Spray-Drying Microencapsulation of Andean Blueberry (Vaccinium meridionale Sw.) Anthocyanins Using Prickly Pear (Opuntia ficus indica L.) Peel Mucilage or Gum Arabic: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Vegetal Materials
2.3. Spray-Drying Microencapsulation
2.4. Physicochemical and Functional Characterization of Microcapsules
2.4.1. Total Anthocyanin Contents
2.4.2. Analysis of Anthocyanins by HPLC–MS
2.4.3. Antioxidant Activity by Oxygen Radical Absorbance Capacity (ORAC)
2.4.4. Dietary Fiber Content
2.4.5. Color Parameters
2.4.6. Particle Size and Polydispersity Index
2.4.7. Scanning Electron Microscopy (SEM)
2.4.8. Thermal Characterization
2.5. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity and Contents of Anthocyanins and Dietary Fiber
3.2. CIELab Color Space
3.3. Anthocyanin Identification by HPLC-MS/MS
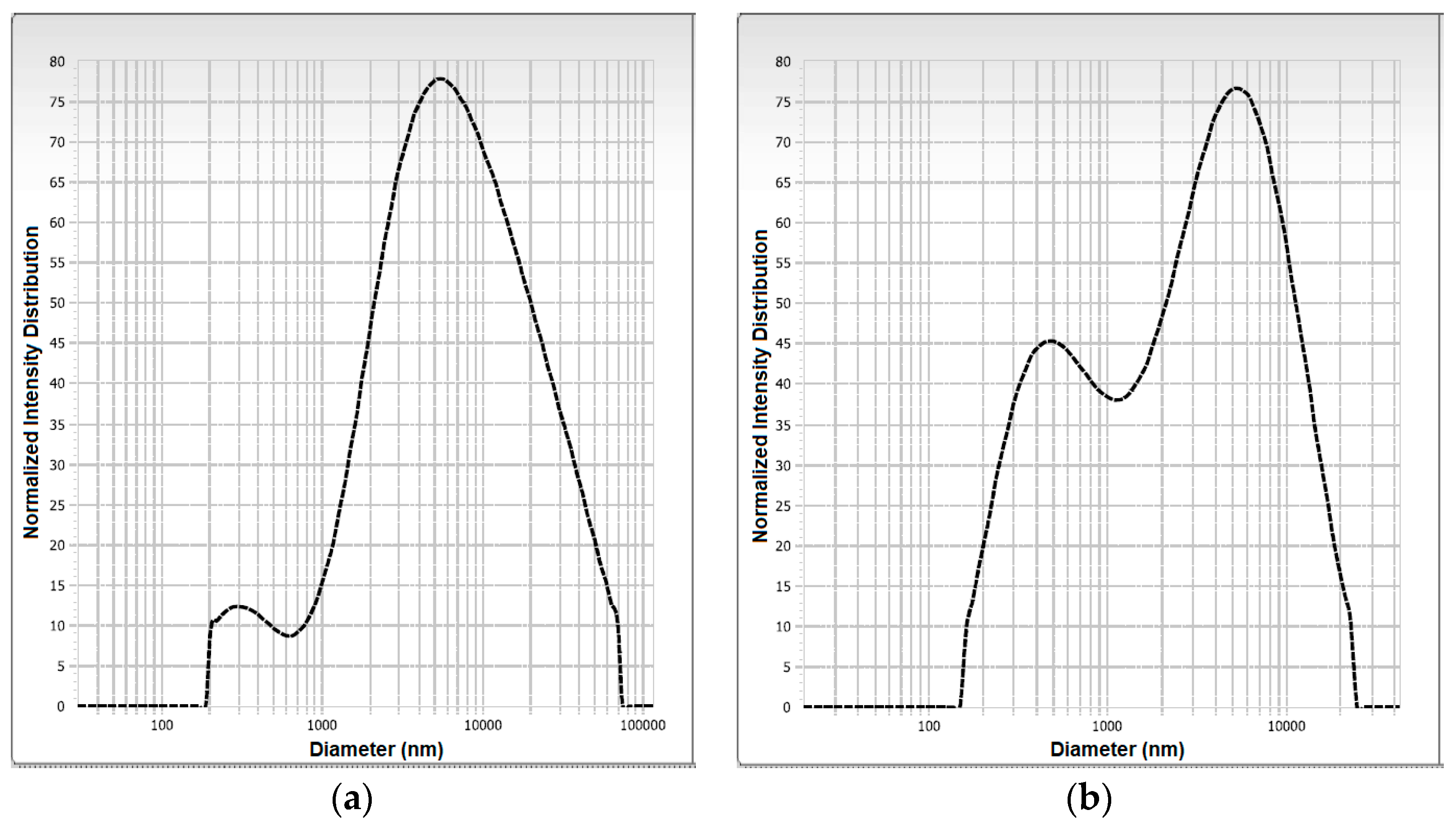
3.4. Microscopic Morphology and Particle Size
3.5. Thermal Characterization
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, S.; Nagababu, B.H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. Advan. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Niaz, K.; Khan, F. Analysis of polyphenolics. In Recent Advances in Natural Products Analysis; Silva, A.S., Nabavi, S.F., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–197. [Google Scholar]
- Garzon, G.A.; Soto, C.Y.; Lopez-R, M.; Riedl, K.M.; Browmiller, C.R.; Howard, L. Phenolic profile, in vitro antimicrobial activity and antioxidant capacity of Vaccinium meridionale Swartz pomace. Heliyon 2020, 6, 03845. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Campos-Vega, R.; Loarca-Piña, G.; Maldonado- Celis, M.E. Bioaccessibility during in vitro digestion and antiproliferative effect of bioactive compounds from Andean Berry (Vaccinium meridionale Swartz) juice. J. Agric. Food Chem. 2018, 66, 7358–7736. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. BlueBerry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin Stability and Recovery: Implications for the Analysis of Clinical and Experimental Samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Ortega-Regules, A.E.; Lozada-Ramírez, J.D.; Pérez-Pérez, M.C.I.; Vernon-Carter, E.J.; Welti-Chanes, J. Color and chemical stability of spray-dried blueBerry extract using mesquite gum as wall material. J. Food Compos. Anal. 2011, 24, 889–894. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueBerry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In vitro release properties of encapsulated blueBerry (Vaccinium ashei) extracts. Food Chem. 2015, 168, 225–232. [Google Scholar] [CrossRef]
