Abstract
The control of chilling injury in peach fruit by a new regulator network, that exogenous γ-aminobutyric acid (GABA) regulates the metabolisms of polyamines (PAs), the GABA shunt, and proline, is still unclear. This study found that GABA induced an increase in the expression of PpADC and PpODC and a decrease in the expression of PpPAO expression, resulting in the accumulation of PAs. There was also an increase in the expression of PpGAD, which improved GABA content, and an increase in the expression of PpP5CS and PpOAT, which improved proline content. The correlation analysis showed that an increase in PpADC/PpP5CS expression was closely associated with the accumulation of putrescine and that the synergistic increase in the expression of PpODC and PpGAD/PpP5CS/PpOAT was closely related to the accumulation of spermine, proline, and GABA induced by GABA. Importantly, arginine and PpADC played a key role in putrescine accumulation, whereas ornithine and PpODC/PpOAT played a crucial role in the synergistic accumulation of spermine, proline, and GABA induced by GABA. This study provides new information on GABA-induced cold tolerance in peach fruit.
1. Introduction
Low temperature (LT) storage is a common and efficient way to keep fruits and vegetables fresh. Appropriate LT can significantly delay fruit tissue senescence, inhibit the growth of pathogenic microorganisms, and extend shelf life [1,2]. Currently, there has been extensive research into the mechanisms of and controls for chilling injury (CI) in peach fruit under LT storage, such as γ-aminobutyric acid (GABA) [3], oxalic acid [4], melatonin [5], 2,4-epibrassinolide [6], methyl jasmonate [7], ethylene [8], nitric oxide (NO) [9], and glycine betaine [10], which can effectively improve cold tolerance in peach fruit.
GABA is a plant signaling molecule that regulates the pH in and among cells, balances the nutrition of carbon and nitrogen, and contributes to plants’ resistance to adverse environments [11]. Studies have shown that GABA can actually postpone the appearance of CI in banana fruits [12], citrus fruits [13], blueberry fruits [14], Nanguo pears [15], Chinese olive fruits [16], ‘Sahebi’ grapes [17], and aonla fruits [18] and maintain their good quality and shelf life. When plants react to LT, dark surroundings, or mechanical damage, the content of endogenous GABA increases rapidly [19]. GABA is generated directly from glutamate acid decarboxylase (GAD) in the GABA shunt pathway [20].
Polyamines (PAs) metabolism and proline metabolism are closely related to the GABA shunt. Some amino acids act as common precursors, establishing a close link with a variety of metabolic pathways. PAs are involved in controlling the tolerance of abiotic stress in plants [21], including putrescine (Put), spermidine (Spd), and spermine (Spm). Ornithine and arginine are indirectly decarboxylated under the catalysis of ornithine decarboxylase (ODC) and arginine decarboxylase (ADC), respectively, to produce Put [22], and Put is added to aminopropyl to synthesize Spd and Spm [23]. PAs degradation is catalyzed by diamine oxidase (DAO) and polyamine oxidase (PAO) [21,24]. Put can be converted into GABA under the catalysis of DAO [21,25], and PAO is the key enzyme for the degradation of Spd and Spm.
Proline, as a soluble osmotic substance, can maintain cellular osmotic equilibrium and protect the subcellular structure, the content of which is closely linked to the cold sensitivity of post-harvest fruits and vegetables [26]. Glutamic acid is a common precursor for the synthesis of proline and GABA. It is indirectly reduced to proline under the catalyst Δ1-pyrroline 5-carboxylate synthetase (P5CS). Ornithine is a common precursor substance for the synthesis of proline and PAs, and is indirectly reduced to proline under the catalysis of ornithine aminotransferase (OAT) [27]. Additionally, glutamic acid is indirectly produced from proline through the catalysis of proline dehydrogenase (PDH) [28].
Therefore, PAs metabolism, the GABA shunt, and proline metabolism play a crucial role in plants’ response to stress. At present, the correlative mechanisms of PAs metabolism, the GABA shunt, and proline metabolism have been reported in GABA-induced disease resistance in apples [29], NO-induced cold tolerance in banana fruit [30], PSKα-induced cold tolerance in banana fruit [31], and melatonin-induced cold tolerance in cucumber fruit [32]. It has been confirmed that PAs metabolism, the GABA shunt, and proline metabolism are involved in regulating melatonin-induced cold tolerance in peach fruit [5]. It is well known that the application of exogenous GABA induces the cold tolerance of fruits by regulating the levels of PAs and proline and maintains the good quality of post-harvest horticultural products [29,33]. However, relevant studies have not been reported in peach fruit. To this end, it is very important to explore the mechanism of the GABA shunt, PA metabolism, and proline metabolism in cold tolerance induced by GABA in peaches. The purpose of this study was to investigate the effects of GABA treatment on the GABA shunt, PAs metabolism, and proline metabolism in post-harvest peaches.
2. Materials and Methods
2.1. Materials and Treatments
Commercially mature (around 120 days after flowering) “Hujing” peaches (Prunus persica L. Batsch) were harvested from Fenghua District, Ningbo City, Zhejiang Province and transported to the laboratory within 1 h. Evenly sized and mature peaches were randomly divided into two groups, each with 100 fruits. The peaches were dipped into either a GABA solution (5 mmol·L−1) or a water solution for 20 min, creating the GABA treatment group (GABA) and the control group (CK), respectively. The peaches were naturally dried by air and stored in an incubator (4 ± 1 °C), which was sampled every 7 days in order to determine the physiological indicators and key gene expression.
2.2. Determination of Fruit Firmness
Six peaches from each treatment group were randomized, and a TMS-Touch Full Touch Properties analyzer was used to test the TPA of equatorial symmetrical parts peeled from the peaches in different treatment groups. The test program parameters included the probe diameter 7.5 mm, test speed 30 mm/min, deformation 15%, tripping force 0.1 N (sensor range 25 N), or 1 N (sensor range 1000 N). The output of firmness is represented by a double-crested curve, where the maximum F value for the first peak is taken as the firmness data and reported in units of N.
2.3. Determination of Fruit Color
Six peaches from each treatment group were randomized. A CR410 color difference meter was used to take three points around the equatorial area of the peeled fruits in order to measure the value of flesh a and b. The colorimeter must be calibrated with a standard color plate prior to use. The Hue angle (h°) was calculated according to the formula Hue angle = arctan (b/a). This value represents the comprehensive chromaticity index, with a value range of 0–180. As the value increases, the color changes from amaranth, red, orange, yellow, yellow-green, green, and blue-green. When the value is 90, the color is yellow.
2.4. Determination of Total Soluble Solids (TSS)
Six peaches were randomly chosen from each treatment group. A small amount of flesh was taken from the symmetric part close to the equator of the fruit. After the juice was compressed, it was completely mixed. The content of the TSS was determined immediately using a handheld digital saccharimeter.
