Selective Activity of an Anthocyanin-Rich, Purified Blueberry Extract upon Pathogenic and Probiotic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Production and Purification
2.2. Microorganisms
2.3. The Effect on Pathogenic Bacteria
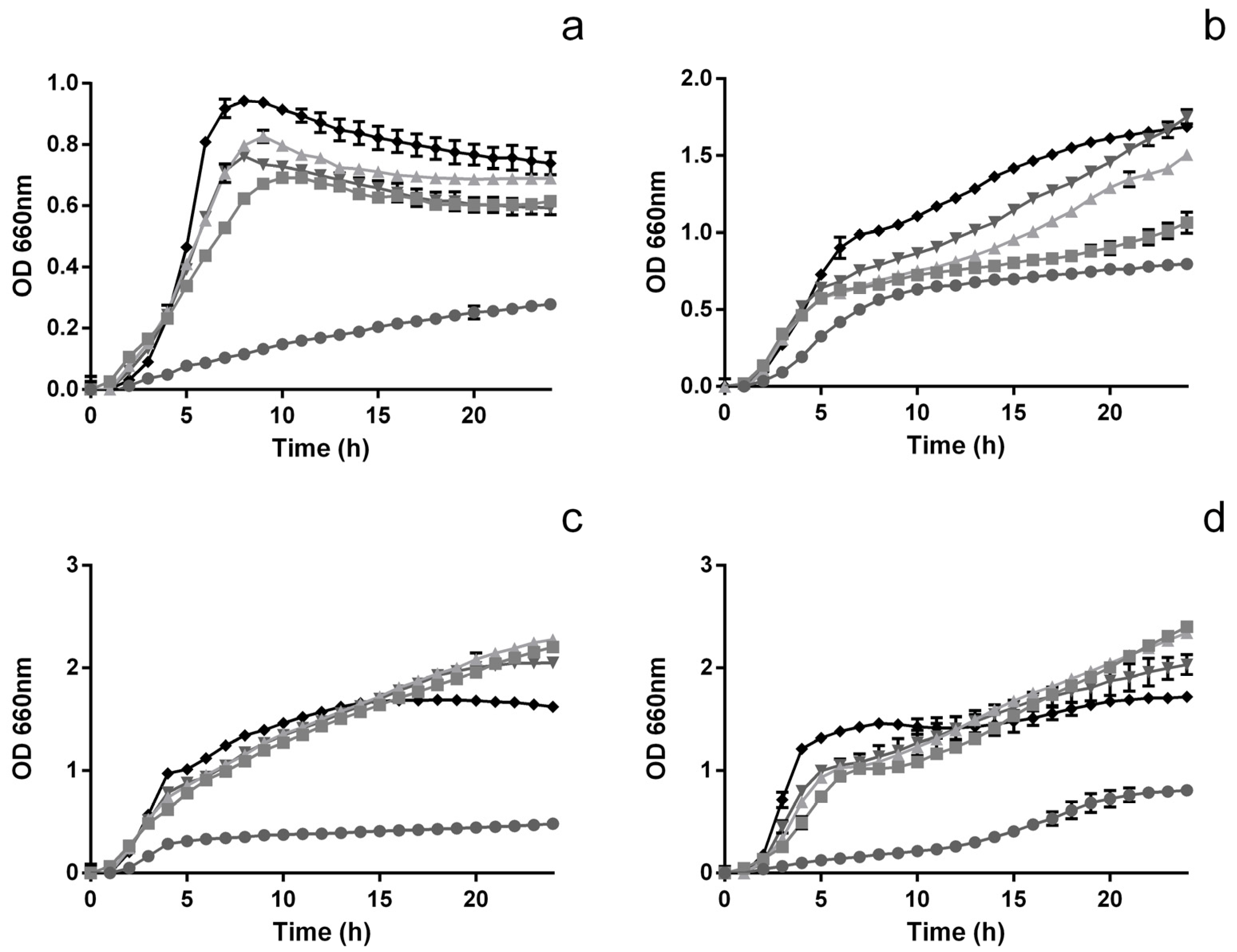
2.3.1. Time-Inhibition Curves
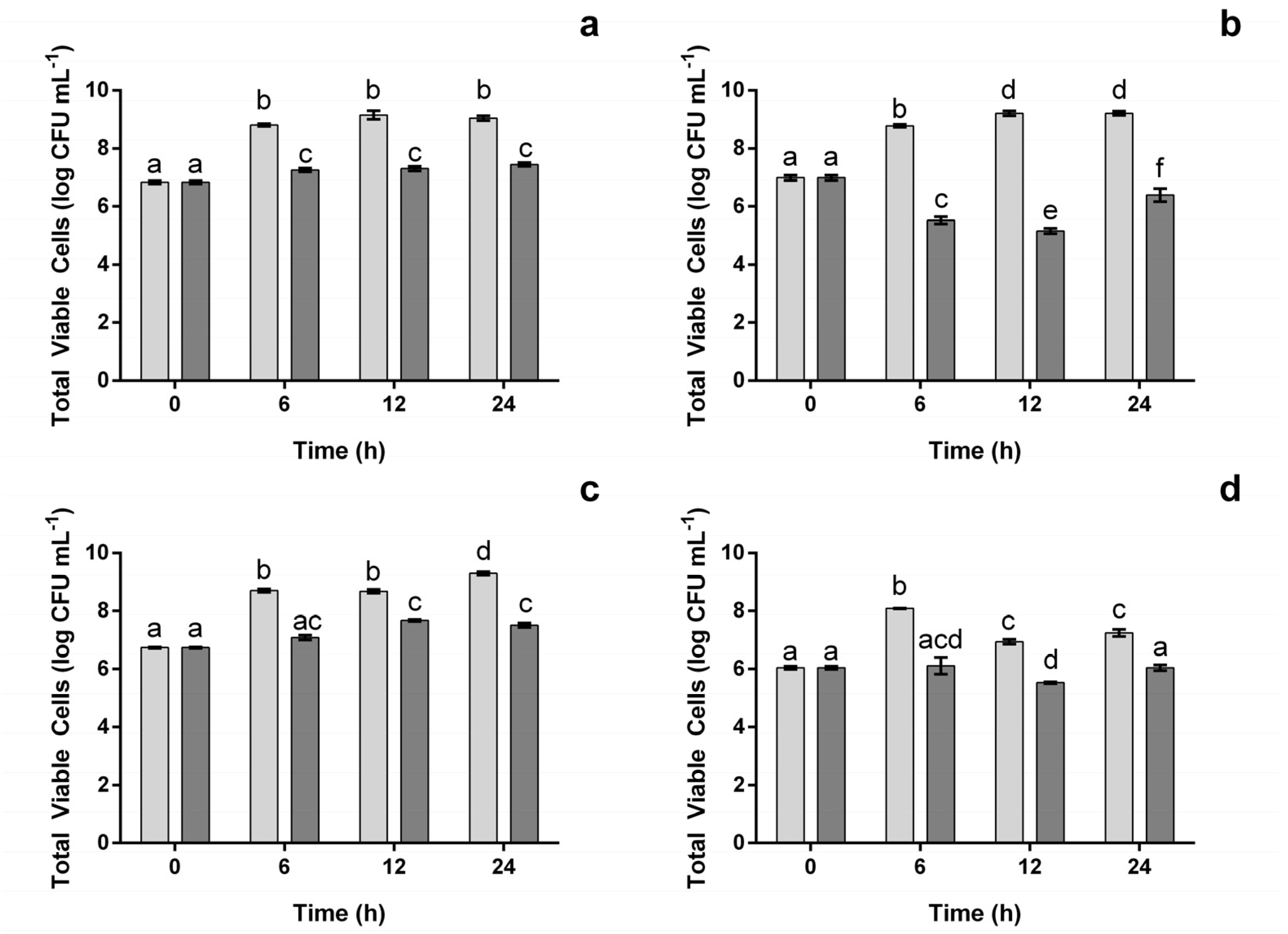
2.3.2. The Impact on Pathogenic Viable Counts
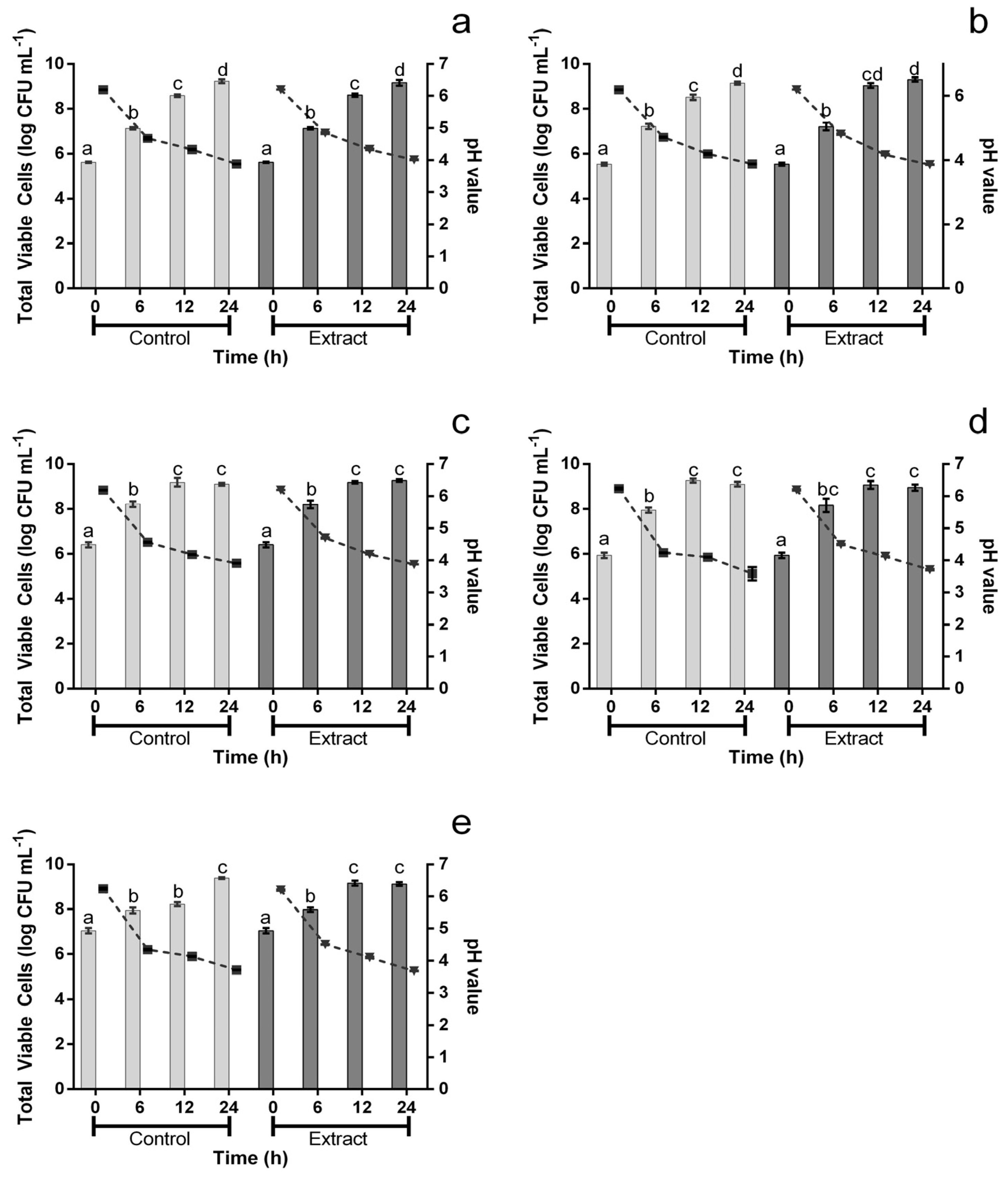
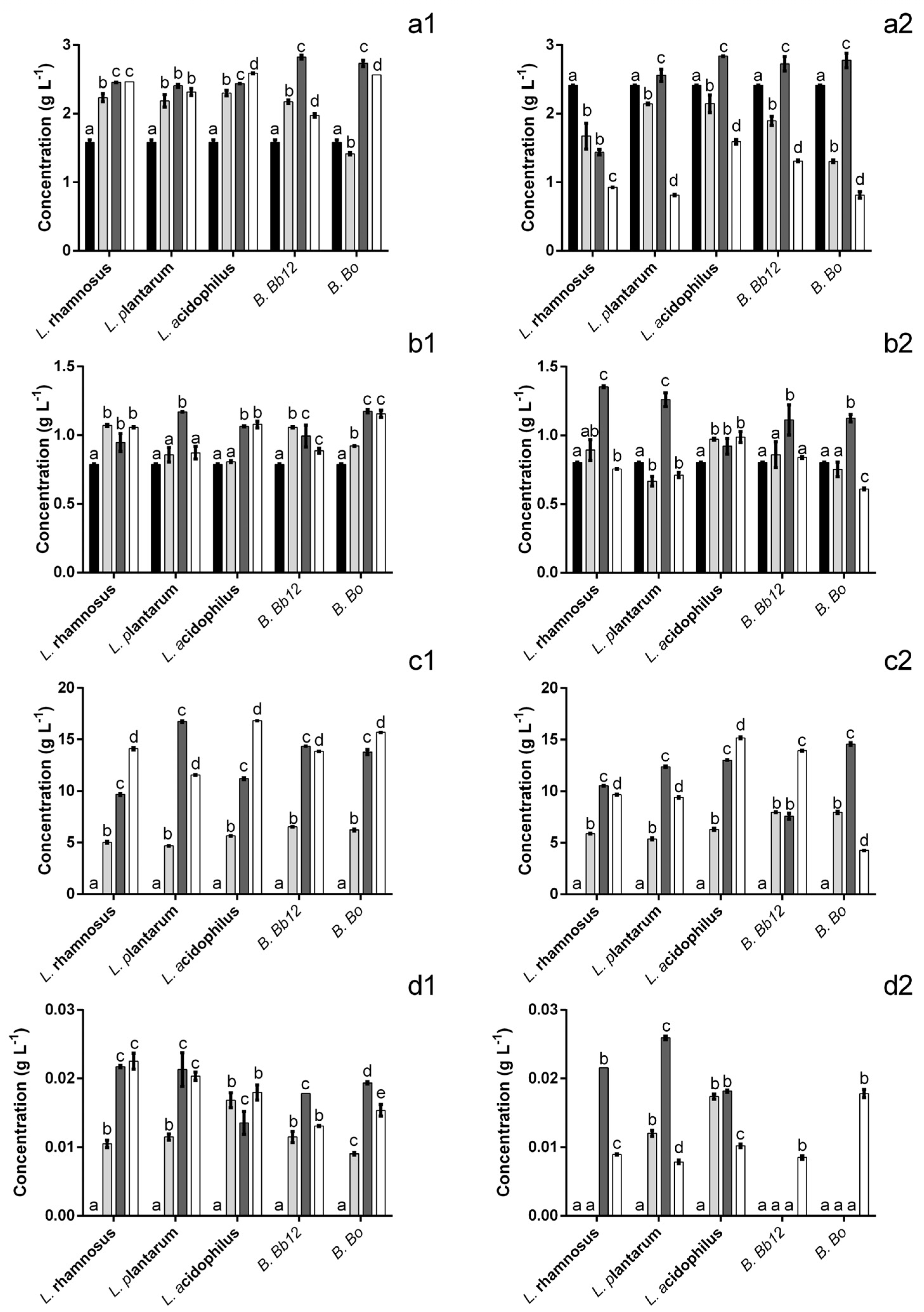
2.4. The Effect on Probiotic Bacteria
2.5. Statistical Analysis
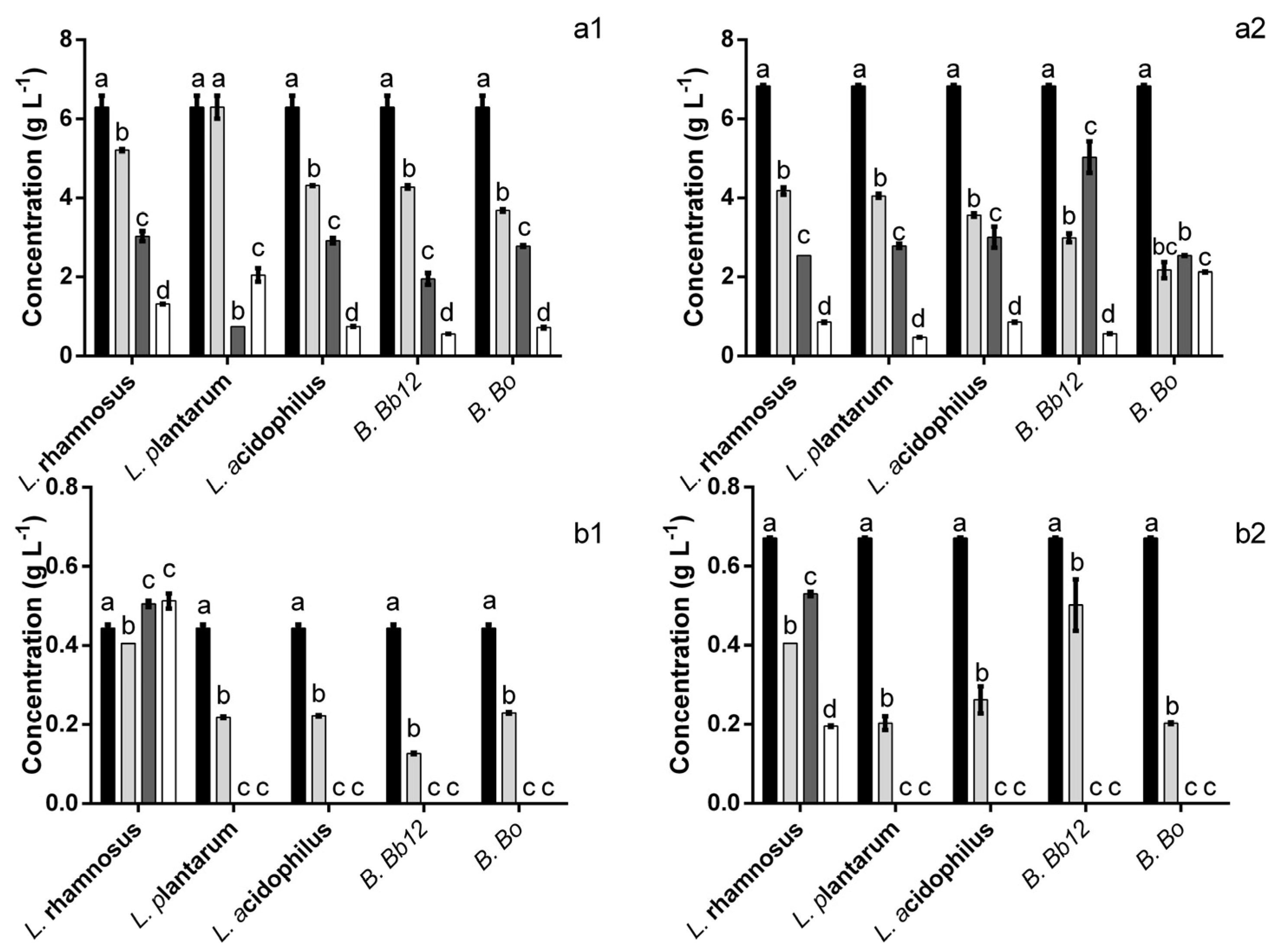
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, S.; Costa, E.; Mendes, M.; Morais, R.; Calhau, C.; Pintado, M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control 2013, 35, 159–165. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, G.; Yue, J.; Qian, B.; Liu, Z.; Wang, D.; Zhong, Y.; Zhao, Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control 2014, 38, 184–191. [Google Scholar] [CrossRef]
- Lacombe, A.; Wu, V.C.; White, J.; Tadepalli, S.; Andre, E.E. The antimicrobial properties of the lowbush blueberry (Vaccinium angustifolium) fractional components against foodborne pathogens and the conservation of probiotic Lactobacillus rhamnosus. Food Microbiol. 2012, 30, 124–131. [Google Scholar] [CrossRef]
- Park, Y.J.; Biswas, R.; Phillips, R.D.; Chen, J. Antibacterial Activities of Blueberry and Muscadine Phenolic Extracts. J. Food Sci. 2011, 76, M101–M105. [Google Scholar] [CrossRef] [PubMed]
