Effect of Pulsed Electric Field on the Chicken Meat Quality and Taste-Related Amino Acid Stability: Flavor Simulation
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Determinations of PEF Power and Intensity
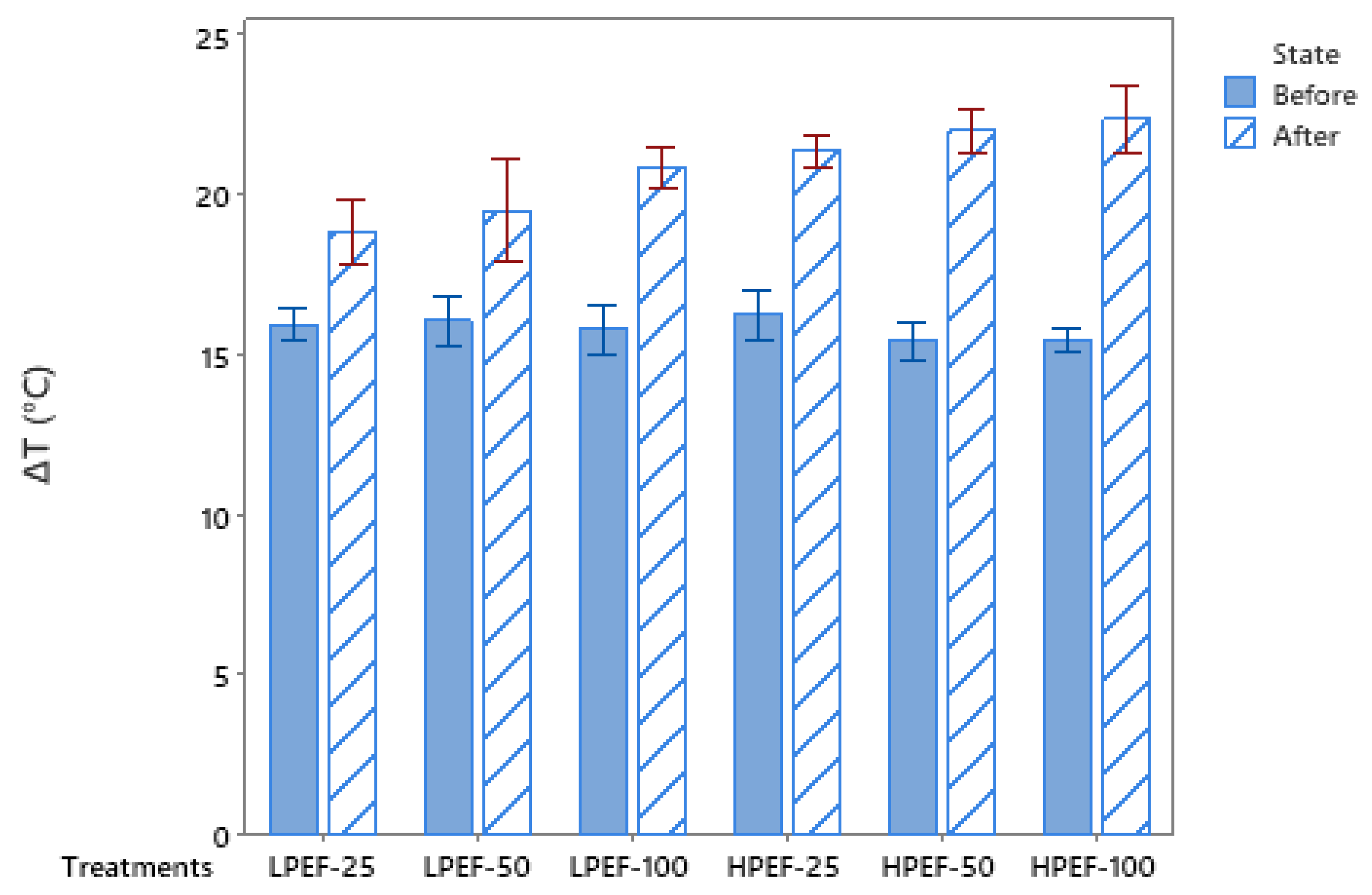
2.3. pH and Temperature Measurements
2.4. Colorimetric Analysis
2.5. Identification and Quantification of FAAs
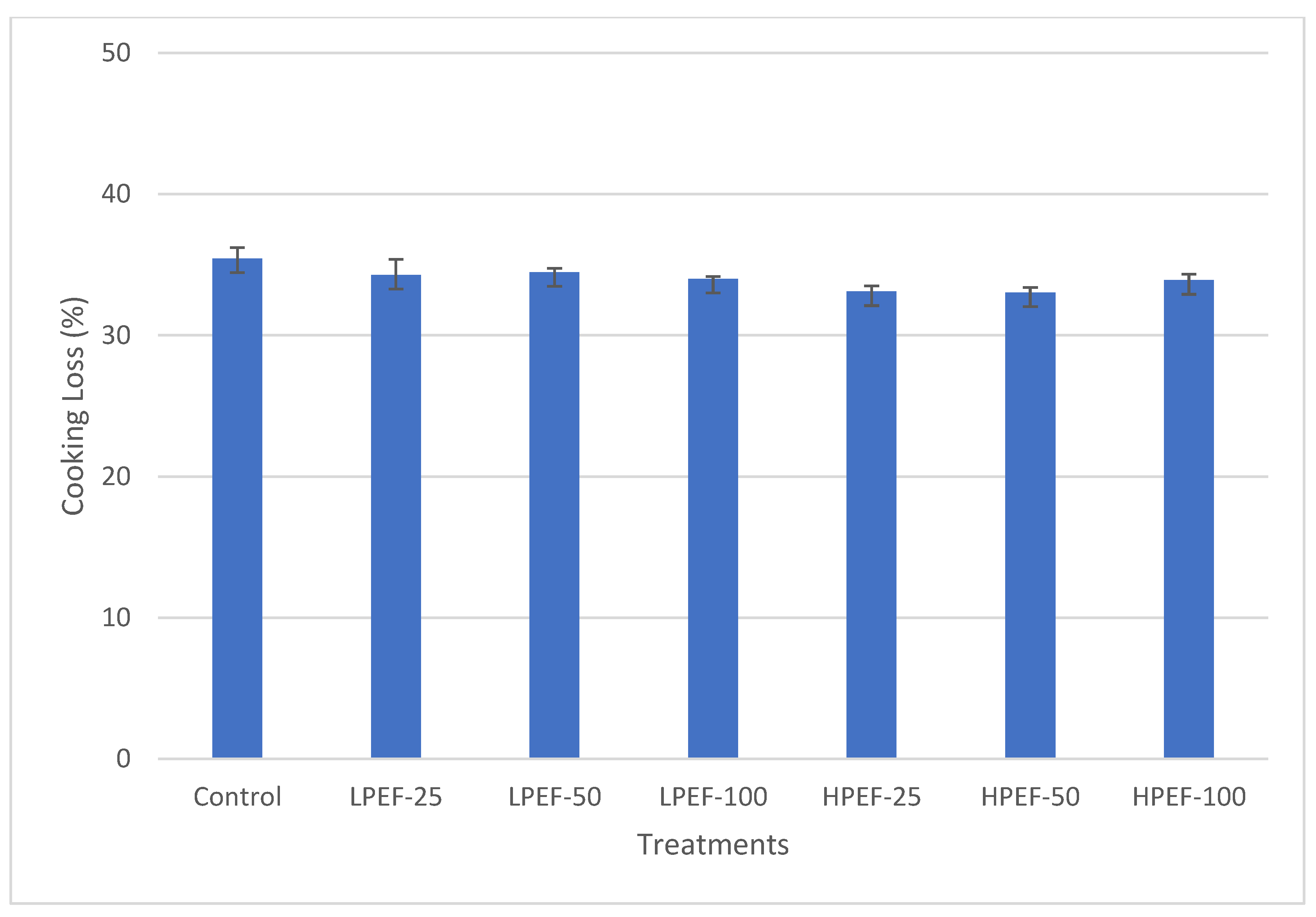
2.6. Determination of Shear Force and Cooking Loss
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of PEF Treatments on pH and Temperature
3.2. Changes in Color
3.3. Effect of PEF Treatments on FAAs
3.4. Effect of PEF Treatments on Texture (Shear Force) and Cooking Loss (%)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldi, G.; D’elia, F.; Soglia, F.; Tappi, S.; Petracci, M.; Rocculi, P. Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat. Foods 2021, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rosas, S.F.; Ballinas-Casarrubias, M.L.; Nevarez-Moorillon, G.V.; Martin-Belloso, O.; Ortega-Rivas, E. Thermal and Pulsed Electric Fields Pasteurization of Apple Juice: Effects on Physicochemical Properties and Flavour Compounds. J. Food Eng. 2007, 83, 41–46. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Oey, I.; Wang, S.; Xu, Y.; Ma, Q.; Faridnia, F.; Farouk, M. Physicochemical and Sensory Properties of Beef Muscles after Pulsed Electric Field Processing. Food Res. Int. 2019, 121, 1–11. [Google Scholar] [CrossRef]
- Faridnia, F.; Ma, Q.L.; Bremer, P.J.; Burritt, D.J.; Hamid, N.; Oey, I. Effect of Freezing as Pre-Treatment Prior to Pulsed Electric Field Processing on Quality Traits of Beef Muscles. Innov. Food Sci. Emerg. Technol. 2015, 29, 31–40. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.D. The Impact of Nonthermal Technologies on the Microbiological Quality of Juices: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Zeng, X.A.; Ahmad, N.; Ahmed, Z.; Rehman, A.; Aadil, R.M.; Roobab, U.; Siddique, R.; Rahaman, A. Effect of Pulsed Electric Field and Thermal Treatments on the Bioactive Compounds, Enzymes, Microbial, and Physical Stability of Almond Milk during Storage. J. Food Process. Preserv. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Chacha, J.S.; Athar, A.; Madni, G.M.; Ranjha, M.M.A.N.; Rusu, A.V.; Zeng, X.A.; Aadil, R.M.; Trif, M. Applications of Innovative Non-Thermal Pulsed Electric Field Technology in Developing Safer and Healthier Fruit Juices. Molecules 2022, 27, 4031. [Google Scholar] [CrossRef]
