Effect of Ultrasound Combinated with Sodium Hypochlorite Treatment on Microbial Inhibition and Quality of Fresh-Cut Cucumber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Treatment
2.3. Quantification of Microorganisms
2.4. Evaluation of Quality Parameters
2.4.1. Weight Loss and Firmness
2.4.2. Malondialdehyde Content
2.4.3. Analysis of Water Mobility and Distribution
2.4.4. Color Evaluation
2.4.5. Chlorophyll Concentration
2.4.6. Flavor Analysis
2.4.7. Volatile Organic Compounds
2.4.8. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbial Analysis
3.2. Texture Properties Analysis
3.2.1. Firmness
3.2.2. Weight Loss
3.2.3. Malondialdehyde Content
3.2.4. Water Mobility and Distribution
3.3. Color Analysis
3.3.1. Color
3.3.2. Chlorophyll Content
3.4. Flavor Analysis
3.4.1. Flavor
3.4.2. Volatile Organic Compounds
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martin-Diana, A.B.; Rico, D.; Frias, J.; Henehan, G.T.M.; Mulcahy, J.; Barat, J.M.; Barry-Ryan, C. Effect of calcium lactate and heat-shock on texture in fresh-cut lettuce during storage. J. Food Eng. 2006, 77, 1069–1077. [Google Scholar] [CrossRef]
- Wang, F.; Mi, S.; Chitrakar, B.; Li, J.; Wang, X. Effect of Cold Shock Pretreatment Combined with Perforation-Mediated Passive Modified Atmosphere Packaging on Storage Quality of Cucumbers. Food Sci. 2022, 11, 1267. [Google Scholar] [CrossRef]
- Jia, B.; Zheng, Q.; Zuo, J.; Gao, L.; Wang, Q.; Guan, W.; Shi, J. Application of postharvest putrescine treatment to maintain the quality and increase the activity of antioxidative enzyme of cucumber. Sci. Hortic. 2018, 239, 210–215. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, Y.; Ma, Y.; Tong, J.; Zhang, J.; Zhang, Y.; Zhao, X. Microbiological and physiological attributes of fresh-cut cucumbers in controlled atmosphere storage. J. Food Prot. 2020, 8310, 1718–1725. [Google Scholar] [CrossRef]
- Ssemanda, J.N.; Joosten, H.; Bagabe, M.C.; Zwietering, M.H.; Reij, M.W. Reduction of microbial counts during kitchen scale washing and sanitization of salad vegetables. Food Control 2018, 85, 495–503. [Google Scholar] [CrossRef]
- Yildiz, G.; Izli, G.; Aadil, R.M. Comparison of chemical, physical, and ultrasound treatments on the shelf life of fresh-cut quince fruit (Cydonia oblonga Mill.). J. Food Process. Preserv. 2020, 44, e14366. [Google Scholar] [CrossRef]
- Demirok, N.T.; Yıkmış, S. Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens. Processes 2022, 10, 2100. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. Lebensm.-Wiss. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Mujumdar, A.S.; Liu, K. Antibacterial mechanism of ultrasound combined with sodium hypochlorite and their application in pakchoi (Brassica campestris L. ssp. chinensis). J. Sci. Food Agric. 2022, 30, 4685–4696. [Google Scholar] [CrossRef]