2.5. Determination of Percentage of Extractable Juice
Six peaches were randomly chosen from each treatment group. A small amount of fruit flesh was taken from the symmetrical part near the equator of the fruit, sliced into small pieces weighing 10 g (6 mm in diameter and 6 mm thick) with a blade, and placed in a centrifuge tube that was previously weighed with absorbent cotton. Then, the small pieces were weighed and centrifuged at 8000 rpm for 10 min. The fragments of peach tissue were removed and weighed again. The percentage of extractable juice is calculated as the rate of weight loss of the fruit after centrifugation.
2.6. Determination of Polyamine Content
The samples of ground peaches with liquid nitrogen were weighed and mixed with pre-cooled perchloric acid in an ice bath at 37 °C for 1 h. After centrifugation, the supernatant was mixed with NaOH and benzoyl chloride, which then reacted in a water bath at 37 °C for 30 min. The reaction was immediately stopped by adding a saturated NaCl solution. The pre-collated ether was added for extraction. After centrifugation, the ether stage was collected, dried with nitrogen, and dissolved by methanol chromatography. The above samples after filtration were used to determine the PAs content.
High Performance Liquid Chromatography (HPLC, Waters 2695, Waters Corporation, Milford, MA, USA) with a model 2998 photodiode array detector (DAD, Waters) and a 4.6 × 150 mm C18 column was used to detect and analyze the PAs at 230 nm. The mobile phase consisted of 64% (v/v) chromatographic methanol with a flow rate of 0.8 mL/min. The column temperature was 30 °C and a sample injection volume of 20 µL was used.
2.7. Determination of GABA Content
The peach sample ground with liquid nitrogen was weighed, mixed with lanthanum chloride, and vortexed at room temperature for 15 min. After centrifugation, the supernatant was mixed with KOH. After centrifugation, the supernatant was mixed with KOH, phosphatic buffer, phenol, and sodium hypochlorite. Then, the mixture was reacted in a boiling water bath for 10 min and immediately placed in ice to cool for 5 min. The above blended solution was blended with 60% ethanol and measured at 645 nm with the Shimadzu UV-1750 ultraviolet spectrophotometer in order to calculate the GABA content.
2.8. Determination of Proline Content
The peach sample ground with liquid nitrogen was weighed and reacted with sulfosalicylic acid in a boiling water bath for 10 min. The supernatant was reacted with glacial acetic acid and ninhydrin color developing solution in a boiling water bath for 40 min. After cooling to room temperature, toluene was added for extraction and the absorbance was measured at 520 nm in order to calculate the proline content.
2.9. Total RNA Preparation and cDNA Synthesis
A plant RNA kit (Omega Bio-Tek, Inc., Norcross, GA, USA) was used to prepare the total RNA. A NanoDrop 2000 spectrophotometer was used for RNA quantification. A SuperRT First Strand cDNA Synthesis Kit (CWBIO, Beijing, China) was used for cDNAs synthesis. A SYBR Green PCR master mix (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) was used for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analyses.
The transcription levels of the key enzyme genes, including PpOAT, PpP5CS, PpPDH, PpADC, PpODC, PpPAO, and PpGAD, of proline, PAs, and GABA shunt metabolism were determined, and information regarding the primes was determined as previously reported [5].
2.10. Statistical Analysis
The study was entirely randomized and replicated at least three times. Data are expressed as means ± standard errors (SE). SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis. Asterisks (*) indicate significant differences between the GABA and CK groups. Student’s unpaired t-test; (*) p < 0.05, (**) p < 0.01. An analysis of correlation was carried out with Omicroshare Tools.
3. Results
3.1. Effect of Exogenous GABA Treatment on Quality Parameters
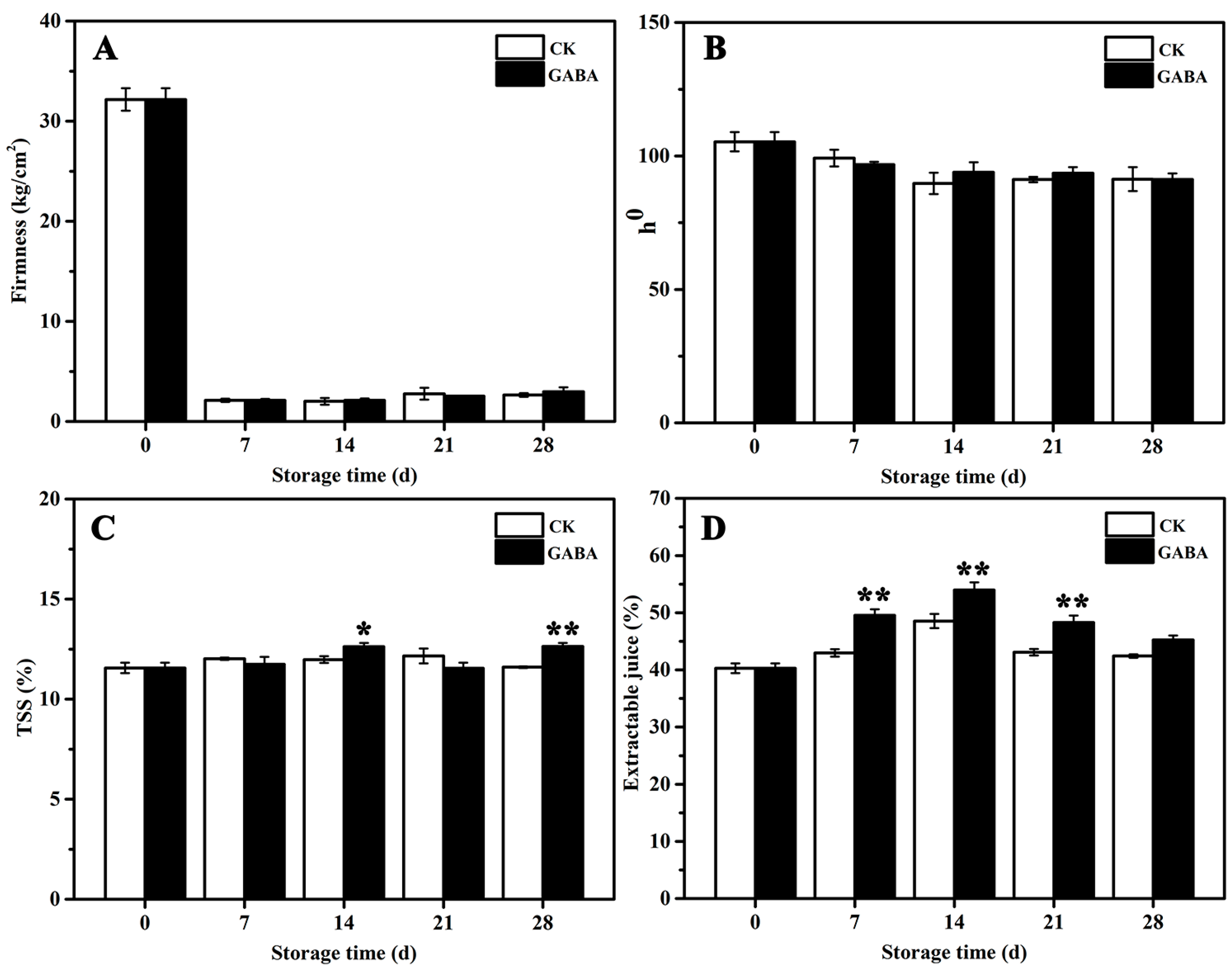
Changes in the quality parameters for the peach fruits in the CK and GABA groups are shown in Figure 1. The firmness of peaches stored for 7 days declined significantly, and no significant differences were found between the treatments during storage (Figure 1A). No significant differences in the hue angle (h°) of the peach color were observed between the treatments throughout the storage process (Figure 1B). Compared to the CK group, the GABA-treated peaches maintained significantly higher TSS levels on days 14 and 28 (Figure 1C), and retained significantly higher extractable juice before days 28 (Figure 1D).
