Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Castanea Sativa Shells
2.3. Preparation of Chestnut Shells Extract by Subcritical Water Extraction
2.4. Preparation of Cookies Enriched with Chestnut Shells Extract
2.5. Approximate Composition
2.6. Extraction of Bioactive Compounds from Cookies
2.7. Total Phenolic and Flavonoid Contents and In Vitro Bioactivity
2.8. Reactive-Oxygen- and Nitrogen-Species-Counteracting Activity
2.8.1. Superoxide Anion Radical Quenching Assay
2.8.2. Hydrogen Peroxide Quenching Assay
2.8.3. Hypochlorous Acid Quenching Assay
2.8.4. Peroxyl Radical Quenching Assay
2.8.5. Peroxynitrite Quenching Assay
2.9. Phenolic Composition by HPLC-PDA
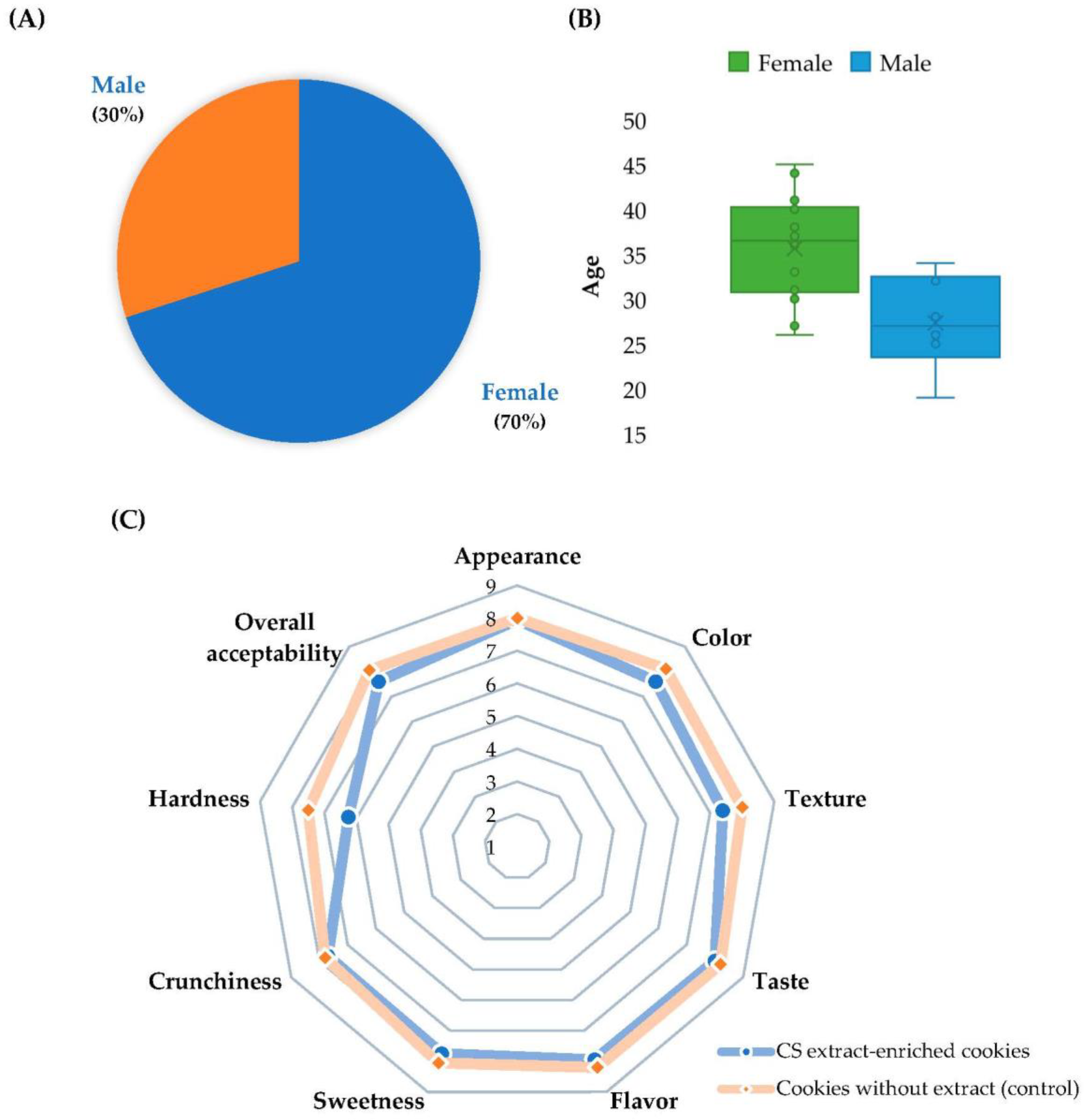
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Approximate Composition of Functional Cookies
3.2. Phenolic Content and In Vitro Bioactivity
3.3. Reactive-Oxygen- and Nitrogen-Species-Counteracting Potential
3.4. Phenolic Composition of Functional Cookies
3.5. Sensory Evaluation of Functional Cookies
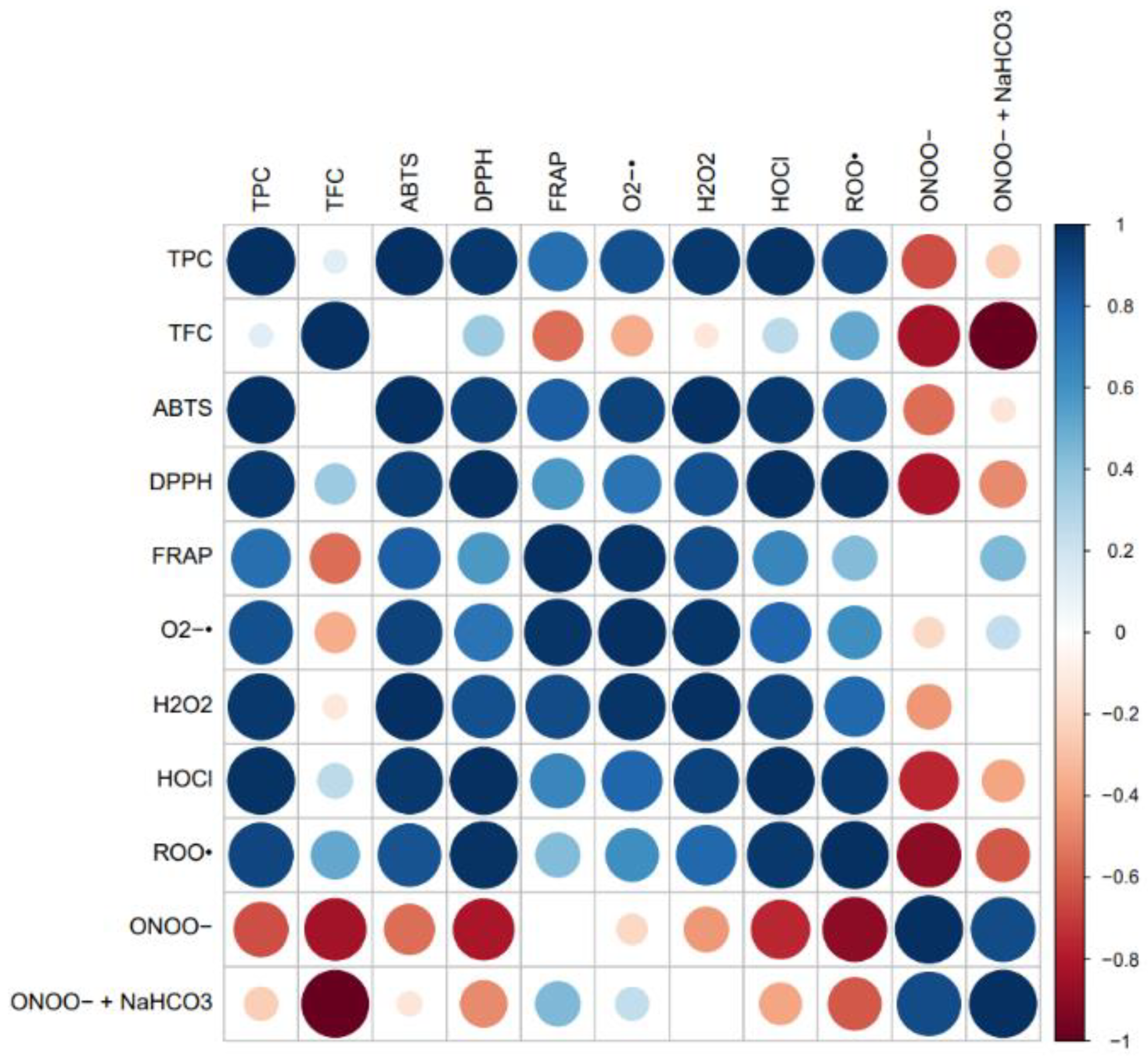
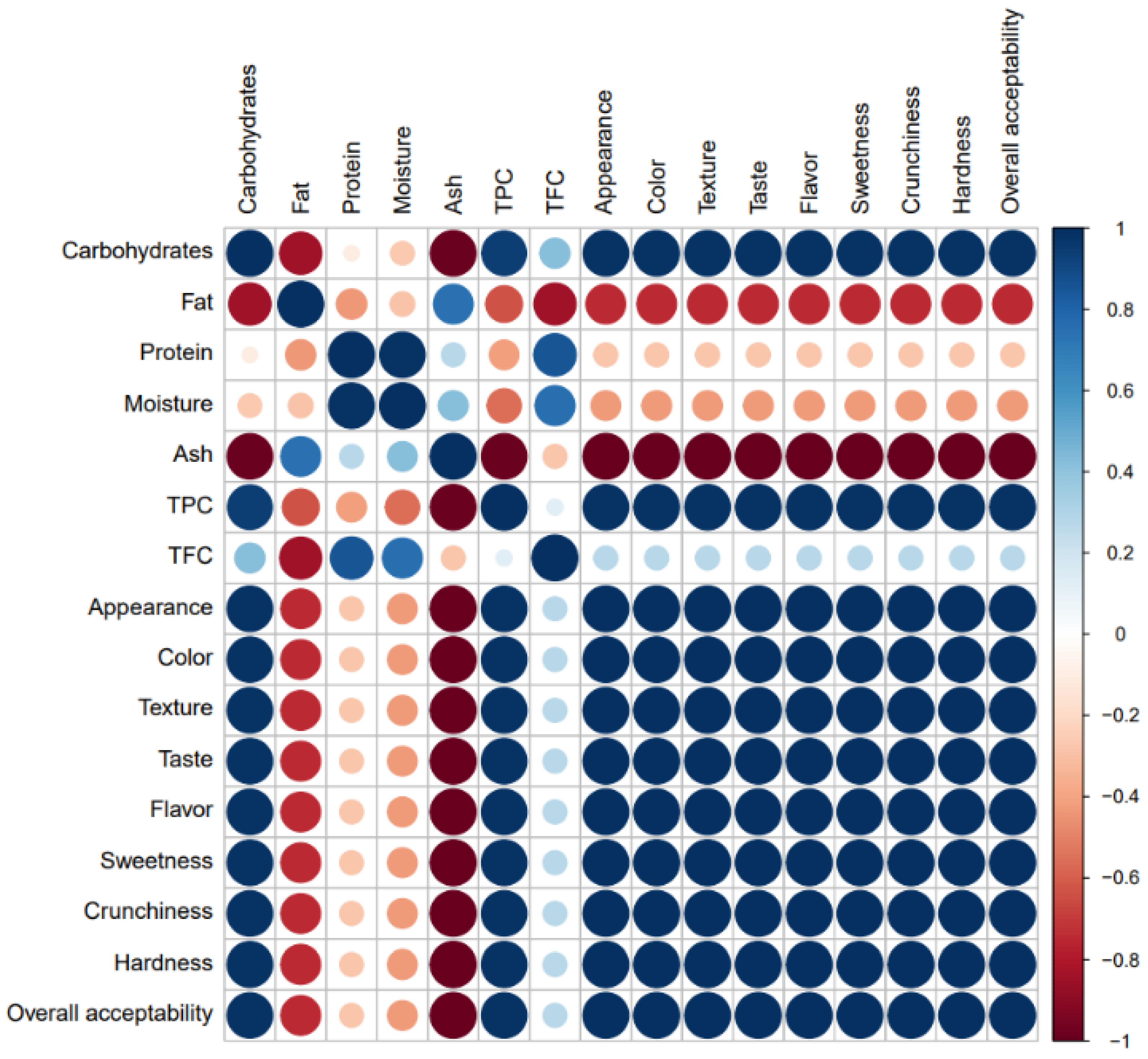
3.6. Correlation Analyses
3.6.1. Phenolic Compounds and Antioxidant/Antiradical Activities
3.6.2. Nutritional Composition, Phenolic Content, and Sensory Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, D.; Cádiz-Gurrea, M.D.L.L.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: A review on phytochemical composition, bioactivity and waste management approaches for industrial valorization. Food Res. Int. 2021, 144, 110364. [Google Scholar] [CrossRef] [PubMed]
- Luvián-Morales, J.; Varela-Castillo, F.O.; Flores-Cisneros, L.; Cetina-Pérez, L.; Castro-Eguiluz, D. Functional foods modulating inflammation and metabolism in chronic diseases: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4371–4392. [Google Scholar] [CrossRef]
