.C.; Liu, S.S.; Zhao, J.Y. Rice Bran Polysaccharide with Lipid Lowering Activity and Preparation Method of Rice Bran Polysaccharide. Br. Patent CN111718971, 29 September 2018. [Google Scholar]
- Huang, W.J.; Cai, Q.Y.; Zhou, J.; Shen, W.Y.; Lu, Q.Y.; Hu, Z.Z. Preparation Method of pH-Responsive Carrier, Drug Delivery System and Preparation Method of Drug Delivery System. Br. Patent CN 111419824, 17 July 2020. [Google Scholar]
- Hong, C.L. A Compound rice bran Polysaccharide Buccal Tablet and a Preparing Method Thereof. Br. Patent CN107041552, 15 August 2017. [Google Scholar]
- Niu, Z.Y.; Wang, X.; Shi, X.F.; Wei, K.P.; Zhu, X.J.; Wang, Z.X.; Gao, C.H.; Qiu, C.Z. Rice Bran Polysaccharide Oral Liquid, Injection and Soluble Powder and Corresponding Preparation Method. Br. Patent CN104306391, 28 January 2015. [Google Scholar]
- Zhang, H.J.; Wang, J.; Cao, X.R. Preparation Method of Rice Bran Flour with high Antioxidization Property. Br. Patent CN109221920, 18 January 2018. [Google Scholar]
- Huang, W.J.; Cai, Q.Y.; Zhou, J.; Shen, W.Y.; Lu, Q.Y.; Hu, Z.Z. Preparation Method of Rice Bran Polysaccharide-Based Composite Nanoparticles. Br. Patent CN111437267, 24 July 2020. [Google Scholar]
- Liu, L.; Wu, S.H.; Pan, X.H.; Yu, H.Y.; Yan, Y.J.; Ni, D.N.; Guan, J.T.; Chen, X. Preparation Method and Applications of Fluores Cently-Labeled Rice Bran Polysaccharide Iron Complex. Br. Patent CN109897118, 18 June 2019. [Google Scholar]
- Li, F. Modified Rice Bran Polysaccharide Synergistic Nano-Titanium Dioxide Microemulsion Additive for Cosmetics. Br. Patent CN106726696, 31 May 2017. [Google Scholar]
- Yazan, A.T.; Aishah, S.; Ayah, K.; Dalal, A.; Dimah, S.; Ghadir, K.; Hiba, S.; Hussein, A.Q.; Joudy, F.; Salam, A.A. Chapter 7-Instrumental analytical techniques for physicochemical characterization of bio-nanomaterials. In Handbook on Nanobiomaterials for Therapeutics and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 133–150. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, H.; Liang, X.D.; Zhang, M.T.; Tang, Y.X.; Wang, J.H.; Mao, J.L. The isolation, structural features and biological activities of polysaccharide from Ligusticum chuanxiong: A review. Carbohydr. Polym. 2022, 285, 118971. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Yu, Y.G.; Li, Y.; Yin, Z.Y.; Liu, X.M.; Lian, B. Molecular mechanism of increasing extracellular polysaccharide production of Paenibacillus mucilaginosus K02 by adding mineral powders. Int. Biodeterior. Biodegrad. 2022, 167, 105340. [Google Scholar] [CrossRef]

| Polysaccharide Fraction | Extraction, Separation, and Purification Procedures | Mw (KDa) | Monosaccharide Composition | Structure Features | Structural Characterization Method | Reference |
|---|---|---|---|---|---|---|
| RBP | Hot water extraction, DEAE-52 ion exchange chromatography, dextran gel tomography | 488 | Glu:Xyl:Gal:Ara = 1.669:1:0.225:1.228 | α-D-glycoside bond. | HPLC, FTIR, NMR, | [30] |
| RHPS | Hot distilled water (90–95 °C, 5 h), DEAE column | 77 | Ara:Gal:Glu:Man:Xyl = 10:44.8:29.8:9.3:6.1 | (1 → 3)-Gal as backbone | HPAEC-PAD, HP-SEC, NMR, GC-MS | [31] |
| M-50-A | Hot water extraction, DEAE Sephadex A-25 anion-exchange and Sephadex G-75 gel-permeation chromatography | 110 | Ara:Xyl:Glu:Man: Gal = 1.7:7.2:1:11:1.4 | α-D-pyranoid polysaccharide and alpha-residues | HPGPC, GC, FT-IR | [32] |
| A-50-G | Hot water extraction, DEAE Sephadex A-25 anion-exchange and Sephadex G-75 gel-permeation chromatography | 14 | Ara:Xyl:Glu:Man: Gal = 1.2:1.5:1:11.1:2.4, | α-D-pyranoid polysaccharide and alpha-residues | HPGPC, GC, FT-IR | [32] |
| RBP1, RBP2 and RBP3 | Distilled water (50–60 °C, 1 h) DEAE-Cellulose and Sephadex G-200 column chromatography | 51.5, 26.1 and 6.95 | GPC, GC-MS | [34] | ||
| RBPS2a | Hot water extraction | 90 | Ara:Xyl:Glu:Gal = 4:2:1:4 | β-(1 → 3)-linked d-galactopyranosyl residues substituted at O-2 with glycosyl residues composed of α-d-xylose–(1 → 4)-α-d-arabinose (1 → and α-d-glucose–(1 → 4)-α-d-arabinose (1 → linked residues | HPGPC, GC, FT-IR, NMR | [35] |
| Rice bran polysaccharide | Hot distilled water (100 °C, 5 h), Sephadex G100 column chromatography | α-1,4 and α-1,6 glycosidic bonds | FT-IR, NMR | [36] | ||
| RBP2 | Water extraction (90 °C, 2 h) | Gal:Ara:Rha:Glu:Xyl:Man = 41.51:22.58:12.89:9.56:7.01:3.75 | Arabingalactan in its main chain and a xylose polymer in its side chain | GC, NMR | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Qiao, Y.; Wang, X.; Zhang, Y.; Fu, L. Extraction, Structural Characterization, Biological Functions, and Application of Rice Bran Polysaccharides: A Review. Foods 2023, 12, 639. https://doi.org/10.3390/foods12030639
Chen B, Qiao Y, Wang X, Zhang Y, Fu L. Extraction, Structural Characterization, Biological Functions, and Application of Rice Bran Polysaccharides: A Review. Foods. 2023; 12(3):639. https://doi.org/10.3390/foods12030639
Chicago/Turabian StyleChen, Bingjie, Yongjin Qiao, Xiao Wang, Yi Zhang, and Linglin Fu. 2023. "Extraction, Structural Characterization, Biological Functions, and Application of Rice Bran Polysaccharides: A Review" Foods 12, no. 3: 639. https://doi.org/10.3390/foods12030639