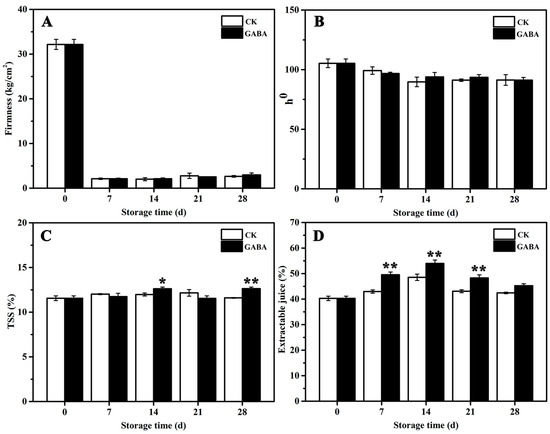
Figure 1.
Effect of exogenous GABA on peach fruit quality. (A) Firmness, (B) L*, (C) TSS, and (D) extractable juice. All data are expressed as means ± standard errors (SE). Asterisks (*) indicate significant differences between the GABA and CK groups. Unpaired Student’s t-test; (*) p < 0.05, (**) p < 0.01.
3.2. Effect of Exogenous GABA Treatment on Polyamines Metabolism
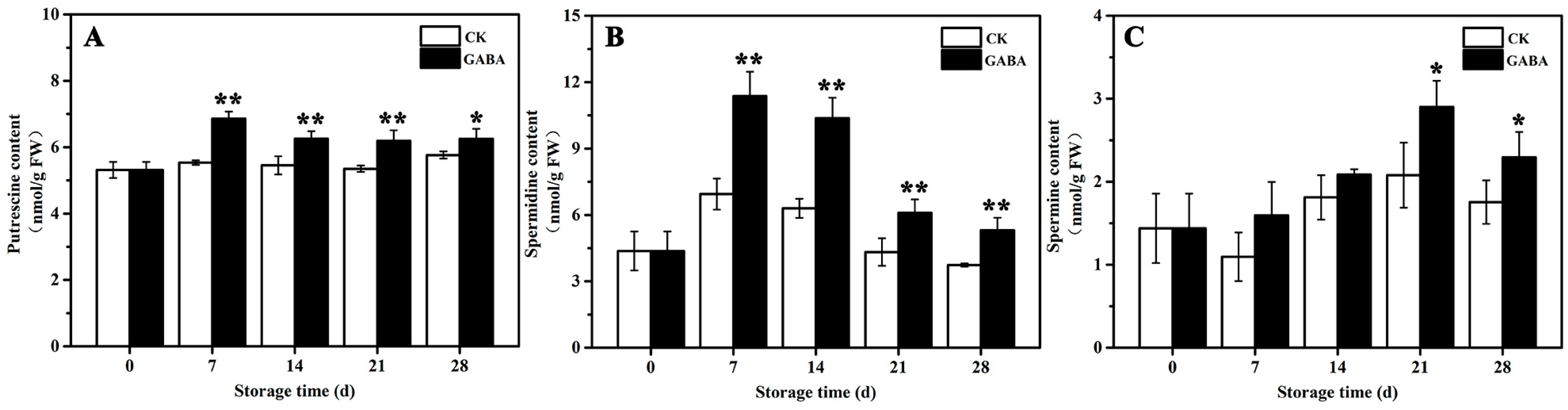
The mechanism of PAs metabolism, which is involved in regulating GABA-induced cold tolerance in peach fruit, was investigated. As shown in Figure 2, in the CK group, Put content tended to be flat during the entire storage period (Figure 2A), Spd content increased first and then decreased (Figure 2B), and Spm content decreased first and then increased (Figure 2C). In the GABA group during the entire storage period, Put content increased first and then tended to be flat (Figure 2A), Spd content first increased and then decreased (Figure 2B), and Spm content increased first and then decreased (Figure 2C). Compared to the CK group, GABA-treated peaches maintained significantly higher Put and Spd levels throughout storage, as well as a significantly higher Spm content on days 21 and 28.
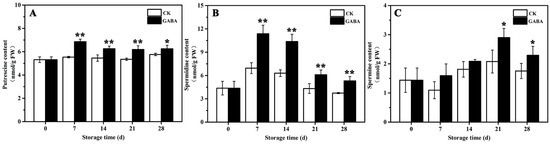
Figure 2.
Change in (A) putrescine, (B) spermidine, and (C) spermine in peach fruit induced by GABA. All data are expressed as means ± standard errors (SE). Asterisks (*) indicate significant differences between the GABA and CK groups. Unpaired Student’s t-test; (*) p < 0.05, (**) p < 0.01.
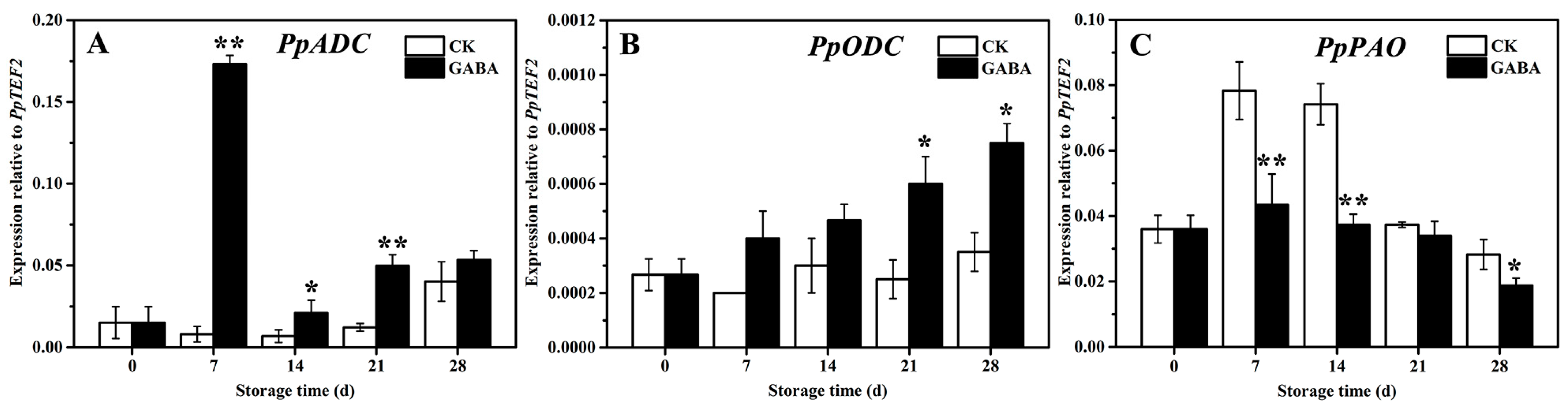
PpADC, PpODC, and PpPAO are key enzyme genes in PAs metabolism in peach fruit. As shown in Figure 3, within the CK group throughout the storage period, the expression of PpADC tended to be flat and then increased on days 28 (Figure 3A), the expression of PpODC tended to be flat (Figure 3B), and the expression of PpPAO initially increased first and then decreased (Figure 3C). In the GABA group during the entire storage period, the expression of PpADC expression increased first, then decreased, and then increased (Figure 3A), the expression of PpODC increased progressively (Figure 3B), and the expression of PpPAO initially increased slowly and then decreased (Figure 3D). Compared with the CK group, GABA significantly increased the expression of PpADC on days 7, 14, and 21, as well as the expression of PpODC on days 21 and 28, and significantly downregulated the expression of PpPAO on days 7, 14, and 28.