- Lucini Mas, A.; Brigante, F.I.; Salvucci, E.; Ribotta, P.; Martinez, M.L.; Wunderlin, D.A.; Baroni, M.V. Novel cookie formulation with defatted sesame flour: Evaluation of its technological and sensory properties. Changes in phenolic profile, antioxidant activity, and gut microbiota after simulated gastrointestinal digestion. Food Chem. 2022, 389, 133122. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) Data Statistics. Available online: https://www.fao.org/faostat/en/#home (accessed on 15 September 2022).
- de Vasconcelos, M.d.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-sustainable recovery of phenolic and antioxidant compounds from industrial chestnut shells using ultrasound-assisted extraction: Optimization and evaluation of biological activities in vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef]
- Rodrigues, F.; Santos, J.; Pimentel, F.B.; Braga, N.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P.P. Promising new applications of Castanea sativa shell: Nutritional composition, antioxidant activity, amino acids and vitamin E profile. Food Funct. 2015, 6, 2854–2860. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Garcia, J.; Saavedra, M.J.; Freitas, V.; Costa, P.; Sarmento, B.; Delerue-Matos, C.; Rodrigues, F. From soil to cosmetic industry: Validation of a new cosmetic ingredient extracted from chestnut shells. Sustain. Mater. Technol. 2021, 29, e00309. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-assisted extraction as a green technology approach to recover polyphenols from Castanea sativa shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Pinto, D.; Almeida, A.; López-Yerena, A.; Pinto, S.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Appraisal of a new potential antioxidants-rich nutraceutical ingredient from chestnut shells through in-vivo assays—A targeted metabolomic approach in phenolic compounds. Food Chem. 2023, 404, 134546. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Sut, S.; Ferreira, A.S.; Leyva-Jimenez, F.J.; Dall’Acqua, S.; Segura-Carretero, A.; Delerue-Matos, C.; Rodrigues, F. Valorisation of underexploited Castanea sativa shells bioactive compounds recovered by supercritical fluid extraction with CO2: A response surface methodology approach. J. CO2 Util. 2020, 40, 101194. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Sigolo, S.; Fortunati, P.; Lucini, L.; Gallo, A. Exploitation of alfalfa seed (Medicago sativa L.) flour into gluten-free rice cookies: Nutritional, antioxidant and quality characteristics. Food Chem. 2018, 239, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Borrás-Linares, I.; Mašković, P. Characterisation of ginger extracts obtained by subcritical water. J. Supercrit. Fluids 2017, 123, 92–100. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Najjar, Z.; Kizhakkayil, J.; Shakoor, H.; Platat, C.; Stathopoulos, C.; Ranasinghe, M. Antioxidant potential of cookies formulated with date seed powder. Foods 2022, 11, 448. [Google Scholar] [CrossRef]
- Pinto, D.; Reis, J.; Silva, A.M.; Salazar, M.; Dall’Acqua, S.; Delerue-Matos, C.; Rodrigues, F. Valorisation of Salicornia ramosissima biowaste by a green approach—An optimizing study using response surface methodology. Sustain. Chem. Pharm. 2021, 24, 100548. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- ISO 11136:2014; Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. ISO (International Organization for Standardization): Geneva, Switzerland, 2014.
- ISO 11136:2014/AMD 1:2020; Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area—Amendment 1. ISO (International Organization for Standardization): Geneva, Switzerland, 2020.
- Nakov, G.; Jukić, M.; Šimić, G.; Šumanovac, F.; Komlenić, D.K.; Lukinac, J. Effect of the addition of hulless barley flour on the quality of short-dough cookies. Foods 2022, 11, 2428. [Google Scholar] [CrossRef]
- Filipović, V.; Lončar, B.; Filipović, J.; Nićetin, M.; Knežević, V.; Šeregelj, V.; Košutić, M.; Solarov, M.B. Addition of combinedly dehydrated peach to the cookies—Technological quality testing and optimization. Foods 2022, 11, 1258. [Google Scholar] [CrossRef]