- Castro, A.J.; Swanson, B.G.; Barbosa-Cánovas, G.V.; Zhang, Q.H. Pulsed Electric Field Modification of Milk Alkaline Phosphatase Activity. Pulsed Electr. Fields Food Process. 2019, 65–82. [Google Scholar] [CrossRef]
- Rashid, M.H.; Khan, M.R.; Roobab, U.; Rajoka, M.S.R.; Inam-ur-Raheem, M.; Anwar, R.; Ahmed, W.; Jahan, M.; Ijaz, M.R.A.; Asghar, M.M.; et al. Enhancing the Shelf Stability of Fresh-Cut Potatoes via Chemical and Nonthermal Treatments. J. Food Process. Preserv. 2021, 45, 1–14. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Bulski, K.; Oziembłowski, M. Effect of Pulsed Electric Field Treatment on Shelf Life and Nutritional Value of Apple Juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The Effect of Pulsed Electric Field as a Pre-Treatment Step in Ultrasound Assisted Extraction of Phenolic Compounds from Fresh Rosemary and Thyme by-Products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.W.; Aadil, R.M. Effective Valorization of Food Wastes and By-Products through Pulsed Electric Field: A Systematic Review. J. Food Process Eng. 2021, 44, 1–14. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. The Application of Pulsed Electric Field as a Sodium Reducing Strategy for Meat Products. Food Chem. 2020, 306, 125622. [Google Scholar] [CrossRef]
- Shuang, L.; Cheng, L.; Anjun, C.; Aiping, L.; Yuntao, L.; Ge, Y.; Liang, Y.; Bi, W. Sterilizing Effect of High-Voltage Pulsed Electric Fields on Prepared Beef. J. Nucl. Agric. Sci. 2019, 33, 722. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Jayawardena, S.R.; Bekhit, A.E.D.A. Pulsed Electric Field: A New Way to Improve Digestibility of Cooked Beef. Meat Sci. 2019, 155, 79–84. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Pulsed Electric Field Operates Enzymatically by Causing Early Activation of Calpains in Beef during Ageing. Meat Sci. 2019, 153, 144–151. [Google Scholar] [CrossRef]