- Burdulis, D.; Sarkinas, A.; Jasutiené, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar]
- Silva, S.; Costa, E.; Pereira, M.; Costa, M.; Pintado, M. Evaluation of the antimicrobial activity of aqueous extracts from dry Vaccinium corymbosum extracts upon food microorganism. Food Control 2013, 34, 645–650. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Oliveira, H.; De Freitas, V.; Morais, R.M.; Calhau, C.; Pintado, M. Impact of a Purified Blueberry Extract on In Vitro Probiotic Mucin-Adhesion and Its Effect on Probiotic/Intestinal Pathogen Systems. Molecules 2022, 27, 6991. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-T.; Huang, J.; Wong, H.-C.; Li, J.; Zhao, D. Metabolic fate of black raspberry polyphenols in association with gut microbiota of different origins in vitro. Food Chem. 2023, 404, 134644. [Google Scholar] [CrossRef] [PubMed]
- Institute Guidelines. M07-08-Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In Clinical and Laboratory Sandards, 8th ed.; Institute Guidelines: Wayne, PA, USA, 2009; pp. 3073–3099. [Google Scholar]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Costa, M.R.; Pereira, M.F.; Pereira, J.O.; Soares, J.C.; Pintado, M.M. Aqueous extracts of Vaccinium corymbosum as inhibitors of Staphylococcus aureus. Food Control 2015, 51, 314–320. [Google Scholar] [CrossRef]