- Rosa, J.R.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; de Menezes, C.R.; da Rosa, C.S. Microencapsulation of anthocyanin compounds extracted from blueBerry (Vaccinium spp.) by spray drying: Characterization, stability and simulated gastrointestinal conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Rocha, J.d.C.G.; de Barros, F.A.R.; Perrone, Í.T.; Viana, K.W.C.; Tavares, G.M.; Stephani, R.; Stringheta, P.C. Microencapsulation by atomization of the mixture of phenolic extracts. Powder Technol. 2019, 343, 317–325. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Cifuentes, G.R.; Gómez Castaño, J.A. Use of Opuntia ficus-indica Fruit Peel as a Novel Source of Mucilage with Coagulant Physicochemical/Molecular Characteristics. Polymers 2022, 14, 3832. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Mucilage from Yellow Pitahaya (Selenicereus megalanthus) Fruit Peel: Extraction, Proximal Analysis, and Molecular Characterization. Molecules 2023, 28, 786. [Google Scholar] [CrossRef]
- Gheribi, R.; Habibi, Y.; Khwaldia, K. Prickly pear peels as a valuable resource of added-value polysaccharide: Study of structural, functional and film forming properties. Int. J. Biol. Macromol. 2019, 126, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; WilchesTorres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers 2022, 14, 310. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, V.K.; Uvanesh, K.; Biswal, D.; Anis, A.; Rana, U.A.; Pal, K. Development of ionic and non-ionic natural gum-based bigels: Prospects for drug delivery application. J. Appl. Polym. Sci. 2015, 132, 42561. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Saenz, C. Microparticles of yellow-orange cactus pear pulp (Opuntia ficus-indica) with cladode mucilage and maltodextrin as a food coloring in yogurt. LWT Food Sci. Technol. 2021, 138, 110672. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L. Assays for hydrophilic and lipophilic antioxidant capacity oxygen radical absorbance capacity (ORAC FL) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Cunniff, P. Enzymatic-gravimetric method. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Application of tristimulus colorimetry to estimate the carotenoids content in ultrafrozen orange juices. J. Agric. Food Chem. 2003, 51, 7266–7270. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, Y. Biopolymer-based encapsulation of anthocyanins as reinforced natural colorants for food applications. J. Sci. Food Agric. 2023, 11, 100488. [Google Scholar] [CrossRef]