- Desai, N.M.; Mallik, B.; Sakhare, S.D.; Murthy, P.S. Prebiotic oligosaccharide enriched green coffee spent cookies and their nutritional, physicochemical and sensory properties. LWT 2020, 134, 109924. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Silva, A.M.; Pinto, D.; Moreira, M.M.; Ferraz, R.; Švarc-Gajić, J.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. New perspectives on the sustainable employment of chestnut shells as active ingredient against oral mucositis: A first screening. Int. J. Mol. Sci. 2022, 23, 14956. [Google Scholar] [CrossRef]
- Uriarte-Frías, G.; Hernández-Ortega, M.M.; Gutiérrez-Salmeán, G.; Santiago-Ortiz, M.M.; Morris-Quevedo, H.J.; Meneses-Mayo, M. Pre-hispanic foods oyster mushroom (Pleurotus ostreatus), nopal (Opuntia ficus-indica) and amaranth (Amaranthus sp.) as new alternative ingredients for developing functional cookies. J. Fungi 2021, 7, 911. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Lombardi, S.; Gaspari, A.; Rubino, M.; Izzo, L.; Narváez, A.; Ritieni, A.; Grosso, M. In vitro bioaccessibility and antioxidant activity of polyphenolic compounds from spent coffee grounds-enriched cookies. Foods 2021, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Argyri, E.-A.; Piromalis, S.-P.; Koutelidakis, A.; Kafetzopoulos, D.; Petsas, A.S.; Skalkos, D.; Nasopoulou, C.; Dimou, C.; Karantonis, H.C. Olive paste-enriched cookies exert increased antioxidant activities. Appl. Sci. 2021, 11, 5515. [Google Scholar] [CrossRef]
- Šťastná, K.; Sumczynski, D.; Yalcin, E. Nutritional composition, in vitro antioxidant activity and phenolic profile of shortcrust cookies supplemented by edible flowers. Foods 2021, 10, 2531. [Google Scholar] [CrossRef] [PubMed]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; De Lucia, A.; Colucci, G.; La Cara, F.