- Sousa, S.; Pinto, J.; Pereira, C.; Malcata, F.X.; Pacheco, M.B.; Gomes, A.M.; Pintado, M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod. Process. 2015, 95, 96–105. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Dong, L.; Shi, R.; Wu, Z.; Liu, L.; Zhang, J.; Wu, Z.; Pan, D. Quantitative determination of Nε-(carboxymethyl)lysine in sterilized milk by isotope dilution UPLC-MS/MS method without derivatization and ion pair reagents. Food Chem. 2022, 385, 132697. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.M.E. Production of a food grade blueberry extract rich in anthocyanins: Selection of solvents, extraction conditions and purification method. J. Food Meas. Charact. 2017, 11, 1248–1253. [Google Scholar] [CrossRef]
- Dalgaard, P.; Ross, T.; Kamperman, L.; Neumeyer, K.; McMeekin, T.A. Estimation of bacterial growth rates from turbidimetric and viable count data. Int. J. Food Microbiol. 1994, 23, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Wu, V.C.; Tyler, S.; Edwards, K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010, 139, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.T.; Le, N.T. Extraction of anthocyanins from Clitoria ternatea L. petals in Vietnam and determination of its antioxidant and antimicrobial activities. Jordan J. Pharm. Sci. 2022, 15, 145–157. [Google Scholar] [CrossRef]
- Li, L.; Zhou, P.; Wang, Y.; Pan, Y.; Chen, M.; Tian, Y.; Zhou, H.; Yang, B.; Meng, H.; Zheng, J. Antimicrobial activity of cyanidin-3-O-glucoside–lauric acid ester against Staphylococcus aureus and Escherichia coli. Food Chem. 2022, 383, 132410. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kahkonen, M.; Heinonen, M.; Maatta-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Biswas, D.; Wideman, N.E.; O’Bryan, C.A.; Muthaiyan, A.; Lingbeck, J.M.; Crandall, P.G.; Ricke, S.C. Pasteurized blueberry (Vaccinium corymbosum) juice inhibits growth of bacterial pathogens in milk but allows survival of probiotic bacteria. J. Food Saf. 2012, 32, 204–209. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.T.Y.; Tang, Q.; Xue, C.; Li, R.W.; Wu, V.C.H. Malvidin 3-Glucoside Modulated Gut Microbial Dysbiosis and Global Metabolome Disrupted in a Murine Colitis Model Induced by Dextran Sulfate Sodium. Mol. Nutr. Food Res. 2019, 63, e1900455. [Google Scholar] [CrossRef]
- Hidalgo, M.; Concha, M.J.O.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; De Pascual-Teresa, S. Metabolism of Anthocyanins by Human Gut Microflora and Their Influence on Gut Bacterial Growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S. Impact of polyphenols on human gut microbiome and associated biomarkers. In Technologies to Recover Polyphenols from AgroFood By-Products and Wastes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–40. [Google Scholar]
- Cheng, J.-R.; Liu, X.-M.; Chen, Z.-Y.; Zhang, Y.-S.; Zhang, Y.-H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota in vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Yoshida, S.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Homo- d -Lactic Acid Fermentation from Arabinose by Redirection of the Phosphoketolase Pathway to the Pentose Phosphate Pathway in l -Lactate Dehydrogenase Gene-Deficient Lactobacillus plantarum. Appl. Environ. Microbiol. 