- Hopkins, D.Z.; Parisi, M.A.; Dawson, P.L.; Northcutt, J.K. Surface decontamination of fresh, whole peaches (Prunus persica) using sodium hypochlorite or acidified electrolyzed water solutions. Int. J. Fruit Sci. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Yang, C.H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef]
- Lee, N.Y.; Kim, S.W.; Ha, S.D. Synergistic effects of ultrasound and sodium hypochlorite (NaOCl) on reducing Listeria monocytogenes ATCC19118 in broth, stainless steel, and iceberg lettuce. Foodborne Pathog. Dis. 2014, 11, 581–587. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Gil, M.I.; Truchado, P.; Selma, M.V.; Allende, A. Cross-contamination of fresh-cut lettuce after a short-term exposure during pre-washing cannot be controlled after subsequent washing with chlorine dioxide or sodium hypochlorite. Food Microbiol. 2010, 27, 199–204. [Google Scholar] [CrossRef]
- Wang, J.; Huang, K.; Wu, Z.; Yu, Y. Effects of ultrasound-assisted low-concentration chlorine washing on ready-to-eat winter jujube (Zizyphus jujuba Mill. cv. Dongzao): Cross-contamination prevention, decontamination efficacy, and fruit quality. Ultrason. Sonochem. 2022, 82, 105905. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, S.; Cai, Y.; Zheng, Y. Combination of salicylic acid and ultrasound to control postharvest blue mold caused by Penicillium expansum in peach fruit. Innov. Food Sci. Emerg. Technol. 2011, 12, 310–314. [Google Scholar] [CrossRef]
- Lagnika, C.; Zhang, M.; Mothibe, K.J. Effects of ultrasound and high pressure argon on physico-chemical properties of white mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2013, 82, 87–94. [Google Scholar] [CrossRef]
- Moosavi, M.H.; Khaneghah, A.M.; Javanmardi, F.; Hadidi, M.; Hadian, Z.; Jafarzadeh, S.; Sant’Ana, A.S. A review of recent advances in the decontamination of mycotoxin and inactivation of fungi by ultrasound. Ultrason. Sonochem. 2021, 79, 105755. [Google Scholar] [CrossRef]
- De São José, J.F.B.; De Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Guo, M.; Jin, T.Z.; Arabi, S.A.; Liu, D. Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157: H7 on cherry tomatoes. Int. J. Food Microbiol. 2021, 337, 108936. [Google Scholar] [CrossRef]
