Content of Trace Elements and Human Health Risk Assessment via Consumption of Commercially Important Fishes from Montenegrin Coast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Trace Element Analyzes
2.3. Risk Assessment for Human Health
2.3.1. Target Hazard Quotient (THQ)
2.3.2. Hazard Index (HI)
2.3.3. Estimated Daily Intake (EDI)
2.4. Statistical Analyses
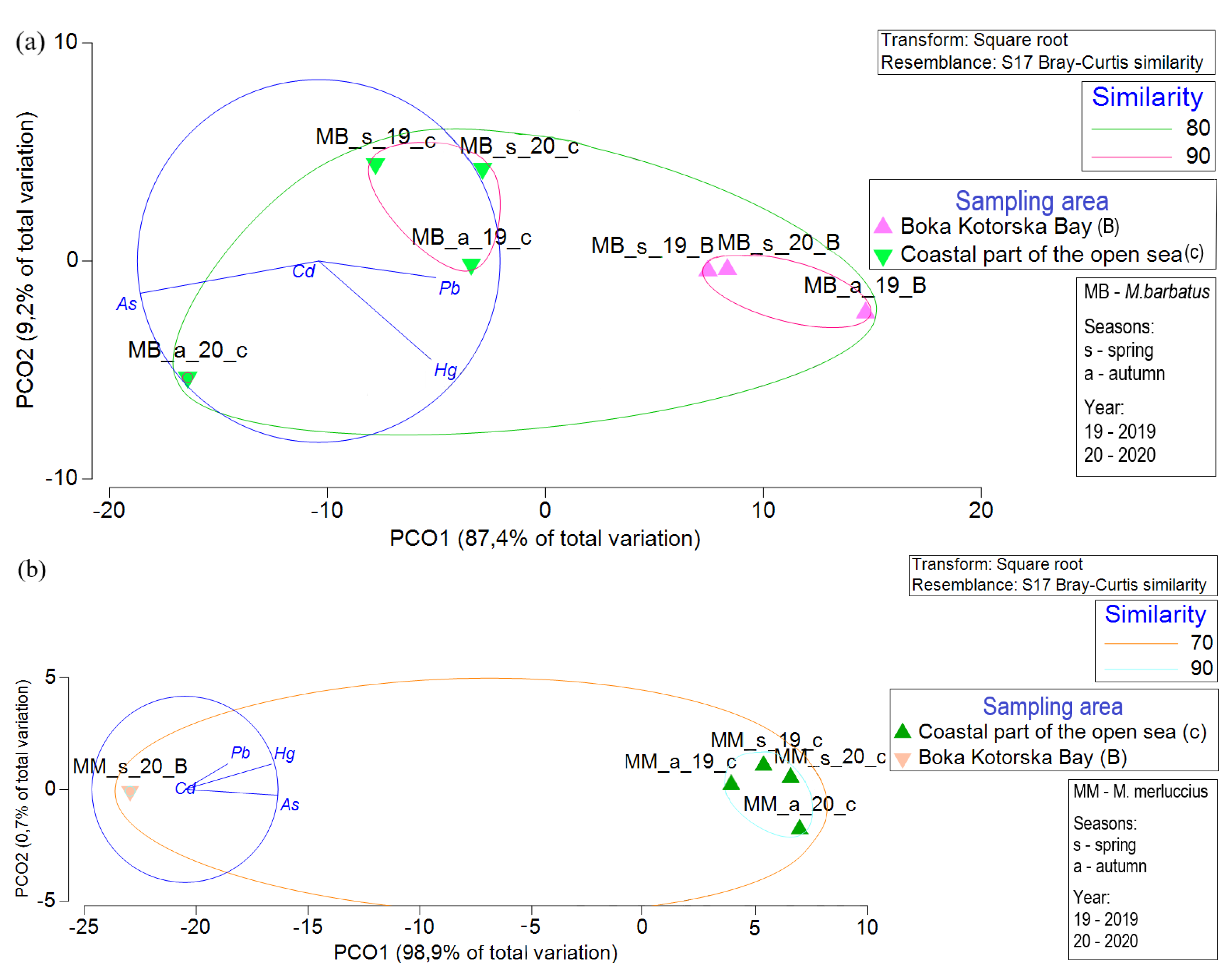
3. Results
3.1. Concentrations of TEs in Fish
3.2. Health Risk Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Milenkovic, B.; Stajic, J.M.; Stojic, N.; Pucarevic, M.; Strbac, S. Evaluation of heavy metals and radionuclides in fish and seafood products. Chemosphere 2019, 229, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Ali, M.L.; Jahan Rakib, M.R.; Islam, M.S.; Bhuyan, M.S.; Senapathi, V.; Chung, S.Y.; Roy, P.D.; Sekar, S.; Towfiqul Islam, A.R.; et al. Seasonal behavior and accumulation of some toxic metals in commercial fishes from Kirtankhola tidal river of Bangladesh—A health risk taxation. Chemosphere 2022, 301, 134660. [Google Scholar] [CrossRef] [PubMed]
- Chahid, A.; Hilali, M.; Benlhachimi, A.; Bouzid, T. Contents of cadmium, mercury and lead in fish from the Atlantic sea (Morocco) determined by atomic absorption spectrometry. Food Chem. 2014, 147, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Nowakowski, P.; Puscion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczynska, J.; Markiewicz-Zukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Zmudzinska, A.; et al. Arsenic, cadmium, lead and mercury content and health risk assessment of consuming freshwater fish with elements of chemometric analysis. Food Chem. 2022, 379, 132167. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Diet, Nutrition and the Prevention of Chronic Diseases. Report of the Joint WHO/FAO Expert Consultation. WHO Technical Report Series, 916. 2003. Available online: http://apps.who.int/iris/bitstream/10665/42665/1/WHO_TRS_916.pdf (accessed on 10 May 2021).