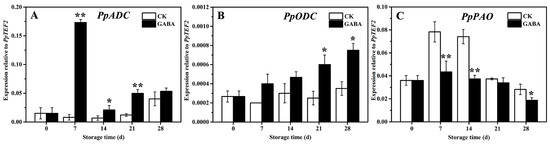
Figure 3.
Change in the expression of (A) PpADC, (B) PpODC, and (C) PpPAO in peach fruit induced by GABA. All data are expressed as means ± standard errors (SE). Asterisks (*) indicate significant differences between the GABA and CK groups. Unpaired Student’s t-test; (*) p < 0.05, (**) p < 0.01.
3.3. Effect of Exogenous GABA Treatment on GABA Shunt
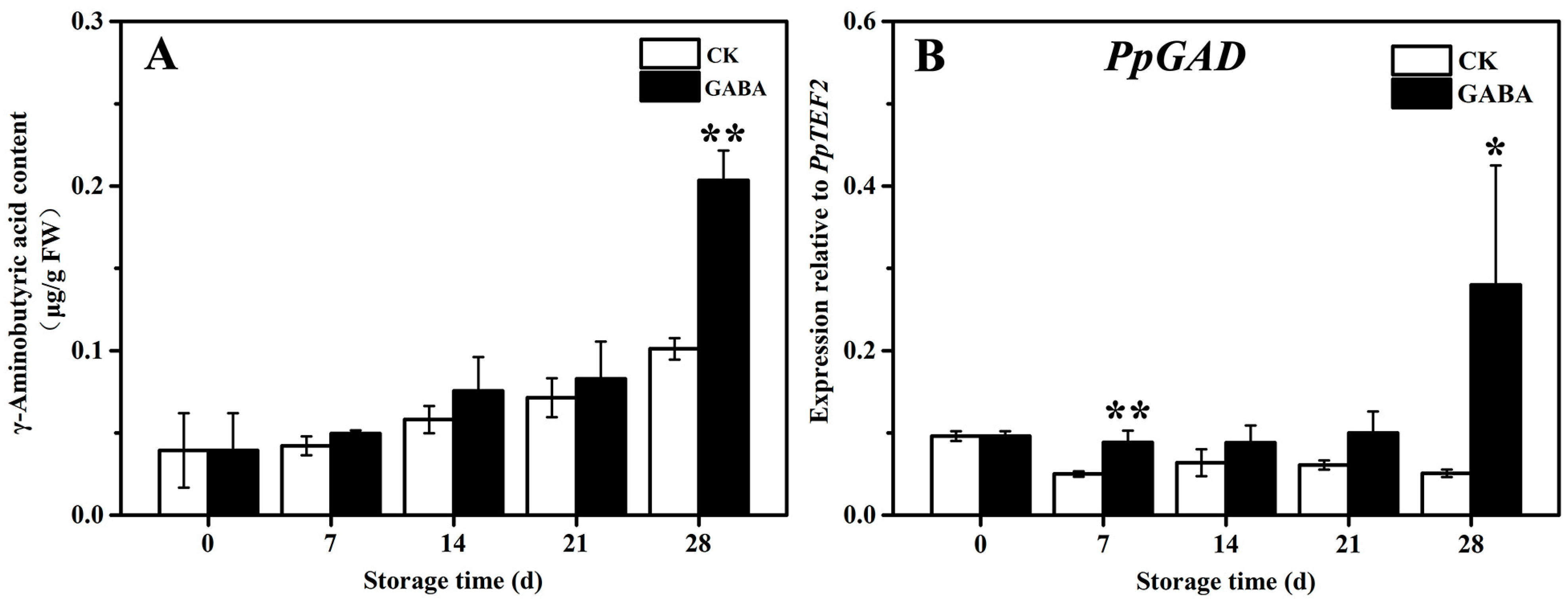
The mechanism of the GABA shunt involved in the regulation of GABA-induced cold tolerance in peach fruit was investigated. As shown in Figure 4, the content of GABA in the CK and GABA groups grew progressively, and the content of GABA in the GABA group was significantly higher than that of the CK group (Figure 4A). In the CK group throughout the storage period, the expression of PpGAD initially declined and then tended to be flat. In the GABA group during the entire storage period, the expression of PpGAD tended to be flat and then increased on days 28. Compared to the CK group, GABA considerably increased the expression of PpGAD on days 7 and 28 (Figure 4B).
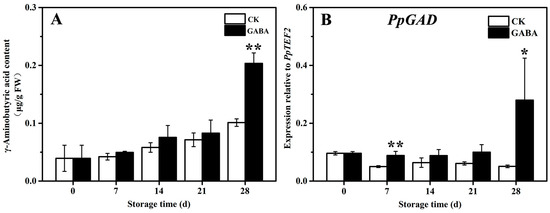
Figure 4.
Change in (A) GABA content and (B) PpGAD expression in peach fruit induced by GABA. All data are expressed as means ± standard errors (SE). Asterisks (*) indicate significant differences between the GABA and CK groups. Unpaired Student’s t-test; (*) p < 0.05, (**) p < 0.01.