- Bang, H.J.; Park, S.Y.; Kim, S.E.; Rahaman, M.M.F.; Ha, S.D. Synergistic effects of combined ultrasound and peroxyacetic acid treatments against Cronobacter sakazakii biofilms on fresh cucumber. Lebensm.-Wiss. Technol. 2017, 84, 91–98. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Jiang, F. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochem. 2019, 54, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, R.B.; Annapure, U.S. Integrated effect of sodium hypochlorite and modified atmosphere packaging on quality and shelf life of fresh-cut cilantro. Food Packag. Shelf Life 2015, 3, 62–69. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Ma, Y.; Liang, H.; Wang, D.; Zhao, X. Shifts in the Bacterial Community Related to Quality Properties of Vacuum-Packaged Peeled Potatoes during Storage. Food Sci. 2022, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Yagoub, A.E.A.; Owusu-Ansah, P.; Wahia, H.; Ma, H.; Zhou, C. Effects of low frequency multi-mode ultrasound and it’s washing solution’s interface properties on freshly cut cauliflower. Food Chem. 2022, 366, 130683. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, L. Effect of ultrasound treatment on microbial inhibition and quality maintenance of green asparagus during cold storage. Ultrason. Sonochem. 2019, 58, 104631. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Zhao, W.; Zheng, Y.; Wang, Y.; Wang, P.; Zhao, X. Low frequency ultrasound treatment enhances antibrowning effect of ascorbic acid in fresh-cut potato slices. Food Chem. 2022, 380, 132190. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, D.; Han, S.; Li, S.; Gong, N.; Fan, Y.; Ji, X. Characterization of the chitinase gene family in mulberry (Morus notabilis) and MnChi18 involved in resistance to botrytis cinerea. Genes 2022, 13, 98. [Google Scholar] [CrossRef]
- Gao, C.; Mumtaz, M.A.; Zhou, Y.; Yang, Z.; Shu, H.; Zhu, J.; Wang, Z. Integrated transcriptomic and metabolomic analyses of cold-tolerant and cold-sensitive pepper species reveal key genes and essential metabolic pathways involved in response to cold stress. Int. J. Mol. Sci. 2022, 23, 6683. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Wei, B.; Zeng, R. Low frequency ultrasound pretreatment of carrot slices: Effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrason. Sonochem. 2018, 40, 619–628. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Ma, Y.; Zhang, M.; Zhao, Y.; Zhao, X. Effect of UV-C treatment on the quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root. Food Chem. 2019, 278, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.L.A.; do Rosário, D.K.A.; Oliveira, S.B.S.; de Souza, H.L.S.; De Carvalho, R.V.; Carneiro, J.C.S.; Bernardes, P.C. Ultrasound improves antimicrobial effect of sodium dichloroisocyanurate to reduce Salmonella Typhimurium on purple cabbage. Int. J. Food Microbiol. 2018, 269, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Hu, Z.; Pang, B. Optimization of postharvest ultrasonic treatment of strawberry fruit. Postharvest Biol. Tec. 2010, 55, 150–153. [Google Scholar] [CrossRef]