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process. Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Karimi-Khouzani, O.; Heidarian, E.; Amini, S.A. Anti-inflammatory and ameliorative effects of gallic acid on fluoxetine-induced oxidative stress and liver damage in rats. Pharmacol. Rep. 2017, 69, 830–835. [Google Scholar] [CrossRef]
- Usta, C.; Ozdemir, S.; Schiariti, M.; Puddu, P.E. The pharmacological use of ellagic acid-rich pomegranate fruit. Int. J. Food Sci. Nutr. 2013, 64, 907–913. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Liang, J.; Ou, M.; Chen, J.; Liet, G. Extraction, purification and anti-radiation activity of persimmon tannin from Diospyros kaki L.f. J. Environ. Radioact. 2016, 162, 182–188. [Google Scholar] [CrossRef]
- Ulvia, B.R.; Andarwulan, N.; Hunaefi, D. Profile of bioactive compounds and antioxidant capacity of Indonesian cocoa Powder: A case of food processing authentication. In Proceedings of the 2nd SEAFAST International Seminar (2nd SIS 2019)—Facing Future Challenges: Sustainable Food Safety, Quality and Nutrition, Bogor, Indonesia, 4–5 September 2019; pp. 97–105. [Google Scholar]
- Ali, F.; Ranneh, Y.; Ismail, A.; Mohd Esa, N. Identification of phenolic compounds in polyphenols-rich extract of Malaysian cocoa powder using the HPLC-UV-ESI-MS/MS and probing their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2103–2111. [Google Scholar] [CrossRef]
- Bucalossi, G.; Fia, G.; Dinnella, C.; De Toffoli, A.; Canuti, V.; Zanoni, B.; Servili, M.; Pagliarini, E.; Toschi, T.G.; Monteleone, E. Functional and sensory properties of phenolic compounds from unripe grapes in vegetable food prototypes. Food Chem. 2020, 315, 126291. [Google Scholar] [CrossRef]
- Wang, H.; Chambers, E. Sensory characteristics of various concentrations of phenolic compounds potentially associated with smoked aroma in foods. Molecules 2018, 23, 780. [Google Scholar] [CrossRef] [PubMed]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef] [PubMed]