2009, 75, 5175–5178. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Ray, R.C.; Joshi, V. Fermented foods: Past, present and future. In Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–36. [Google Scholar]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Hadinejad, M.; Emam-Djomeh, Z.; Mirzapour, M. Effect of Fermentation of Pomegranate Juice by Lactobacillus plantarum and Lactobacillus acidophilus on the Antioxidant Activity and Metabolism of Sugars, Organic Acids and Phenolic Compounds. Food Biotechnol. 2013, 27, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, H.; Shao, S.; Zhao, L.; Zhang, R.; Zhang, S. Anthocyanins from Opuntia ficus-indica Modulate Gut Microbiota Composition and Improve Short-Chain Fatty Acid Production. Biology 2022, 11, 1505. [Google Scholar] [CrossRef]
- Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; Van Sinderen, D. Fermented functional foods based on probiotics and their biogenic metabolites. Curr. Opin. Biotechnol. 2005, 16, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Lankaputhra, W.; Shah, N. Antimutagenic properties of probiotic bacteria and of organic acids. Mutat. Res. Mol. Mech. Mutagen. 1998, 397, 169–182. [Google Scholar] [CrossRef]
- Makras, L.; De Vuyst, L. The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int. Dairy J. 2006, 16, 1049–1057. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G941–G950. [Google Scholar]