- Ghosh, S.; Gillis, A.; Levkov, K.; Vitkin, E.; Golberg, A. Saving Energy on Meat Air Convection Drying with Pulsed Electric Field Coupled to Mechanical Press Water Removal. Innov. Food Sci. Emerg. Technol. 2020, 66, 102509. [Google Scholar] [CrossRef]
- Kantono, K.; Hamid, N.; Ma, Q.; Oey, I.; Farouk, M. Changes in the Physicochemical Properties of Chilled and Frozen-Thawed Lamb Cuts Subjected to Pulsed Electric Field Processing. Food Res. Int. 2021, 141. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hamid, N.; Oey, I.; Leong, S.Y.; Kantono, K.; Alfaro, A.; Lu, J. Physicochemical Changes in New Zealand Abalone (Haliotis Iris) with Pulsed Electric Field (PEF) Processing and Heat Treatments. Lwt 2019, 115. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Current and Future Prospects for the Use of Pulsed Electric Field in the Meat Industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.D.A.; Suwandy, V.; Carne, A.; van de Ven, R.; Hopkins, D.L. Effect of Repeated Pulsed Electric Field Treatment on the Quality of Hot-Boned Beef Loins and Topsides. Meat Sci. 2016, 111, 139–146. [Google Scholar] [CrossRef]
- Shimamura, Y.; Shinke, M.; Hiraishi, M.; Tsuchiya, Y.; Masuda, S. The Application of Alkaline and Acidic Electrolyzed Water in the Sterilization of Chicken Breasts and Beef Liver. Food Sci. Nutr. 2016, 4, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Randhawa, M.A.; Carne, A.; Mohamed Ahmed, I.A.; Barr, D.; Reid, M.; Bekhit, A.E.D.A. Quality and Nutritional Minerals in Chicken Breast Muscle Treated with Low and High Pulsed Electric Fields. Food Bioprocess Technol. 2018, 11, 122–131. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments - Douglas C. Montgomery - Google Books. Available online: https://books.google.com.pk/books?hl=en&lr=&id=Py7bDgAAQBAJ&oi=fnd&pg=PA1&dq=Montgomery,+D.C.+2017.+Design+and+analysis+of+experiments.+9th+Ed.+John+Wiley+%26+Sons.+Inc.+Hoboken,+NJ,+USA.+5:162-264&ots=X7t-n5SO_a&sig=_R-FW6cwK1zOJjVSTpVjPdwFVFI&redir_esc= (accessed on 14 September 2022).
- Faridnia, F.; Bremer, P.; Burritt, D.J.; Oey, I. Effects of Pulsed Electric Fields on Selected Quality Attributes of Beef Outside Flat (Biceps Femoris). IFMBE Proc. 2016, 53, 51–54. [Google Scholar] [CrossRef]