3.4. Effect of Exogenous GABA Treatment on Proline Metabolism
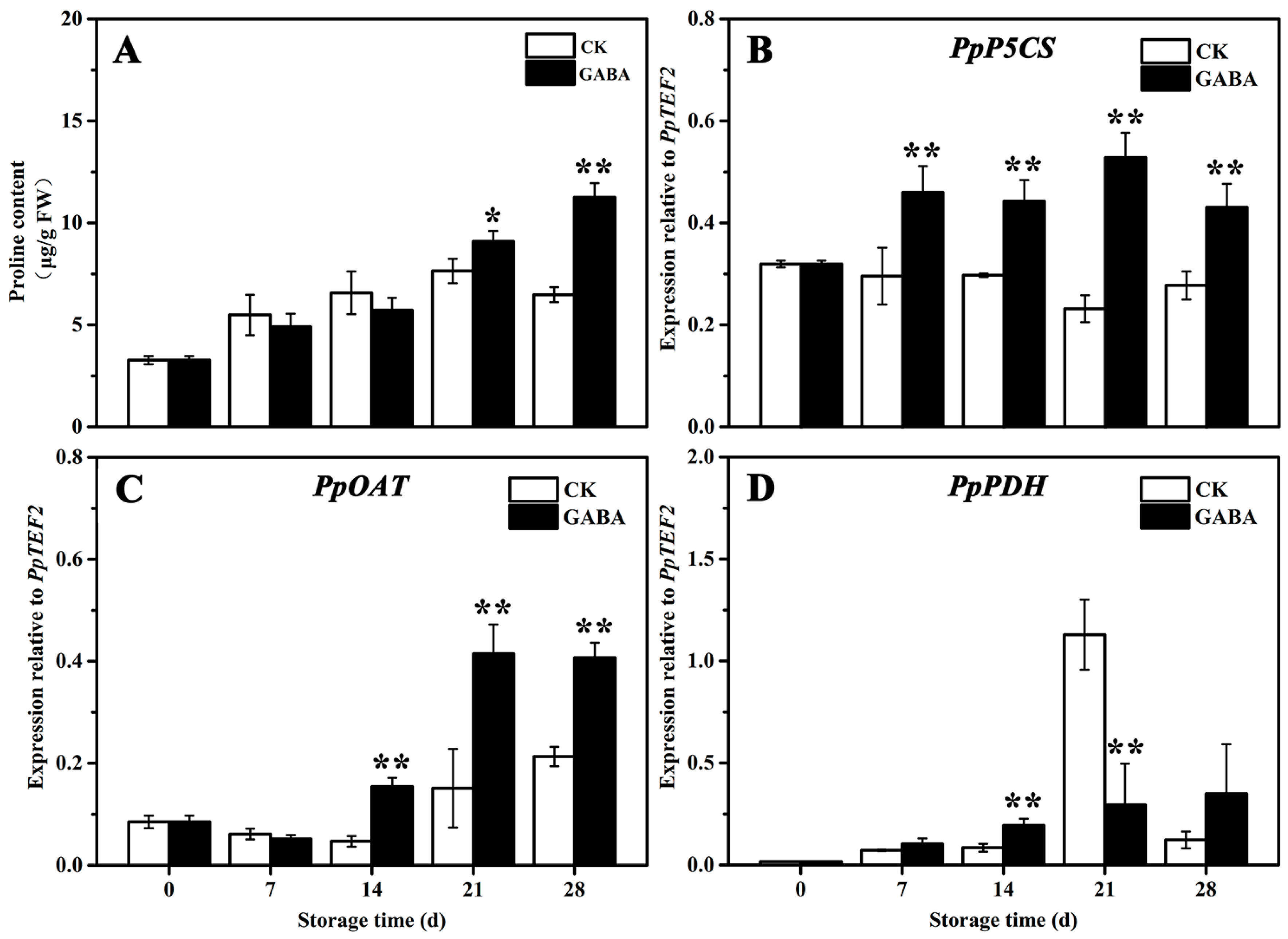
The mechanism of proline metabolism involved in regulating GABA-induced cold tolerance in peaches was studied. As shown in Figure 5, the proline levels in the CK and GABA groups increased gradually, but the proline levels in the GABA group were significantly higher than that of the CK group on days 21 and 28 (Figure 5A). In the CK group throughout the storage period, the expression of PpP5CS tended to be flat (Figure 5B), the expression of PpOAT initially decreased slowly and then increased (Figure 5C), and the expression of PpPDH initially increased slowly, increased significantly on day 21, and then declined significantly on days 28 (Figure 5D). In the GABA group for the entire storage period, the expression of PpP5CS initially increased significantly and then tended to be flat (Figure 5B), and the expression of PpOAT and PpPDH gradually increased (Figure 5C,D). Compared with the CK group, GABA considerably improved the expression of PpP5CS throughout storage, as well as the expression of PpOAT on days 14, 21, and 28, and the expression of PpPDH on day 14, but significantly decreased the expression of PpPDH on day 21.
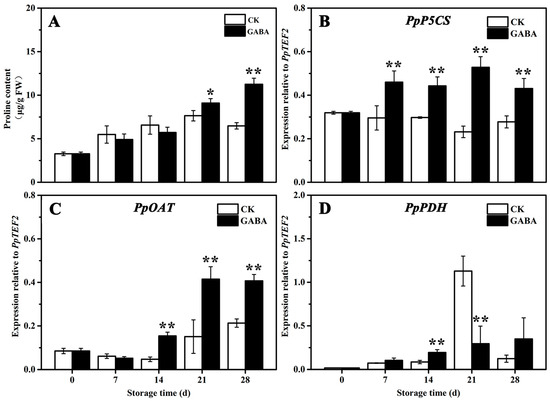
Figure 5.
Change in (A) proline content and the expression of (B) PpP5CS, (C) PpOAT, and (D) PpPDH in peach fruit induced by GABA. All data are expressed as means ± standard errors (SE). Asterisks (*) indicate significant differences between the GABA and CK groups. Unpaired Student’s t-test; (*) p < 0.05, (**) p < 0.01.