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Spadafora, P.; Eshuis, H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991, 53, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Brul, S. Preservative agents in foods Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pizzocaro, F.; Torreggiani, D.; Gilardi, G. Inhibition of apple polyphenoloxidase (ppo) by ascorbic acid, citric acid and sodium chloride. J. Food Process. Preserv. 1993, 17, 21–30. [Google Scholar] [CrossRef]
- Eswaranandam, S.; Hettiarachchy, N.S.; Johnson, M.G. Antimicrobial Activity of Citric, Lactic, Malic, or Tartaric Acids and Nisin-incorporated Soy Protein Film Against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J. Food Sci. 2004, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Sallam, K.I.; Sallam, K.I. Chemical, sensory and shelf life evaluation of sliced salmon treated with salts of organic acids. Food Chem. 2007, 101, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Minervini, G.; Rizzello, C.G.; De Angelis, M.; Gobbetti, M. Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiol. 2011, 28, 1062–1071. [Google Scholar] [CrossRef]
- Fong, R.A.; Kepner, R.E.; Webb, A.D. Acetic-Acid-Acylated Anthocyanin Pigments in the Grape Skins of a Number of Varieties of Vitis Vinifera. Am. J. Enol. Vitic. 1971, 22, 150–155. [Google Scholar]
- Anderson, D.; Gueffroy, D.; Webb, A.; Kepner, R. Identification of acetic acid as an acylating agent of anthocyanin pigments in grapes. Phytochemistry 1970, 9, 1579–1583. [Google Scholar] [CrossRef]