- Faridnia, F.; Bekhit, A.E.D.A.; Niven, B.; Oey, I. Impact of Pulsed Electric Fields and Post-Mortem Vacuum Ageing on Beef Longissimus Thoracis Muscles. Int. J. Food Sci. Technol. 2014, 49, 2339–2347. [Google Scholar] [CrossRef]
- Arroyo, C.; Lascorz, D.; O’Dowd, L.; Noci, F.; Arimi, J.; Lyng, J.G. Effect of Pulsed Electric Field Treatments at Various Stages during Conditioning on Quality Attributes of Beef Longissimus Thoracis et Lumborum Muscle. Meat Sci. 2014, 99, 52–59. [Google Scholar] [CrossRef]
- Wideman, N.; O’Bryan, C.A.; Crandall, P.G. Factors Affecting Poultry Meat Colour and Consumer Preferences—A Review. Worlds. Poult. Sci. J. 2016, 72, 353–366. [Google Scholar] [CrossRef]
- Min, T.; Zhou, L.; Sun, X.; Du, H.; Zhu, Z.; Wen, Y. Electrospun Functional Polymeric Nanofibers for Active Food Packaging: A Review. Food Chem. 2022, 391, 133239. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.G.; Harvey, D.; Zahniser, S.; Gale, F.; Liefert, W. Assessing the Growth of U.S. Broiler and Poultry Meat Exports; US Department of Agriculture: Washington, DC, USA, 2013. [Google Scholar]
- Fu, Q.; Shi, H.; Zhou, L.; Li, P.; Wang, R. Effects of Ultrasound Power on the Properties of Non-Salt Chicken Myofibrillar Protein Emulsions. Food Sci. Technol. Int. 2022, 57, 2523–2534. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of Pulsed Electric Fields in Meat and Fish Processing Industries: An Overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Clarke, F.M.; Purslow, P.P.; Warner, R.D. Meat Color Is Determined Not Only by Chromatic Heme Pigments but Also by the Physical Structure and Achromatic Light Scattering Properties of the Muscle. Compr. Rev. Food Sci. Food Saf. 2020, 19, 44–63. [Google Scholar] [CrossRef]
- Arroyo, C.; Eslami, S.; Brunton, N.P.; Arimi, J.M.; Noci, F.; Lyng, J.G. An Assessment of the Impact of Pulsed Electric Fields Processing Factors on Oxidation, Color, Texture, and Sensory Attributes of Turkey Breast Meat. Poult. Sci. 2015, 94, 1088–1095. [Google Scholar] [CrossRef]