- Us-Medina, U.; Julio, L.M.; Segura-Campos, M.R.; Ixtaina, V.Y.; Tomas, M.C. Development and characterization of spray-dried chia oil microcapsules using by- products from chia as wall material. Powder Technol. 2018, 334, 1–8. [Google Scholar] [CrossRef]
- Kaushik, V.; Roos, Y. Limonene encapsulation in freeze-drying of gum Arabic–sucrose-gelatin systems. LWT Food Sci. Technol. 2006, 40, 1381–1391. [Google Scholar] [CrossRef]
- Tabio-García, D.; Paraguay-Delgado, F.; Lardizabal Gutiérrez, D.; Quintero-Ramos, A.; Meléndez-Pizarro, C.O.; Ochoa-Martínez, L.A.; Sánchez-Madrigal, M.A.; Ruiz-Gutiérrez, M.G.; Espinoza-Hicks, J.C. Effectiveness of Opuntia ficus-indica mucilage as a carrier agent in microencapsulation of bioactive compounds of Amaranthus hypochondriacus var. Nutrisol. Food Biosci. 2023, 52, 102368. [Google Scholar] [CrossRef]
- Mariod, A.A. 20—Gum Arabic Dietary Fiber. In Gum Arabic: Structure, Properties, Application and Economics; Mariod, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 237–243. [Google Scholar]
- Antigo, J.L.D.; Stafussa, A.P.; Bergamasco, R.D.C.; Madrona, G.S. Chia seed mucilage as a potential encapsulating agent of a natural food dye. J. Food Eng. 2022, 285, 110101. [Google Scholar] [CrossRef]
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crop. Prod. 2018, 111, 846–855. [Google Scholar] [CrossRef]
- de Araujo Santiago, M.C.P.; Nogueira, R.I.; Paim, D.R.S.F.; Gouvea, A.C.M.S.; de Oliveira Godoy, R.L.; Peixoto, F.M.; Pacheco, S.; Freitas, S.P. Effects of encapsulating agents on anthocyanin retention in pomegranate powder obtained by the spray drying process. LWT Food Sci. Technol. 2016, 73, 551–556. [Google Scholar] [CrossRef]
- Ahmada, M.; Ashrafa, B.; Ganib, A.; Gania, A. Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behavior antioxidant potential during in-vitro digestion. J. Biol. Macromol. 2018, 109, 432–445. [Google Scholar] [CrossRef]