- Kopjar, M.; Jakšić, K.; Piližota, V. Influence of sugars and chlorogenic acid addition on anthocyanin content, antioxidant activity and color of blackberry juice during storage. J. Food Process. Preserv. 2012, 36, 545–552. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.Y.; Tung, M.A.; Speers, R.A. Anthocyanins as food colorants? A review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Yue, W.; Han, F. Effects of monoglucoside and diglucoside anthocyanins from Yan 73 (Vitis vinifera L.) and spine grape (Vitis davidii Foex) skin on intestinal microbiota in vitro. Food Chem. X 2022, 16, 100501. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Díaz, A.; Ng, K.M.; Thomsen, T.; Real-Ramírez, I.; Dahan, D.; Dittmar, S.; Gonzalez, C.G.; Chavez, T.; Vasquez, K.S.; Nguyen, T.H.; et al. Establishment and characterization of stable, diverse, fecal-derived in vitro microbial communities that model the intestinal microbiota. Cell Host Microbe 2022, 30, 260–272.e5. [Google Scholar] [CrossRef] [PubMed]
- Rosales, T.K.O.; Hassimotto, N.M.A.; Lajolo, F.M.; Fabi, J.P. Nanotechnology as a Tool to Mitigate the Effects of Intestinal Microbiota on Metabolization of Anthocyanins. Antioxidants 2022, 11, 506. [Google Scholar] [CrossRef]
- Gui, H.; Sun, L.; Liu, R.; Si, X.; Li, D.; Wang, Y.; Shu, C.; Sun, X.; Jiang, Q.; Qiao, Y.; et al. Current knowledge of anthocyanin metabolism in the digestive tract: Absorption, distribution, degradation, and interconversion. Crit. Rev. Food Sci. Nutr. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E.; Zduńczyk, Z.; Juśkiewicz, J.; Klewicki, R. Effects of Lactofermented Beetroot Juice Alone or with N-nitroso-N-methylurea on Selected Metabolic Parameters, Composition of the Microbiota Adhering to the Gut Epithelium and Antioxidant Status of Rats. Nutrients 2015, 7, 5905–5915. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Zhao, J.; Zhang, H.; Chen, W. Untargeted metabolomics reveals metabolic state of Bifidobacterium bifidum in the biofilm and planktonic states. Lwt 2019, 118, 108772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.; Costa, E.M.; Machado, M.; Morais, R.M.; Calhau, C.; Pintado, M. Selective Activity of an Anthocyanin-Rich, Purified Blueberry Extract upon Pathogenic and Probiotic Bacteria. Foods 2023, 12, 734. https://doi.org/10.3390/foods12040734
Silva S, Costa EM, Machado M, Morais RM, Calhau C, Pintado M. Selective Activity of an Anthocyanin-Rich, Purified Blueberry Extract upon Pathogenic and Probiotic Bacteria. Foods. 2023; 12(4):734. https://doi.org/10.3390/foods12040734
Chicago/Turabian StyleSilva, Sara, Eduardo M. Costa, Manuela Machado, Rui M. Morais, Conceição Calhau, and Manuela Pintado. 2023. "Selective Activity of an Anthocyanin-Rich, Purified Blueberry Extract upon Pathogenic and Probiotic Bacteria" Foods 12, no. 4: 734. https://doi.org/10.3390/foods12040734
APA StyleSilva, S., Costa, E. M., Machado, M., Morais, R. M., Calhau, C., & Pintado, M. (2023). Selective Activity of an Anthocyanin-Rich, Purified Blueberry Extract upon Pathogenic and Probiotic Bacteria. Foods, 12(4), 734. https://doi.org/10.3390/foods12040734