- Park, S.Y.; Mizan, M.F.R.; Ha, S.D. Inactivation of Cronobacter sakazakii in head lettuce by using a combination of ultrasound and sodium hypochlorite. Food Control 2016, 60, 582–587. [Google Scholar] [CrossRef]
- Rosario, D.K.; Duarte, A.L.A.; Madalao, M.; Libardi, M.C.; Teixeira, L.J.; Conte-Junior, C.A.; Bernardes, P.C. Ultrasound improves antimicrobial effect of sodium hypochlorite and instrumental texture on fresh-cut yellow melon. J. Food Quality 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Toivonen, P.M.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Li, N.; Chen, F.; Cui, F.; Sun, W.; Zhang, J.; Qian, L.; Yang, H. Improved postharvest quality and respiratory activity of straw mushroom (Volvariella volvacea) with ultrasound treatment and controlled relative humidity. Sci. Hortic. 2017, 225, 56–64. [Google Scholar] [CrossRef]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control. 2009, 20, 508–514. [Google Scholar] [CrossRef]
- Zhou, R.; Mo, Y.; Li, Y.; Zhao, Y.; Zhang, G.; Hu, Y. Quality and internal characteristics of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) treated with different kinds of coatings during storage. Postharvest Biol. Technol. 2008, 491, 171–179. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; Muy-Rangel, M.D.; Mascorro, A.G. Fruit size and stage of ripeness affect postharvest water loss in bell pepper fruit (Capsicum annuum L.). J. Sci. Food Agric. 2007, 87, 68–73. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Adhikari, B.; Guo, Z. Effect of ultrasound combined with controlled atmosphere on postharvest storage quality of cucumbers (Cucumis sativus L.). Food Bioprocess Technol. 2018, 11, 1328–1338. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Murtaza, A.; Iqbal, A.; Zhang, J.; Zhu, L.; Hu, W. High-pressure carbon dioxide treatment alleviates browning development by regulating membrane lipid metabolism in fresh-cut lettuce. Food Control 2022, 134, 108749. [Google Scholar] [CrossRef]
- Wu, S.; Nie, Y.; Zhao, J.; Fan, B.; Huang, X.; Li, X.; Tang, X. The synergistic effects of low-concentration acidic electrolyzed water and ultrasound on the storage quality of fresh-sliced button mushrooms. Food Bioprocess Technol. 2018, 11, 314–323. [Google Scholar] [CrossRef]
- Lu, R.; Ma, Y.; Wang, X.; Zhao, X.; Liang, H.; Wang, D. Study of texture properties of ‘laba’ garlic in different color states and their change mechanisms. Int. J. Food Sci. Technol. 2021, 56, 4710–4721. [Google Scholar] [CrossRef]