- Pittari, E.; Piombino, P.; Andriot, I.; Cheynier, V.; Cordelle, S.; Feron, G.; Gourrat, K.; Le Quéré, J.-L.; Meudec, E.; Moio, L.; et al. Effects of oenological tannins on aroma release and perception of oxidized and non-oxidized red wine: A dynamic real-time in-vivo study coupling sensory evaluation and analytical chemistry. Food Chem. 2022, 372, 131229. [Google Scholar] [CrossRef] [PubMed]
- Abu-Salem, F.M.; Abou-Arab, A.A. Effect of supplementation of Bambara groundnut (Vigna subterranean L.) flour on the quality of biscuits. Afr. J. Food Sci. 2011, 5, 376–383. [Google Scholar]

| Ingredients | Amount (%) | |
|---|---|---|
| Functional Cookies Enriched with CS Extract | Control Cookies (Without Extract) | |
| Sugar | 32.20 | 32.20 |
| White flour | 29.40 | 29.40 |
| Butter | 20.70 | 20.70 |
| Eggs (approx. 2) | 7.80 | 7.80 |
| Cocoa powder | 7.08 | 7.08 |
| Lyophilized chestnut shells extract | 1.77 | − |
| Distilled water | − | 1.77 |
| Sodium bicarbonate (NaHCO3) | 0.71 | 0.71 |
| Sodium chloride (NaCl) | 0.34 | 0.34 |
| Approximate Composition | Content (% dw) | |
|---|---|---|
| Functional Cookies Enriched with CS Extract | Control Cookies (Without Extract) | |
| Carbohydrates | 53.92 ± 2.67 a | 59.73 ± 0.34 a |
| Fat | 32.62 ± 2.83 a | 25.04 ± 0.21 b |
| Protein | 6.17 ± 0.57 a | 7.59 ± 0.54 a |
| Fiber | 5.15 ± 0.05 a | 4.96 ± 0.04 a |
| Moisture | 4.77 ± 0.96 a | 3.95 ± 0.08 a |
| Ash | 2.14 ± 0.04 b | 2.93 ± 0.13 a |
| Energy in kJ/100 g (kcal/100 g) | 2242.48 (533.92) a | 2189.19 (521.24) a |
| TPC (mg GAE/100 g Cookies) | TFC (mg CE/100 g Cookies) | ABTS (mg AAE/100 g Cookies) | DPPH (mg TE/100 g Cookies) | FRAP (mg FSE/100 g Cookies) | |
|---|---|---|---|---|---|
| CS-extract-enriched cookies | 163.53 ± 11.00 a | 51.49 ± 4.16 a | 146.59 ± 8.10 a | 67.24 ± 7.04 a | 730.16 ± 65.10 a |
| Control cookies (without extract) | 55.89 ± 1.89 b | 16.31 ± 1.00 b | 58.64 ± 2.79 b | 30.56 ± 2.33 b | 262.69 ± 3.31 b |