- Toldrá, F. The Role of Muscle Enzymes in Dry-Cured Meat Products with Different Drying Conditions. Trends Food Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Franco, D. Fat Effect on Physico-Chemical, Microbial and Textural Changes through the Manufactured of Dry-Cured Foal Sausage Lipolysis, Proteolysis and Sensory Properties. Meat Sci. 2012, 92, 704–714. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Q.; Sun, Q.; Kong, B.; Xiong, Y. Flavour Formation from Hydrolysis of Pork Sarcoplasmic Protein Extract by a Unique LAB Culture Isolated from Harbin Dry Sausage. Meat Sci. 2015, 100, 110–117. [Google Scholar] [CrossRef]
- Liu, Y.F.; Oey, I.; Bremer, P.; Silcock, P.; Carne, A. Proteolytic Pattern, Protein Breakdown and Peptide Production of Ovomucin-Depleted Egg White Processed with Heat or Pulsed Electric Fields at Different PH. Food Res. Int. 2018, 108, 465–474. [Google Scholar] [CrossRef]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Effects of PH, Temperature and Pulsed Electric Fields on the Turbidity and Protein Aggregation of Ovomucin-Depleted Egg White. Food Res. Int. 2017, 91, 161–170. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Wang, M.; Lu, R. Effects of Pulsed Electric Fields on Bioactive Components, Colour and Flavour of Green Tea Infusions. Int. J. Food Sci. Technol. 2009, 44, 312–321. [Google Scholar] [CrossRef]
- Sánchez-Vega, R.; Garde-Cerdán, T.; Rodríguez-Roque, M.J.; Elez-Martínez, P.; Martín-Belloso, O. High-Intensity Pulsed Electric Fields or Thermal Treatment of Broccoli Juice: The Effects of Processing on Minerals and Free Amino Acids. Eur. Food Res. Technol. 2020, 246, 539–548. [Google Scholar] [CrossRef]
- Siddeeg, A.; Zeng, X.A.; Rahaman, A.; Manzoor, M.F.; Ahmed, Z.; Ammar, A.F. Effect of Pulsed Electric Field Pretreatment of Date Palm Fruits on Free Amino Acids, Bioactive Components, and Physicochemical Characteristics of the Alcoholic Beverage. J. Food Sci. 2019, 84, 3156–3162. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Pulsed Electric Field: Role in Protein Digestion of Beef Biceps Femoris. Innov. Food Sci. Emerg. Technol. 2018, 50, 132–138. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Liu, Q.; Xu, L. The Role of Bacterial Fermentation in the Hydrolysis and Oxidation of Sarcoplasmic and Myofibrillar Proteins in Harbin Dry Sausages. Meat Sci. 2016, 121, 196–206. [Google Scholar] [CrossRef]