- Francisco, C.A.I.; Araújo Naves, E.A.; Ferreira, D.C.; Rosário, D.K.A.D.; Cunha, M.F.; Bernardes, P.C. Synergistic effect of sodium hypochlorite and ultrasound bath in the decontamination of fresh arugulas. J. Food Saf. 2018, 38, e12391. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Bhandari, B.; Jiang, F. A combination treatment of ultrasound and ε-polylysine to improve microorganisms and storage quality of fresh-cut lettuce. Lebensm.-Wiss. Technol. 2019, 113, 108315. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.; Ayim, I.; Lu, F.; Zhou, C. Efficacy of sweep ultrasound on natural microbiota reduction and quality preservation of Chinese cabbage during storage. Ultrason. Sonochem. 2019, 59, 104712. [Google Scholar] [CrossRef] [PubMed]
- Berna, A. Metal oxide sensors for electronic noses and their application to food analysis. Sensors 2010, 10, 3882–3910. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cai, D.; Li, W.; Blank, I.; Liu, Y. Application of gas chromatography-ion mobility spectrometry (GC-IMS) and ultrafast gas chromatography electronic-nose (uf-GC E-nose) to distinguish four Chinese freshwater fishes at both raw and cooked status. J. Food Biochem. 2022, 46, e13840. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent advance in aromatic volatile research in tomato fruit: The metabolisms and regulations. Food Bioprocess Technol. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, X.; Ma, Y.; Guan, H.; Liang, H.; Wang, D. Inhibitory effect of modified atmosphere packaging on Escherichia coli O157: H7 in fresh-cut cucumbers (Cucumis sativus L.) and effectively maintain quality during storage. Food Chem. 2022, 369, 130969. [Google Scholar] [CrossRef] [PubMed]




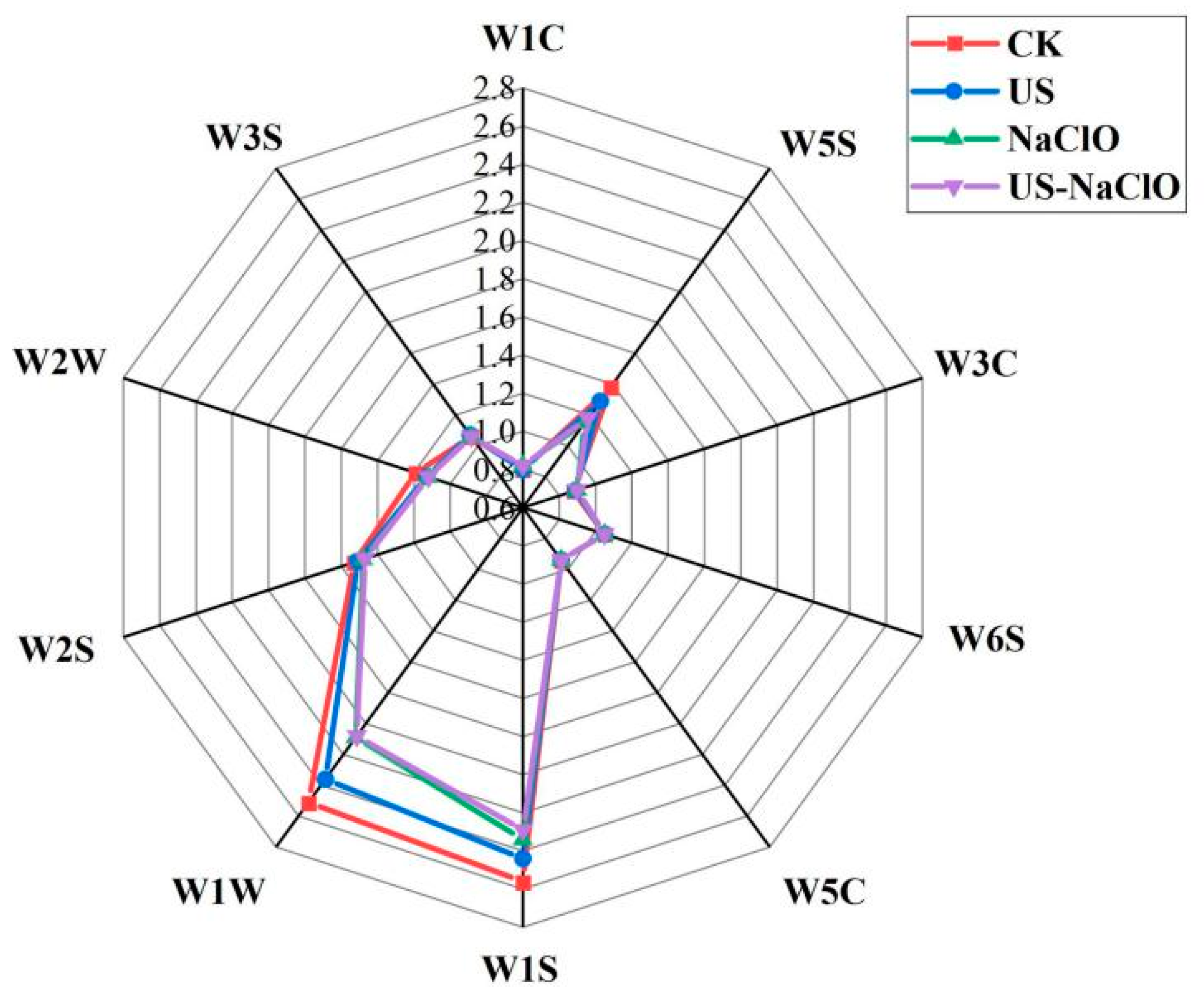



| Storage Time (d) | Treatment | Weight Loss (%) | Firmness (N) |
|---|---|---|---|
| 0 | CK | 0 | 4.88 ± 0.36 a |
| US | 0 | 4.87 ± 0.34 a | |
| NaClO | 0 | 4.90 ± 0.43 a | |
| US-NaClO | 0 | 4.78 ± 0.23 a | |
| 4 | CK | 3.02 ± 0.37 a | 4.09 ± 0.18 a |
| US | 3.03 ± 0.52 a | 4.25 ± 0.29 ab | |
| NaClO | 2.67 ± 0.19 ab | 4.33 ± 0.35 ab | |
| US-NaClO | 2.04 ± 0.14 b | 4.43 ± 0.23 b | |
| 8 | CK | 4.57 ± 0.19 a | 3.85 ± 0.26 a |
| US | 4.65 ± 0.59 a | 4.20 ± 0.24 b | |
| NaClO | 4.09 ± 0.45 a | 4.29 ± 0.37 b | |
| US-NaClO | 3.21 ± 0.26 b | 4.34 ± 0.21 b |
| Storage Time (d) | CK | US | NaClO | US-NaClO | |
|---|---|---|---|---|---|
| T23 (ms) | 0 | 1072.27 ± 0.00 a | 908.31 ± 54.32 b | 1125.79 ± 92.71 a | 1112.41 ± 80.29 a |
| 4 | 1002.44 ± 98.76 b | 1125.79 ± 92.71 ab | 1232.85 ± 0.00 a | 1232.85 ± 0.00 a | |
| 8 | 871.87 ± 85.89 a | 851.62 ± 70.13 a | 871.87 ± 85.89 a | 758.31 ± 74.71 a | |
| A23 (%) | 0 | 92.87 ± 0.26 b | 94.23 ± 0.33 a | 94.22 ± 0.70 a | 93.81 ± 0.30 a |