- Machado, M.H.; Almeida, A.D.R.; Maciel, M.V.D.O.B.; Vitorino, V.B.; Bazzo, G.C.; da Rosa, C.G.; Sganzerla, W.G.; Mendes, C.; Barreto, P.L.M. Microencapsulation by spray drying of red cabbage anthocyanin-rich extract for the production of a natural food colorant. Biocatal. Agric. Biotechnol. 2022, 39, 102287. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Microencapsulation of betacyanin from dragon fruit peel by complex coacervation: Physicochemical characteristics, thermal stability, and release profile of microcapsules. Food Biosci. 2022, 49, 101882. [Google Scholar] [CrossRef]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.A.; Calderas, F.; González- Laredo, R.F.; Rocha-Guzmán, N.E.; Ochoa-Martínez, L.A.; Bernad-Bernad, M.J. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; González-Laredo, R.F.; Minjares-Fuentes, R.; Valadez-García, M.A.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation of gallic acid by spray drying with aloe vera mucilage (Aloe barbadensis miller) as wall material. Ind. Crop. Prod. 2019, 138, 111461. [Google Scholar] [CrossRef]
- Kwak, H.-S.; Al Mijan, M.; Ganesan, P. Application of nanomaterials, nano-and microencapsulation to milk and dairy products. Nano-Microencapsul. Foods 2014, 1, 273–300. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- de Campo, C.; Dick, M.; dos Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.; Rios, A.D.O.; Flôres, S.H. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Colloids Surf. A 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Mohammed, J.K.; AL-Ansi, W.; Ghaleb, A.D.S.; Al-Maqtari, Q.-A.; Ma, M.; Ahmed, M.I.; Wang, H. Microencapsulation of fingered citron extract with gum arabic, modified starch, whey protein, and maltodextrin using spray drying. Int. J. Biol. Macromol. 2020, 152, 1125–1134. [Google Scholar] [CrossRef]
- Soto-Castro, D.; Gutiérrez Miguel Chávez, M.; León-Martínez, F.; Santiago-García, P.A.; Aragón-Lucero, I.; Antonio-Antonio, F. Spray drying microencapsulation of betalain rich extracts from Escontria chiotilla and Stenocereus queretaroensis fruits using cactus mucilage. Food Chem. 2019, 272, 715–722. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of curcumin using coconut milk whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Dellarosa, N.; Tylewicz, U.; Laghi, L.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. The influence of carrier material on some physical and structural properties of carrot juice microcapsules. Food Chem. 2017, 236, 134–141. [Google Scholar] [CrossRef] [PubMed]
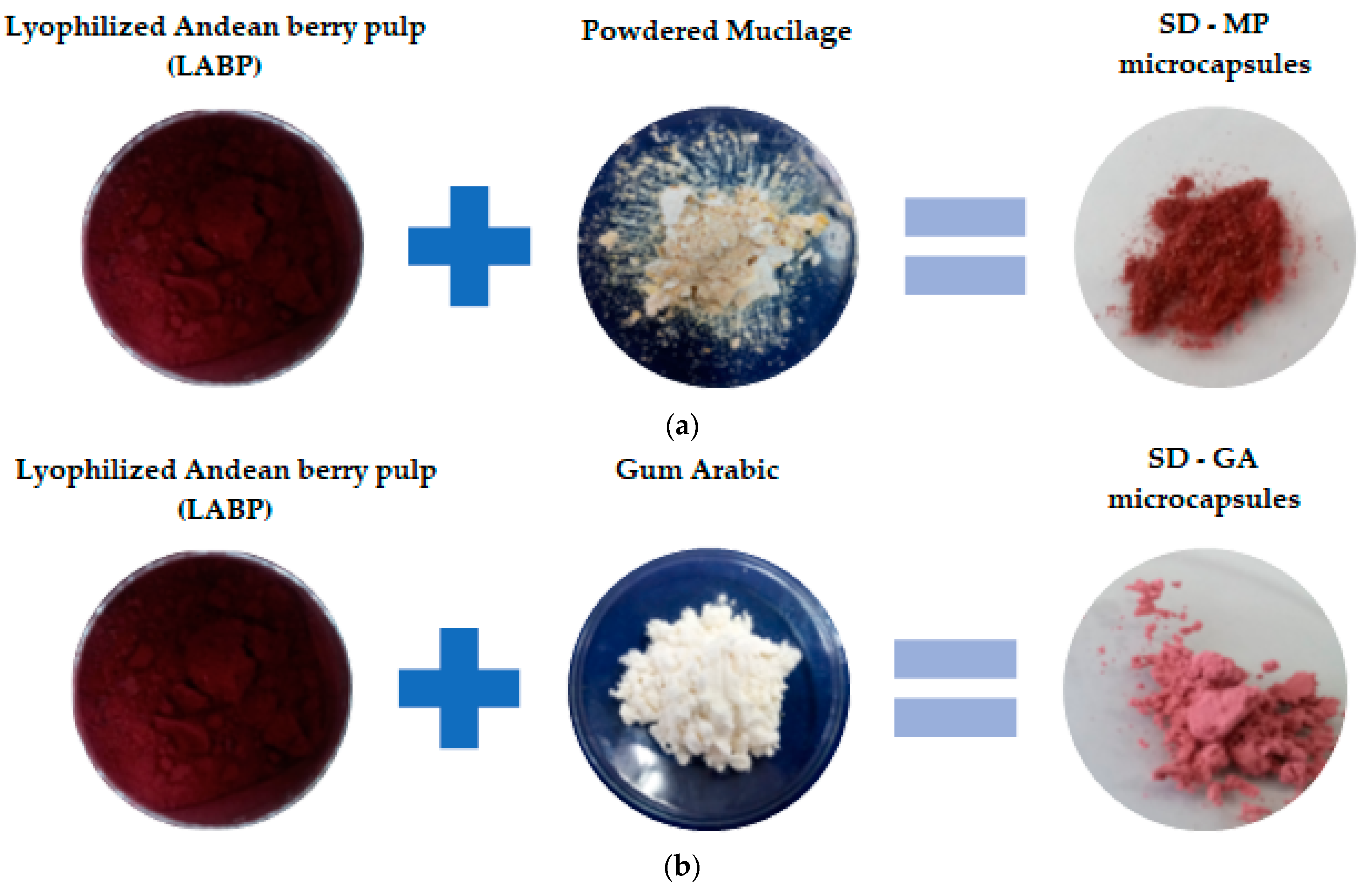