- Gutsche, K.A.; Tran, T.B.T.; Vogel, R.F. Production of Volatile Compounds by Lactobacillus Sakei from Branched Chain α-Keto Acids. Food Microbiol. 2012, 29, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Ardö, Y. Flavour Formation by Amino Acid Catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Bekard, I.; Dunstan, D.E. Electric Field Induced Changes in Protein Conformation. Soft Matter 2014, 10, 431–437. [Google Scholar] [CrossRef]
- O’Dowd, L.P.; Arimi, J.M.; Noci, F.; Cronin, D.A.; Lyng, J.G. An Assessment of the Effect of Pulsed Electrical Fields on Tenderness and Selected Quality Attributes of Post Rigour Beef Muscle. Meat Sci. 2013, 93, 303–309. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; van de Ven, R.; Suwandy, V.; Fahri, F.; Hopkins, D.L. Effect of Pulsed Electric Field Treatment on Cold-Boned Muscles of Different Potential Tenderness. Food Bioprocess Technol. 2014, 7, 3136–3146. [Google Scholar] [CrossRef]
- Toepfl, S. Pulsed Electric Fields (PEF) for Permeabilization of Cell Membranes in Food-and Bioprocessing–Applications, Process and Equipment Design and Cost Analysis. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2006. [Google Scholar] [CrossRef]
- Lopp, A.; Weber, H. Untersuchungen Zur Optimierung Der Zartheit von Rindfleisch. Fleischwirtschaft 2005, 85, 111–116. [Google Scholar]
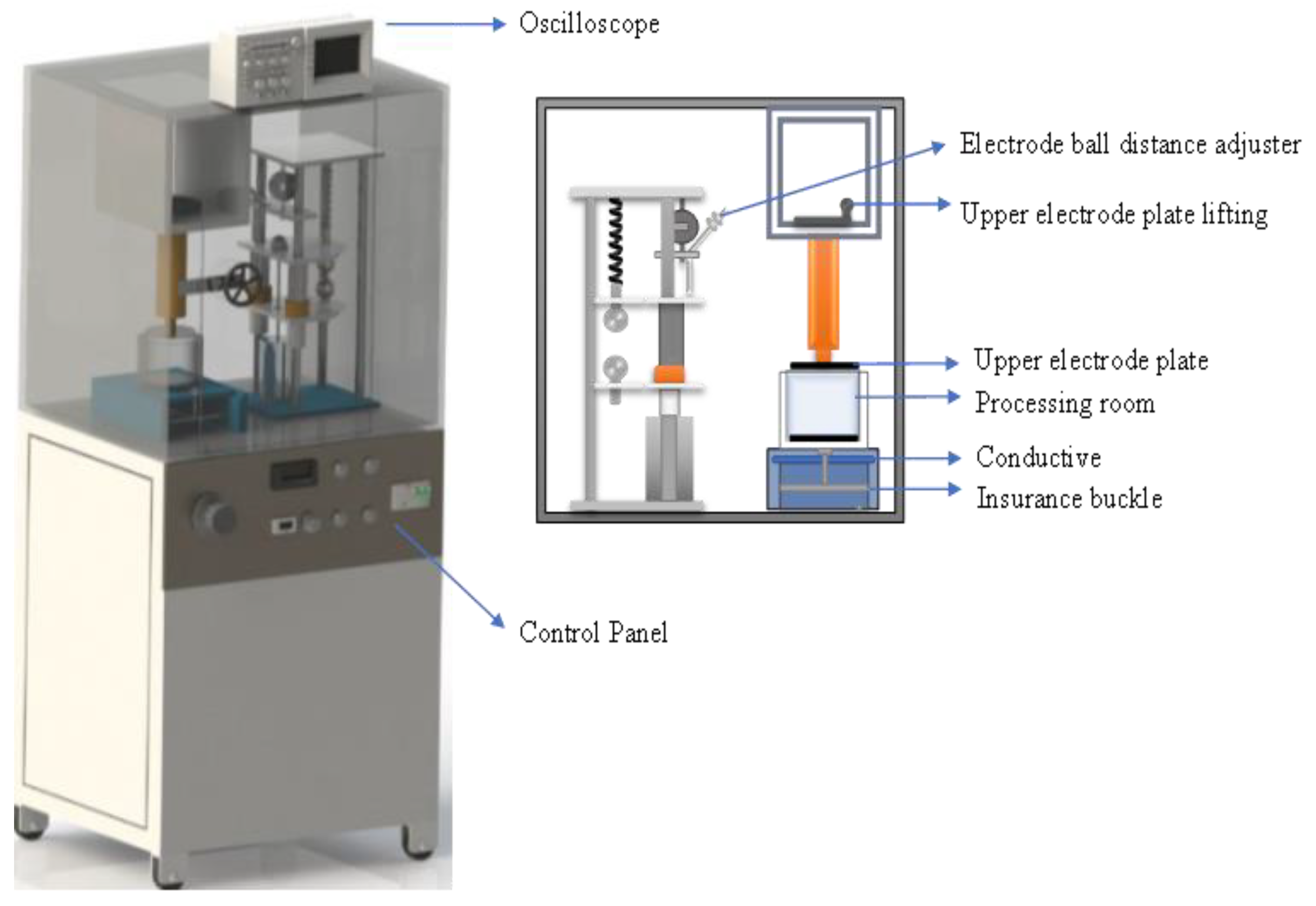

| Parameters | Specifications |
|---|---|
| Input power supply voltage | 220 V, 50 Hz |
| Adjustable discharge mode | Direct high-voltage rapid discharge. |
| Output maximum discharge voltage | 0–20 kV/cm |
| Electrodes | Stainless steel, parallel plate, 200 × 200 mm |
| Electrode gap | 0–10 cm, adjustable distance. |
| Treatment chamber material | Plexiglass |
| Output Power | ≥500 W |
| Output frequency | 0–1.5 Hz (the higher the voltage, the longer the charging time, and then the lower the frequency) |
| Processing chamber volume | Configuration: 100 mL, 400 mL, and 700 mL, three kinds of containers. |