3.5. Correlation Analysis among Polyamines Metabolism, the GABA Shunt and Proline Metabolism
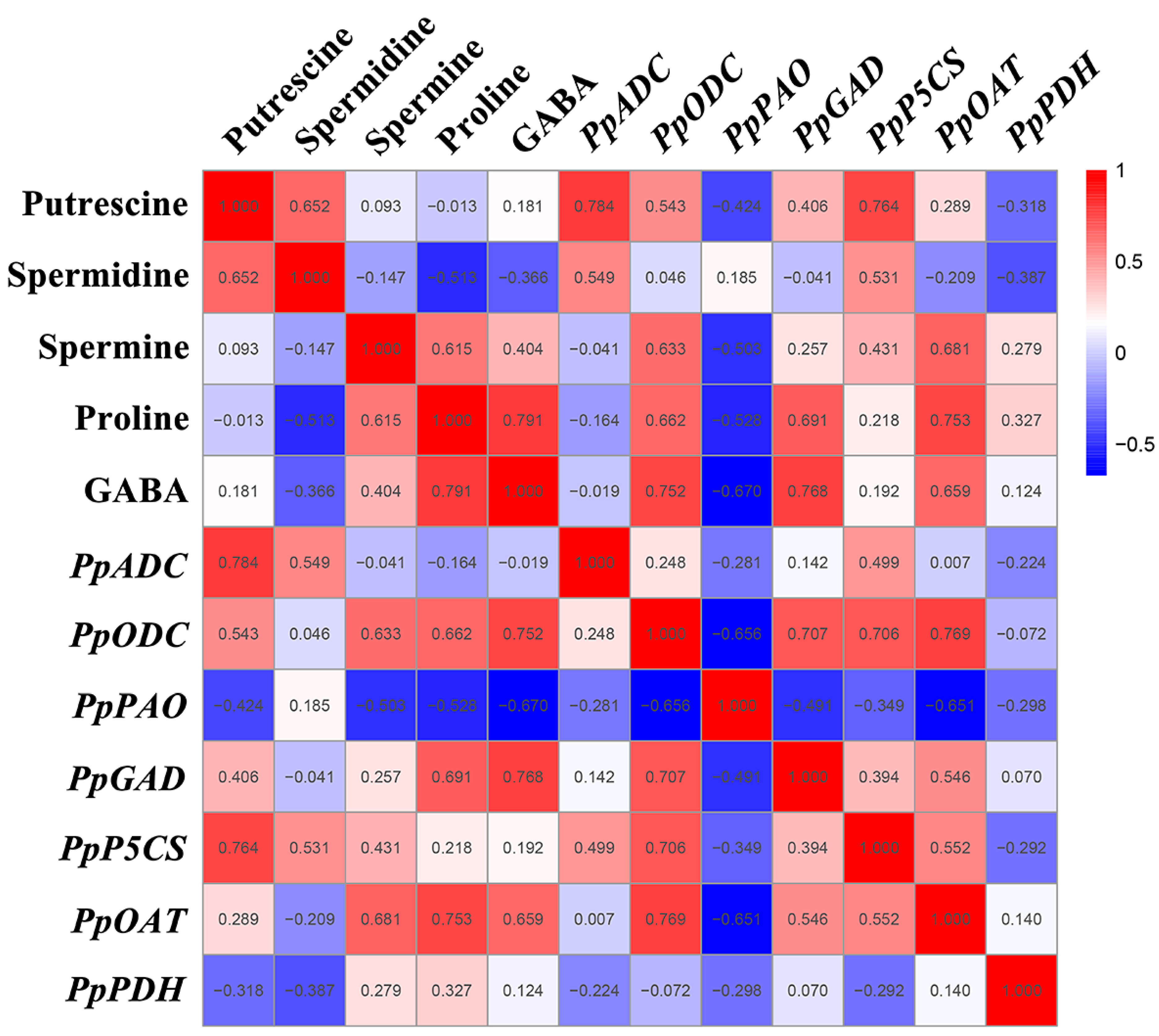
A Pearson correlation analysis was performed in order to further investigate the mechanism of the GABA shunt, PAs metabolism, and proline metabolism in the regulation of GABA-induced cold tolerance in peach fruit. As shown in Figure 6, the correlation analysis between the metabolites and gene expression showed that Put content was significantly positively correlated with PpADC/PpP5CS expression (r = 0.7–0.8, p < 0.05) and was positively correlated with PpODC expression (r = 0.543, p < 0.05). The proline and GABA levels were significantly positively correlated with PpODC, PpGAD, and PpOAT expression (r = 0.6–0.8, p < 0.05). The content of GABA was significantly negatively correlated with PpPAO expression (r = −0.670, p < 0.05), and the level of proline was negatively correlated with PpPAO expression (r = −0.528, p < 0.05). The Spm content was significantly positively correlated with PpODC/PpOAT expression (r = 0.6–0.7, p < 0.05) and was negatively correlated with PpPAO expression (r = −0.503, p < 0.05). The Spd content was positively correlated with PpADC expression (r = 0.549, p < 0.05) and PpP5CS expression (r = 0.531, p < 0.05).
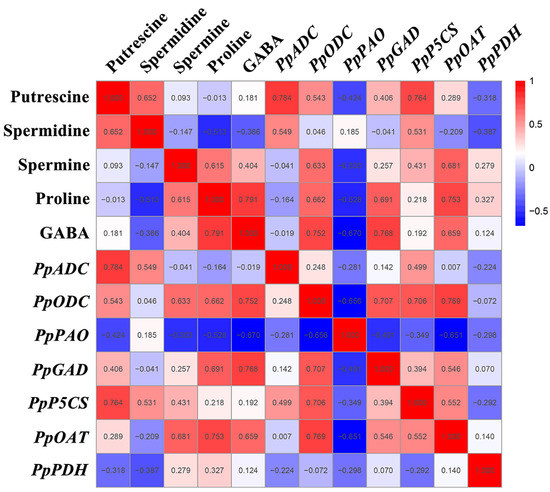
Figure 6.
Thermal map analysis of the correlation of gene expression and PA, GABA, and proline content. The number represents the correlation coefficient, the red ground represents positive correlation, and the blue ground represents negative correlation.
The metabolite correlation analysis found a significant positive correlation between Put content and Spd content (r = 0.652, p < 0.05), between proline content and Spm content (r = 0.615, p < 0.05), and between proline content and GABA content (r = 0.791, p < 0.05). The correlation analysis of gene expression showed that PpODC expression was significantly positively correlated with PpGAD/PpP5CS/PpOAT expression (r = 0.7–0.8, p < 0.05), and that PpPAO expression was significantly negatively correlated with PpODC/PpOAT expression (r = −0.7–−0.6, p < 0.05). PpOAT expression was positively correlated with PpGAD expression (r = 0.546, p < 0.05) and PpP5CS expression (r = 0.552, p < 0.05).
4. Discussion
GABA is a natural, safe, and bio-active compound. GABA accumulates rapidly when plants respond to external stress [11]. In addition, GABA has a variety of signal transduction functions, playing a pivotal role in plant growth and development, cell osmotic regulation, cell nitrogen supply, free radical sweeping, and biological and abiotic stress conditions [34]. GABA is useful for human health and is widely used in food [35]. In particular, GABA helps to regulate the post-harvest physiological process of fruits and vegetables and to maintain the post-harvest quality of fruits and vegetables [36]. For example, GABA treatment maintains high levels of chitinase, b-1,3-glucanase, phenylalanine ammonialyase, peroxidase, and polyphenol oxidase activities in pear fruits in order to withstand pathogen damage and to reduce the rate of decay [37]. 1-Methylcyclopropene (1-MCP) and GABA can effectively maintain post-harvest acidity and storage quality for apples and citrus fruits [38]. Exogenous GABA treatment can be directly involved in regulating malic acid metabolism, keeping organic acid levels in ‘Crisps Pink’ apples, and maintaining good flavor [38]. Exogenous GABA is involved in regulating ethylene anabolism, polyamine metabolism, and the GABA shunt in order to maintain fruit quality in “Golden Delicious” apples [33]. GABA + CaO composite treatment can improve the quality attributes and lifespan of fresh pistachios with shells in cold storage by decreasing the activity of polyphenol oxidase (PPO) in the shell of pistachios, whole fruit respiratory frequency, and microbial activity, and maintaining seed antioxidant activity, anthocyanin content in the seed shell, and phenol in the pistachio shell [39]. In this study, exogenous GABA treatment maintained higher levels of TSS on days 14 and 28 and higher extractable juice before days 28, leading to the preservation of peach quality (Figure 1C,D). Similar results were observed in melatonin-treated peach fruits [5].