| 4 | 94.88 ± 0.17 a | 94.91 ± 0.15 a | 93.60 ± 1.23 a | 89.05 ± 0.81 b | |
| 8 | 95.85 ± 0.46 a | 94.95 ± 0.33 a | 95.39 ± 0.35 a | 93.37 ± 0.79 b |
| Count | Compound | CAS | Formula | MW | RI | RT/s | DT/ms |
|---|---|---|---|---|---|---|---|
| 1 | Hexanal | C66251 | C6H12O | 100.2 | 791.7 | 252.978 | 1.55912 |
| 2 | Hexanal | C66251 | C6H12O | 100.2 | 800.9 | 260.662 | 1.25972 |
| 3 | trans-2-pentenal | C1576870 | C5H8O | 84.1 | 746.7 | 218.579 | 1.35592 |
| 4 | trans-2-pentenal | C1576870 | C5H8O | 84.1 | 750.2 | 221.082 | 1.10492 |
| 5 | 3-Pentanone | C96220 | C5H10O | 86.1 | 686.5 | 180.136 | 1.35242 |
| 6 | 3-Pentanone | C96220 | C5H10O | 86.1 | 690 | 181.898 | 1.11129 |
| 7 | 3-Methylbutanal | C590863 | C5H10O | 86.1 | 640.4 | 160.822 | 1.39938 |
| 8 | 3-Methylbutanal | C590863 | C5H10O | 86.1 | 641.3 | 161.168 | 1.19132 |
| 9 | Isopropyl alcohol | C67630 | C3H8O | 60.1 | 500.7 | 114.007 | 1.23512 |
| 10 | Isopropyl alcohol | C67630 | C3H8O | 60.1 | 497.6 | 113.143 | 1.08602 |
| 11 | Heptanal | C111717 | C7H14O | 114.2 | 899.3 | 359.656 | 1.68588 |
| 12 | Heptanal | C111717 | C7H14O | 114.2 | 901.2 | 362.014 | 1.33124 |
| 13 | 2-hexen-1-ol | C2305217 | C6H12O | 100.2 | 860.5 | 316.271 | 1.51138 |
| 14 | 2-hexen-1-ol | C2305217 | C6H12O | 100.2 | 865.9 | 321.93 | 1.17926 |
| 15 | (E)-2-Heptenal | C18829555 | C7H12O | 112.2 | 957.7 | 442.956 | 1.65787 |
| 16 | (E)-2-Heptenal | C18829555 | C7H12O | 112.2 | 958.1 | 443.574 | 1.25437 |
| 17 | 2-pentyl furan | C3777693 | C9H14O | 138.2 | 993.1 | 502.694 | 1.25191 |
| 18 | 2,4-Heptadienal | C5910850 | C7H10O | 110.2 | 1004.7 | 524.576 | 1.19846 |
| 19 | (E,E)-2,4-heptadienal | C4313035 | C7H10O | 110.2 | 1018.2 | 551.77 | 1.19481 |
| 20 | (E,E)-2,4-heptadienal | C4313035 | C7H10O | 110.2 | 1018.4 | 552.195 | 1.61153 |
| 21 | 2,4-Heptadienal | C5910850 | C7H10O | 110.2 | 1004.2 | 523.727 | 1.61639 |
| 22 | 3-Octanone | C106683 | C8H16O | 128.2 | 988.9 | 495.258 | 1.30659 |
| 23 | 3-Octanone | C106683 | C8H16O | 128.2 | 989.9 | 496.958 | 1.71237 |
| 24 | (Z)-6-nonenal | C2277192 | C9H16O | 140.2 | 1103.6 | 758.983 | 1.16983 |
| 25 | (Z)-6-nonenal | C2277192 | C9H16O | 140.2 | 1101.9 | 754.022 | 1.76907 |
| 26 | Nonanal | C124196 | C9H18O | 142.2 | 1105.6 | 764.653 | 1.47671 |
| 27 | Nonanal | C124196 | C9H18O | 142.2 | 1104.9 | 762.527 | 1.93431 |
| 28 | (E,Z)-2,6-nonadienal | C557482 | C9H14O | 138.2 | 1144 | 882.299 | 1.37321 |
| 29 | (E,Z)-2,6-nonadienal | C557482 | C9H14O | 138.2 | 1144.3 | 883.297 | 1.88471 |
| 30 | octanal | C124130 | C8H16O | 128.2 | 1009.1 | 533.24 | 1.80799 |
| 31 | octanal | C124130 | C8H16O | 128.2 | 1008.5 | 532.022 | 1.40866 |
| 32 | Hexyl acetate | C142927 | C8H16O2 | 144.2 | 1023.5 | 562.699 | 1.3826 |