| Pulse shape | Exponential decay pulses, monopolar |
| System capacity | 1 μF |
| Treatment | Code | Electric Field Strength (kV/cm) | Pulse Number | pH | L* | a* | b* |
|---|---|---|---|---|---|---|---|
| Control | Control | – | – | 5.98 ± 0.12 | 42.51 ± 0.36 ab | 1.58 ± 0.08 | 6.12 ± 0.71 |
| Low-PEF | LPEF-25 | 1.5 ± 0.13 | 25 | 5.96 ± 0.01 | 45.17 ± 0.85 ab | 1.29 ± 0.17 | 6.75 ± 0.46 |
| LPEF-50 | 1.5 ± 0.17 | 50 | 5.93 ± 0.02 | 45.78 ± 0.94 a | 0.93 ± 0.24 | 5.78 ± 0.31 | |
| LPEF-100 | 1.5 ± 0.12 | 100 | 5.95 ± 0.03 | 43.12 ± 1.04 ab | 1.22 ± 0.22 | 5.98 ± 0.6 | |
| High-PEF | HPEF-25 | 3.3 ± 0.15 | 25 | 5.91 ± 0.02 | 43.04 ± 0.56 ab | 1.17 ± 0.2 | 6.59 ± 0.51 |
| HPEF-50 | 3.3 ± 0.16 | 50 | 5.93 ± 0.03 | 42.26 ± 0.55 b | 1.38 ± 0.11 | 6.82 ± 0.41 | |
| HPEF-100 | 3.3 ± 0.10 | 100 | 5.90 ± 0.03 | 43.38 ± 0.71 ab | 1.04 ± 0.18 | 6.19 ± 0.22 |
| FAAs Type (ng/20 µL) | Control | LPEF-25 | LPEF-50 | LPEF-100 | HPEF-25 | HPEF-50 | HPEF-100 |
|---|---|---|---|---|---|---|---|
| Umami taste FAAs | |||||||
| Aspartic acid | 454.07 ± 4.73 ab | 434.97 ± 0.93 b | 552.2 ± 1.33 a | 513.8 ± 10.2 ab | 525.1 ± 28 ab | 436.1 ± 5.92 ab | 462.7 ± 45.7 ab |
| Glutamic acid | 875.5 ± 5.59 d | 1219.7 ± 2.87 a | 1130.4 ± 5.81 ab | 951.1 ± 19.3 cd | 1047.7 ± 57.6 bc | 945.4 ± 6.55 cd | 1031.3 ± 3.09 bc |
| Sweet taste FAAs | |||||||
| Threonine | 443.2 ± 10.7 c | 379.6 ± 8.52 d | 478.7 ± 6.49 abc | 465.1 ± 14.4 bc | 504.7 ± 18 ab | 508.02 ± 1.97 ab | 531.4 ± 2.2 a |
| Serine | 468.6 ± 1.05 c | 491.3 ± 1.21 bc | 512.8 ± 3.05 b | 500 ± 11.8 b | 507.1 ± 4.41 b | 516.99 ± 1.58 b | 565.5 ± 4.2 a |
| Glycine | 400.2 ± 1.54 ab | 371.3 ± 0.63 ab | 414.9 ± 2.4 ab | 409.5 ± 8.26 ab | 439.8 ± 19 a | 358.7 ± 1.24 b | 425.3 ± 25.3 ab |
| Alanine | 717.7 ± 4.59 a | 577 ± 136 a | 732.4 ± 3.79 a | 726.8 ± 16.4 a | 838.6 ± 23.4 a | 762.8 ± 9.8 a | 848.8 ± 4.68 a |
| Bitter taste FAAs | |||||||
| Isoleucine | 248.7 ± 2.16 ab | 229.7 ± 0.39 b | 288.8 ± 1.31 a | 274.1 ± 6.1 a | 291.6 ± 7.02 a | 254.9 ± 2.36 ab | 264.9 ± 18 ab |
| Leucine | 472.5 ± 3.21 cd | 443 ± 0.32 d | 544.7 ± 3.02 ab | 511 ± 11.5 bc | 559.5 ± 2.03 a | 523.6 ± 6.95 ab | 522.2 ± 14.2 ab |