The GABA shunt, PAs metabolism, and proline metabolism have been associated with regulating fruit disease resistance and cold tolerance. For example, exogenous GABA regulated the GABA shunt, active oxygen, and PAs metabolism in order to enhance apple disease resistance by enhancing the expression of MdMT, MdMS, MdSAMS, MdSAMDC, MdODC, MdADC, and MdSPDS and decreasing the expression of MdPAO and MdDAO [29]. NO improves the cold tolerance of banana fruit by enhancing ADC and ODC activities, which promotes PAs accumulation, increasing DAO, PAO, and GAD activities and reducing the activity of GABA transaminase, which promotes GABA accumulation, and enhancing OAT activity, which increases proline content [30]. PSKα maintains the quality of banana fruit and delayes the occurrence of banana CI by increasing ADC and ODC activities and decreasing DAO and PAO activities, which promotes the accumulation of Put, Spm, and Spd, as well as an increase in proline and GABA content [31]. Melatonin treatment improves the cold tolerance of cucumber fruit by increasing ADC and ODC activities and CsADC and CsODC expression, which promotes PA accumulation, enhancing P5CS and OAT activities, as well as CsOAT and CsP5CS expression, decreasing PDH activity in order to increase proline content, and enhancing GABA-T and GAD activities and CsGAD expression in order to improve GABA content [32]. Recent research has shown that exogenous melatonin treatment maintains the extractable juice and TSS of peach fruit in order to keep their quality and improves cold tolerance in peach fruit by increasing the expression of PpADC, PpODC, and PpGAD in order to increase GABA and PAs content, enhancing the expression of PpP5CS and PpOAT, and decreasing PpPDH expression in order to increase proline content [5]. In this study, GABA treatment improved cold tolerance in peach fruit by increasing the expression of PpADC and PpODC and decreasing the expression of PpPAO in order to enhance Put, Spd, and Spm content (Figure 2 and Figure 3), enhancing PpGAD expression in order to increase GABA content on days 28 (Figure 4), and decreasing PpPDH expression on days 21 and increasing PpP5CS and PpOAT expression in order to improve proline content (Figure 5).
GABA treatment maintains the quality of many horticulture crops and improves the cold tolerance of fruits by promoting the synergistic accumulation of metabolites or by promoting the synergistic expression of key genes in order to induce the synergistic accumulation of important metabolites. In the latest research report, it was found that GABA induced the synergistic accumulation of photosensitive choline (PC), diacylglycerol (DAG), and triacylglycerol (TAG) components in order to maintain the stability and fluidity of cell membranes and improves the cold tolerance of peach fruit. In addition, GABA induced the synergistic downward regulation of genes associated with phospholipid degradation and the biosynthesis of phospholipid acid in order to inhibit the accumulation of phospholipid acid and maintain a high level of phospholipids, thereby enhancing the cold tolerance of peaches [3]. In this study, the correlation mechanism of the GABA shunt, PAs metabolism, and proline metabolism in GABA-induced cold tolerance in peach fruit was analyzed. Significant positive correlation was found between the Put content and the Spd content and between the proline content and the Spm/GABA content (r = 0.6–0.8, p < 0.05) (Figure 6), showing that GABA induced the synergistic accumulation of PAs, proline, and GABA, further indicating that the GABA shunt, PAs metabolism, and proline metabolism were implicated in and closely related to the regulation of GABA-induced cold tolerance in peaches. Significant negative correlation was found between the GABA content and PpPAO expression (r = −0.670, p < 0.05) (Figure 6), which indicated that PpPAO was not the key gene for the degradation of polyamines in order to generate GABA. However, a significant positive correlation was found between the Put content and PpADC/PpP5CS expression, between the proline content and PpODC/PpGAD/PpOAT expression, between the GABA content and PpODC/PpGAD/PpOAT expression, between the Spm content and PpODC/PpOAT expression, and between PpODC expression and PpGAD/PpP5CS/PpOAT expression (r = 0.6–0.8, p < 0.05) (Figure 6), indicating that GABA induced an increase in the expression of PpADC/PpP5CS in order to contribute to Put accumulation, an increase in the expression of PpODC/PpGAD/PpOAT in order to contribute to the accumulation of proline and GABA content, an increase in the expression of PpODC/PpOAT in order to contribute to Spm accumulation, and a synergistic increase in the expression of PpODC and PpGAD/PpP5CS/PpOAT in order to contribute to an increase in Spm, proline, and GABA content. Importantly, arginine was a crucial substrate and PpADC was a crucial gene for GABA-induced Put accumulation, whereas ornithin was a key substrate and PpODC/PpOAT were key genes for the GABA-induced synergistic accumulation of proline, GABA, and Spm, especially PpODC.
5. Conclusions
This study revealed that GABA improved the cold tolerance of peaches by regulating the GABA shunt, PAs metabolism, and proline metabolism. GABA treatment increased the expression of PpADC and PpODC and decreased the expression of PpPAO in order to increase PAs content. Furthermore, GABA treatment increased PpGAD expression in order to improve the GABA content and enhanced PpP5CS and PpOAT expression in order to increase the proline content. In addition, arginine and PpADC played a key role in GABA-induced Put accumulation, whereas ornithine and PpODC/PpOAT played a crucial role in the GABA-induced synergistic accumulation of proline, GABA, and Spm. This research provided a theoretical scientific basis for amino acid metabolism and revealed the important key genes in the GABA shunt, PAs metabolism, and proline metabolism that are involved in regulating GABA-induced cold tolerance in peach fruit. Thus, this study lays the basis for further research into the functional roles of the key genes in the GABA shunt, PA metabolism, and proline metabolism.
Author Contributions
C.S.: methodology, software, validation, formal analysis, visualization, investigation, data curation, project administration, funding acquisition, and writing—original draft preparation; C.Z.: investigation and supervision; Y.P.: investigation and supervision; Z.Y.: supervision, project administration, funding acquisition, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 32102449 (C.S.), 32172646 (Z.Y.), and Zhejiang Provincial Natural Science Foundation of China, grant number LQ22C150001 (C.S.).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
There are no competing interest in this paper.