| Microcapsules | Proportion (LABP (g): Wall Material (mL)) [g LABP:g Wall Material] |
|---|---|
| SD-MP | (6.0:100) [6.0:1.0] |
| SD-GA | (6.0:100) [6.0:1.2] |
| Parameter | LABP | SD-MP | SD-GA |
|---|---|---|---|
| PY (%) | 23.90 | 69.22 | |
| TAC 1 | 2057.54 | 1082.08 | 216.13 |
| ORAC 2 | 97.95 ± 0.54 b | 100.05 ± 0.07 a | 84.31 ± 4.00 c |
| TDF 3 | - | 34.81 | 63.24 |
| Color Parameter | SD-MP | SD-GA |
|---|---|---|
| L* (luminosity) | 30.78 ± 0.49 b | 41.52 ± 0.05 a |
| a* | 18.13 ± 0.14 b | 24.18 ± 0.00 a |
| b* | 8.27 ± 0.01 a | 4.70 ± 0.00 b |
| (chroma) | 19.93 ± 0.13 b | 24.64 ± 0.01 a |
| hab* (hue) | 24.51 ± 0.16 a | 11.02 ± 0.02 b |
| Basic Structure | Name | Formula | R3 | R5 | R6 | R7 | R3′ | R4′ | R5′ | [M + H]+ | LABP | SD-MP | SD-GA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | Peonidin-3-O-alpha-arabinoside | C21H20O10 | O-α-Ara | OH | H | =O | H | OH | OMe | 433.11 | 3.5% | 0.1% | 0.1% |
| Delphinidin-3-arabinoside | C20H19O11 | O-α-Ara | OH | H | OH | OH | OH | OH | 435.09 | 7.4% | 0.5% | 0.1% | |
| Cyanidin-3-O-alpha-arabinopyranoside | C20H19O10 | O-Arapyr | OH | H | OH | H | OH | H | 419.09 | 40.4% | 60.0% | 52.7% | |
| Delphinidin-3-pyranoside | C21H21O12 | O-Pyr | OH | H | OH | OH | OH | OH | 465.10 | 5.6% | 0.5% | 0.1% | |
| Cyanidin-3-O-galactoside (Ideain) | C21H21O11 | O-Gal | OH | H | OH | OH | OH | H | 449,11 | 42.9% | 38.8% | 46.9% | |
| Delphinidin-3-(6-p-coumaroyl-glucoside) | C30H27O14 | O-6p-Cou | OH | H | OH | OH | OH | OH | 611,14 | 0.1% | 0.1% | 0.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Andean Blueberry (Vaccinium meridionale Sw.) Anthocyanins Using Prickly Pear (Opuntia ficus indica L.) Peel Mucilage or Gum Arabic: A Comparative Study. Foods 2023, 12, 1811. https://doi.org/10.3390/foods12091811
Otálora MC, Wilches-Torres A, Gómez Castaño JA. Spray-Drying Microencapsulation of Andean Blueberry (Vaccinium meridionale Sw.) Anthocyanins Using Prickly Pear (Opuntia ficus indica L.) Peel Mucilage or Gum Arabic: A Comparative Study. Foods. 2023; 12(9):1811. https://doi.org/10.3390/foods12091811
Chicago/Turabian StyleOtálora, Maria Carolina, Andrea Wilches-Torres, and Jovanny A. Gómez Castaño. 2023. "Spray-Drying Microencapsulation of Andean Blueberry (Vaccinium meridionale Sw.) Anthocyanins Using Prickly Pear (Opuntia ficus indica L.) Peel Mucilage or Gum Arabic: A Comparative Study" Foods 12, no. 9: 1811. https://doi.org/10.3390/foods12091811