| Tyrosine | 294.6 ± 3.26 b | 274.7 ± 0.18 b | 338.1 ± 0.9 a | 339.03 ± 9.06 a | 353.2 ± 5.16 a | 340.02 ± 0.45 a | 348.09 ± 4.35 a |
| Phenylalanine | 967.8 ± 4.87 a | 151.4 ± 2.4 c | 208.3 ± 0.49 bc | 262.7 ± 8.59 bc | 309.5 ± 38.4 b | 169.6 ± 6.86 c | 241.4 ± 44.3 bc |
| Histidine | 296.7 ± 2.56 b | 297.98 ± 0.62 b | 382.4 ± 0.15 b | 378.2 ± 7.45 b | 407.8 ± 44 b | 808.1 ± 34.6 a | 507 ± 156 ab |
| Bitter/sweet/sulfurous taste FAAs | |||||||
| Cysteine | 25.8 ± 0.18 a | 19.5 ± 0.24 ab | 18.6 ± 0.06 ab | 19.7 ± 0.24 ab | 14.3 ± 4.48 ab | 11.5 ± 0.12 b | 18.1 ± 3.33 ab |
| Valine | 355.98 ± 2.11 bc | 331.9 ± 0.58 c | 407.4 ± 2.22 ab | 384.97 ± 8.02 abc | 416.5 ± 1.7 a | 349.5 ± 0.52 c | 378.8 ± 24.1 abc |
| Methionine | 200.6 ± 1.57 de | 195.08 ± 0.52 e | 229.6 ± 1.19 ab | 213.4 ± 4.03 cd | 224.9 ± 4.1 abc | 217.5 ± 1.94 bc | 233.6 ± 0.59 a |
| Lysine | 377.3 ± 8.55 b | 334.6 ± 5.19 b | 401.8 ± 4.9 b | 505.3 ± 6.87 a | 417.8 ± 29.4 a | 342.5 ± 5.26 b | 374.9 ± 33.8 b |
| Arginine | 467.7 ± 3.31 ab | 432.6 ± 0.26 b | 493.6 ± 2.18 ab | 521.5 ± 11.8 a | 497.2 ± 5.23 ab | 460.2 ± 4.35 ab | 490.4 ± 32.9 ab |
| Proline | 271.9 ± 16.5 a | 225.5 ± 0.86 a | 260.36 ± 1.73 a | 277.51 ± 7.14 a | 333 ± 44.1 a | 364.7 ± 16.2 a | 317.7 ± 54.8 a |
| Total FAAs | 7338.9 | 6410.3 | 7395.3 | 7253.8 | 7688.3 | 7370.4 | 7562.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roobab, U.; Zeng, X.-A.; Ahmed, W.; Madni, G.M.; Manzoor, M.F.; Aadil, R.M. Effect of Pulsed Electric Field on the Chicken Meat Quality and Taste-Related Amino Acid Stability: Flavor Simulation. Foods 2023, 12, 710. https://doi.org/10.3390/foods12040710
Roobab U, Zeng X-A, Ahmed W, Madni GM, Manzoor MF, Aadil RM. Effect of Pulsed Electric Field on the Chicken Meat Quality and Taste-Related Amino Acid Stability: Flavor Simulation. Foods. 2023; 12(4):710. https://doi.org/10.3390/foods12040710
Chicago/Turabian StyleRoobab, Ume, Xin-An Zeng, Waqar Ahmed, Ghulam Muhammad Madni, Muhammad Faisal Manzoor, and Rana Muhammad Aadil. 2023. "Effect of Pulsed Electric Field on the Chicken Meat Quality and Taste-Related Amino Acid Stability: Flavor Simulation" Foods 12, no. 4: 710. https://doi.org/10.3390/foods12040710