References
- Song, C.B.; Wang, K.; Xiao, X.; Liu, Q.L.; Yang, M.J.; Li, X.; Feng, Y.B.; Li, S.S.; Shi, L.Y.; Chen, W.; et al. Membrane lipid metabolism influences chilling injury during cold storage of peach fruit. Food Res. Int. 2022, 157, 111249. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Y.; Zhou, J.Y.; Huang, Q.H.; Pan, Y.J.; Shi, Q.Q.; Ni, H.X.; Yang, Z.F.; Song, C.B. The relationship between membrane lipid metabolism and chilling injury of postharvest peach fruit induced by low temperature. Acta Hortic. Sin. 2022, 1–13. [Google Scholar]
- Song, C.B.; Yang, Z.F.; Xiao, X. Membrane lipid metabolism is involved in regulating γ-aminobutyric acid-mediated cold tolerance in peach fruit. Food Front. 2022, 1–11. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.; Zheng, Y. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef]
- Cao, S.F.; Song, C.B.; Shao, J.R.; Bian, K.; Chen, W.; Yang, Z.F. Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage. J. Agric. Food Chem. 2016, 64, 5215–5222. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Lv, X.G.; Cheng, N.; Peng, B.Z.; Cao, W. Effect of 24-epi brassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Chen, M.; Guo, H.; Chen, S.; Li, T.; Li, M.; Rashid, A.; Xu, C.J.; Wang, K. Methyl jasmonate promotes phospholipid remodeling and jasmonic acid signaling to alleviate chilling injury in peach fruit. J. Agric. Food Chem. 2019, 67, 9958–9966. [Google Scholar] [CrossRef]
- Chen, S.Q.; Chen, M.S.; Li, Y.L.; Huang, X.; Niu, D.Q.; Rashid, A.; Xu, C.J.; Wang, K. Adjustments of both phospholipids and sphingolipids contribute to cold tolerance in stony hard peach fruit by continuous ethylene. Postharvest Biol. Technol. 2021, 171, 111332. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, J.; Song, C.; Qi, S.; Lin, Q.; Cui, Y.; Ling, J.G.; Duan, Y.Q. Nitric oxide alleviates chilling injury by regulating the metabolism of lipid and cell wall in cold-storage peach fruit. Plant Physiol. Biochem. 2021, 169, 63–69. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y.; Wang, L.; Zheng, Y.; Jin, P. Amino acid metabolomic analysis involved in flavor quality and cold tolerance in peach fruit treated with exogenous glycine betaine. Food Res. Int. 2022, 157, 111204. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, H.; Han, Y.; He, Y.; Nan, Y.; Qu, W.; Rao, J.P. Effects of calcium treatment on malate metabolism and γ-aminobutyric acid (GABA) pathway in postharvest apple fruit. Food Chem. 2021, 334, 127479. [Google Scholar] [CrossRef]
- Wang, Y.H.; Luo, Z.S.; Huang, X.D.; Yang, K.L.; Gao, S.J.; Du, R.X. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.L.; Xu, J.; Deng, X.X.; Cheng, Y.J. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef]
- Ge, Y.H.; Duan, B.; Li, C.Y.; Tang, Q.; Li, X.; Wei, M.L.; Chen, Y.R.; Li, J.R. γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 2018, 240, 303–309. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, Q.; Ji, S. GABA application improves the mitochondrial antioxidant system and reduces peel browning in ‘Nanguo’ pears after removal from cold storage. Food Chem. 2019, 297, 124903. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, B.; Lin, H.; Lin, M.; Chen, J.; Lin, Y. γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chem. 2022, 13, 100208. [Google Scholar] [CrossRef]
- Asgarian, Z.S.; Karimi, R.; Ghabooli, M.; Maleki, M. Biochemical changes and quality characterization of cold-stored ‘Sahebi’ grape in response to postharvest application of GABA. Food Chem. 2022, 373, 131401. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Ejaz, S.; Anwar, R.; Khaliq, G.; Hussain, S.; Ullahd, S.; Hussain, R.; Saleem, M.S.; et al. Postharvest γ-aminobutyric acid application mitigates chilling injury of aonla (Emblica officinalis Gaertn.) fruit during low temperature storage. Postharvest Biol. Technol. 2022, 185, 111803. [Google Scholar] [CrossRef]
- Wallace, W.; Secor, J.; Schrader, L.E. Rapid accumulation of γ-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiol. 1984, 75, 170–175. [Google Scholar] [CrossRef]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H.M. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Q.; Chen, H.; Gu, Z.X. Factors influencing diamine oxidase activity and gamma-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J. Agric. Food Chem. 2011, 59, 11616–11620. [Google Scholar] [CrossRef]
- Purvis, A.C. Free proline in peel of grapefruit and resistance to chilling injury during cold storage. HortScience 1981, 16, 160–161. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Sánchez, E.; López-Lefebre, L.R.; García, P.C.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike). J. Plant Physiol. 2001, 158, 593–598. [Google Scholar] [CrossRef]
- Zhu, J.; Li, C.Y.; Sun, L.; Cheng, Y.; Hou, J.B.; Fan, Y.T.; Ge, Y.H. Application of γ-aminobutyric acid induces disease resistance in apples through regulation of polyamine metabolism, GABA shunt and reactive oxygen species metabolism. Sci. Hortic. 2022, 291, 110588. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Mao, L.; Ying, T. Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem. 2016, 197, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, H.; Jiang, Y.M.; Duan, X.W.; Lin, X.Y.; Aghdam, M.S.; Luo, Z.S. Exogenous phytosulfokine α(PSKα) alleviates chilling injury of banana by modulating metabolisms of nitric oxide, polyamine, proline, and γ-aminobutyric acid. Food Chem. 2022, 380, 132179. [Google Scholar] [CrossRef]
- Madebo, M.P.; Luo, S.M.; Wang, L.; Zheng, Y.H.; Jin, P. Melatonin treatment induces chilling tolerance by regulating the contents of polyamine, γ-aminobutyric acid, and proline in cucumber fruit. J. Agric. Sci. 2021, 20, 3060–3074. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhu, J.; Sun, L.; Cheng, Y.; Hou, J.B.; Fan, Y.T.; Ge, Y.H. Exogenous γ-aminobutyric acid maintains fruit quality of apples through regulation of ethylene anabolism and polyamine metabolism. Plant Physiol. Biochem. 2021, 169, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bown, A.W.; Zarei, A. 4-Aminobutyrate (GABA): A metabolite and signal with practical significance. Botany 2017, 95, 1015–1032. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, H.S.; Lim, S.T.; Reddy, C.K. Enhanced accumulation of gamma-aminobutyric acid in rice bran using anaerobic incubation with various additives. Food Chem. 2019, 271, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Niazi, Z.; Razavi, F.; Khademi, O.; Aghdam, M.S. Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 2021, 285, 110198. [Google Scholar] [CrossRef]
- Yu, C.; Zeng, L.; Sheng, K.; Chen, F.X.; Zhou, T.; Zheng, X.D.; Yu, T. γ-aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014, 159, 29–37. [Google Scholar] [CrossRef]
- Han, S.K.; Nan, Y.Y.; Qu, W.; He, Y.H.; Ban, Q.Y.; Lv, Y.R.; Rao, J.P. Exogenous gamma-aminobutyric acid treatment that contributes to regulation of malate metabolism and ethylene synthesis in apple fruit during storage. J. Agric. Food Chem. 2018, 66, 13473–13482. [Google Scholar] [CrossRef]
- Saeedi, M.; Mirdehghan, S.H.; Nazoori, F.; Esmaeilizadeh, M.; Saba, M.K. Impact of calcium and γ-aminobutyric acid (GABA) on qualitative attributes and shelf life characteristics of fresh in-hull pistachio during cold storage. Postharvest Biol. Technol. 2022, 187, 111863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).