| 33 | 4,5-dihydro-3(2H)-thiophenone | C1003049 | C4H6OS | 102.2 | 946.5 | 425.656 | 1.21219 |
| 34 | n-hexanol | C111273 | C6H14O | 102.2 | 879.1 | 336.019 | 1.98374 |
| 35 | Hexanenitrile | C628739 | C6H11N | 97.2 | 875.6 | 332.251 | 1.57373 |
| 36 | n-hexanol | C111273 | C6H14O | 102.2 | 882.6 | 339.859 | 1.63788 |
| 37 | 1,3-butanediol | C107880 | C4H10O2 | 90.1 | 788.7 | 250.523 | 1.36979 |
| 38 | Butyl formate | C592847 | C5H10O2 | 102.1 | 720.4 | 200.71 | 1.22857 |
| 39 | 2-methyl-1-Pentanol | C105306 | C6H14O | 102.2 | 827.3 | 283.957 | 1.59164 |
| 40 | pentanal | C110623 | C5H10O | 86.1 | 703.3 | 189.923 | 1.40474 |
| 41 | ethyl propanoate | C105373 | C5H10O2 | 102.1 | 704.2 | 190.471 | 1.47653 |
| 42 | 2-methylbutanol | C137326 | C5H12O | 88.1 | 739.5 | 213.543 | 1.46241 |
| 43 | isoamyl acetate | C123922 | C7H14O2 | 130.2 | 875.8 | 332.381 | 1.7438 |
| 44 | 2,6-Dimethylpyrazine | C108509 | C6H8N2 | 108.1 | 915.6 | 381.223 | 1.54727 |
| 45 | (E)-2-nonenal | C18829566 | C9H16O | 140.2 | 1150.1 | 902.785 | 1.41067 |
| 46 | 3-Nonen-2-one | C14309570 | C9H16O | 140.2 | 1147 | 892.481 | 1.92945 |
| 47 | (E)-2-nonenal | C18829566 | C9H16O | 140.2 | 1149.1 | 899.519 | 1.97249 |
| 48 | (E)-2-octenal | C2548870 | C8H14O | 126.2 | 1064.6 | 656.046 | 1.33373 |
| 49 | 2-nonanone | C821556 | C9H18O | 142.2 | 1092.7 | 728.544 | 1.40615 |
| 50 | Ethyl 3-hydroxybutanoate | C5405414 | C6H12O3 | 132.2 | 943.4 | 420.887 | 1.17274 |
| 51 | 2-Methyl-2-propanol | C75650 | C4H10O | 74.1 | 521.7 | 120.069 | 1.12829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Hou, W.; Zhao, W.; Zhao, S.; Wang, P.; Zhao, X.; Wang, D. Effect of Ultrasound Combinated with Sodium Hypochlorite Treatment on Microbial Inhibition and Quality of Fresh-Cut Cucumber. Foods 2023, 12, 754. https://doi.org/10.3390/foods12040754
Zhang C, Hou W, Zhao W, Zhao S, Wang P, Zhao X, Wang D. Effect of Ultrasound Combinated with Sodium Hypochlorite Treatment on Microbial Inhibition and Quality of Fresh-Cut Cucumber. Foods. 2023; 12(4):754. https://doi.org/10.3390/foods12040754
Chicago/Turabian StyleZhang, Chunhong, Wanfu Hou, Wenting Zhao, Shuang Zhao, Pan Wang, Xiaoyan Zhao, and Dan Wang. 2023. "Effect of Ultrasound Combinated with Sodium Hypochlorite Treatment on Microbial Inhibition and Quality of Fresh-Cut Cucumber" Foods 12, no. 4: 754. https://doi.org/10.3390/foods12040754
APA StyleZhang, C., Hou, W., Zhao, W., Zhao, S., Wang, P., Zhao, X., & Wang, D. (2023). Effect of Ultrasound Combinated with Sodium Hypochlorite Treatment on Microbial Inhibition and Quality of Fresh-Cut Cucumber. Foods, 12(4), 754. https://doi.org/10.3390/foods12040754