| Reactive Oxygen Species | Reactive Nitrogen Species | |||||
|---|---|---|---|---|---|---|
| O2●− | H2O2 | HOCl | ROO● | ONOO− | ||
| IC50 (µg/mL) | Trolox Equivalents (µg TE/mg dw) | In Presence of NaHCO3 IC50 (µg/mL) | In Absence of NaHCO3 IC50 (µg/mL) | |||
| CS-extract-enriched cookies | 35.85 ± 1.04 *,b | 28.23 ± 0.56 *,b | 81.81 ± 5.01 a | 5.81 ± 0.59 c | 108.15 ± 8.45 a,1 | 115.00 ± 3.59 a,1 |
| Control cookies (without extract) | 33.41 ± 1.49 *,b | 13.39± 1.03 *,d | 47.00 ± 3.34 *,b | 0.76 ± 0.05 d | 45.56 ± 2.66*,b,1 | 38.48 ± 2.35*,b,2 |
| Positive controls | ||||||
| Catechin | 48.21 ± 4.79 a | 20.78 ± 0.91 c | 0.37 ± 0.02 d | 368.95 ± 26.39 a | 0.23 ± 0.01 c,1 | 0.16 ± 0.02 b,2 |
| Gallic acid | 10.95 ± 1.40 c | 106.03 ± 1.14 a | 1.81 ± 0.02 c | 247.09 ± 51.44 b | 0.27 ± 0.04 c,1 | 0.15 ± 0.02 b,2 |
| Phenolic Compounds | Peak Number | Amount (mg/100 g Cookies) | |
|---|---|---|---|
| Functional Cookies Enriched with CS Extract | Control Cookies (without Extract) | ||
| Phenolic acids–Hydroxybenzoic acids | |||
| Gallic acid | 1 | 89.4 ± 4.47 a | 36.5 ± 1.83 b |
| Protocatechuic acid | 2 | 2.02 ± 0.10 a | 2.02 ± 0.10 a |
| Syringic acid | 11 | 0.48 ± 0.02 a | 0.13 ± 0.01 b |
| Vanillic acid | 9 | 0.16 ± 0.01 | n.d. |
| ∑ Hydroxybenzoic acids | 92.06 a | 38.65 b | |
| Phenolic acids–Hydroxycinnamic acids | |||
| 4-O-caffeyolquinic acid | 8 | 3.63 ± 0.18 a | 3.25 ± 0.16 b |
| 3,4-di-O-caffeoylquinic acid | 25 | 1.35 ± 0.07 | n.d. |
| 3,5-di-O-caffeoylquinic acid | 18 | 0.41 ± 0.02 | n.d. |
| Caffeic acid | 10 | 0.79 ± 0.04 | n.d. |
| Caftaric acid | 5 | 1.28 ± 0.06 a | 0.47 ± 0.02 b |
| Chlorogenic acid | 7 | 2.68 ± 0.13 a | 2.22 ± 0.11 b |
| Neochlorogenic acid | 3 | 1.02 ± 0.05 | <LOD |
| p-Coumaric acid | 13 | 0.19 ± 0.01 | n.d. |
| ∑ Hydroxycinnamic acids | 11.35 a | 5.94 b | |
| ∑ Phenolic acids | 103.41 a | 44.59 b | |
| Flavanols | |||
| (+)-Catechin | 4 | 5.17 ± 0.26 a | 4.64 ± 0.23 b |
| (−)-Epicatechin | 12 | n.d. | 3.43 ± 0.17 |
| ∑ Flavanols | 5.17 b | 8.07 a | |
| Flavonols | |||
| Kaempferol | 38 | 0.068 ± 0.003 | n.d. |
| Kaempferol-3-O-glucoside | 29 | 0.81 ± 0.04 | n.d. |
| Kaempferol-3-O-rutinoside | 31 | 0.031 ± 0.002 | n.d. |
| Myricetin | 26 | 0.36 ± 0.02 | n.d. |
| Quercetin | 35 | 0.19 ± 0.01 | n.d. |
| Quercetin-3-O-galactoside | 19 | 0.33 ± 0.02 | n.d. |
| Quercetin-3-O-glucopyranoside | 21 | 0.97 ± 0.05 | n.d. |
| Tiliroside | 37 | 0.018 ± 0.001 | n.d. |
| ∑ Flavonols | 2.78 | − | |
| Flavones | |||
| Apigenin | 39 | 0.057 ± 0.003 | n.d. |
| Chrysin | 40 | 0.028 ± 0.001 | n.d. |
| ∑ Flavones | 0.085 | − | |
| ∑ Flavonoids | 8.04 a | 8.07 a | |
| Hydrolyzable tannins | |||
| Ellagic acid | 24 | 40.0 ± 2.00 | n.d. |
| ∑ Hydrolysable tannins | 40.0 | − | |
| Others–Alkaloids | |||
| Caffeine | 6 | 2.71 ± 0.14 | n.d. |
| ∑ Phenolic compounds | 154.09 a | 52.66 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, D.; Moreira, M.M.; Vieira, E.F.; Švarc-Gajić, J.; Vallverdú-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds. Foods 2023, 12, 640. https://doi.org/10.3390/foods12030640
Pinto D, Moreira MM, Vieira EF, Švarc-Gajić J, Vallverdú-Queralt A, Brezo-Borjan T, Delerue-Matos C, Rodrigues F. Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds. Foods. 2023; 12(3):640. https://doi.org/10.3390/foods12030640
Chicago/Turabian StylePinto, Diana, Manuela M. Moreira, Elsa F. Vieira, Jaroslava Švarc-Gajić, Anna Vallverdú-Queralt, Tanja Brezo-Borjan, Cristina Delerue-Matos, and Francisca Rodrigues. 2023. "Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds" Foods 12, no. 3: 640. https://doi.org/10.3390/foods12030640
APA StylePinto, D., Moreira, M. M., Vieira, E. F., Švarc-Gajić, J., Vallverdú-Queralt, A., Brezo-Borjan, T., Delerue-Matos, C., & Rodrigues, F. (2023). Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds. Foods, 12(3), 640. https://doi.org/10.3390/foods12030640