- Taş, E.C.; Filipuçi, I.; Çakır, D.T.; Beyaztaş, S.; Sunlu, U.; Toğulga, M.; Özaydın, O.; Arslan, O. Heavy metal concentrations in tissues of edible fish (Mullus barbatus L., 1758) from the Çandarli Bay (Turkey). Fresenius Environ. Bull. 2011, 20, 2834–2839. [Google Scholar]
- Xie, Q.; Gui, D.; Liu, W.; Wu, Y. Risk for Indo-Pacific humpback dolphins (Sousa chinensis) and human health related to the heavy metal levels in fish from the Pearl River Estuary, China. Chemosphere 2020, 240, 124844. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Authman, M.M.N.; Gaber, H.S.; El-Kasheif, M.A. Bioaccumulation of heavy metals and their histopathological impact on muscles of Clarias gariepinus from El-Rahawy drain, Egypt. Int. J. Environ. Sci. Eng. 2013, 4, 51–73. [Google Scholar]
- Burger, J.; Gochfeld, M. Perceptions of the risks and benefits of fish consumption: Individual choices to reduce risk and increase health benefits. Environ. Res. 2009, 109, 343–349. [Google Scholar] [CrossRef]
- Authman, M.M. Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aquac. Res. Dev. 2015, 6, 4. [Google Scholar] [CrossRef]
- Hosseini, M.; Nabavi, S.M.B.; Nabavi, S.N.; Pour, N.A. Heavy metals (Cd, Co, Cu, Ni, Pb, Fe, and Hg) content in four fish commonly consumed in Iran: Risk assessment for the consumers. Environ. Monit. Assess. 2015, 187, 237. [Google Scholar] [CrossRef]
- Zaza, S.; de Balogh, K.; Palmery, M.; Pastorelli, A.A.; Stacchini, P. Human exposure in Italy to lead, cadmium and mercury through fish and seafood product consumption from Eastern Central Atlantic Fishing Area. J. Food Compost. Anal. 2015, 40, 148–153. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Preventing Disease through Healthy Environments. In Action Is Needed on Chemicals of Major Public Health Concern; Public Health and Environment; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Burgeot, T.; Bocquéné, G.; Porte, C.; Dimeet, J.; Santella, R.; Garcia de la Parra, L.; Pfhol Leszkowicz, A.; Raoux, C.; Galgani, F. Bioindicators of pollutant exposure in the north-western Mediterranean Sea. Mar. Ecol. Prog. 1996, 131, 125–141. [Google Scholar] [CrossRef]
- FAO; WHO (Food Administration Organization; World Health Organization). Evaluation of Certain Food Additives and the Contaminants Mercury, Lead and Cadmium; Sixteeth Report of the Joint FAO/WHO Expert Committee on Food Additives; FAO: Rome, Italy, 1972. [Google Scholar]
- Moxness Reksten, A.; Rahman, Z.; Kjellevold, M.; Garrido Gamarro, E.; Thilsted, S.H.; Pincus, L.M.; Aakre, I.; Ryder, J.; Ariyawansa, S.; Nordhagen, A. Metal Contents in Fish from the Bay of Bengal and Potential Consumer Exposure—The EAF-Nansen Programme. Foods 2021, 10, 1147. [Google Scholar] [CrossRef]
- Perošević, A.; Pezo, L.; Joksimović, D.; Đurović, D.; Milašević, I.; Radomirović, M.; Stanković, S. The impacts of seawater physicochemical parameters and sediment metal contents on trace metal concentrations in mussels-a chemometric approach. Environ. Sci. Pollut. Res. 2018, 25, 28248–28263. [Google Scholar] [CrossRef]
- Bilandžić, N.; Dokić, M.; Sedak, M. Metal content determination in four fish species from the Adriatic Sea. Food Chem. 2011, 124, 1005–1010. [Google Scholar] [CrossRef]
- Liubartseva, S.; Coppini, G.; Lecci, R.; Creti, S. Regional approach to modeling the transport of floating plastic debris in the Adriatic Sea. Mar. Pollut. Bull. 2016, 103, 115–127. [Google Scholar] [CrossRef]
- Liubartseva, S.; Coppinib, G.; Leccib, R.; Clementic, E. Tracking plastics in the Mediterranean: 2D Lagrangian model. Mar. Pollut. Bull. 2018, 129, 151–162. [Google Scholar] [CrossRef]
- Joksimović, D.; Perošević-Bajčeta, A.; Pestorić, B.; Martinovič, R.; Bošković, N. Introduction (The Montenegrin Adriatic Coast, Marine Chemistry Pollution); The Handbook of Environmental Chemistry Series; Springer: Berlin, Heidelberg, 2021; p. 690. [Google Scholar]
- Bošković, N.; Joksimović, D.; Bajt, O. Microplastics in fish and sediments from the Montenegrin coast (Adriatic Sea): Similarities in accumulation. Sci. Total Environ. 2022, 850, 158074. [Google Scholar] [CrossRef]
- Traina, A.; Bono, G.; Bonsignore, M.; Falco, F.; Giuga, M.; Quinci, E.M.; Sprovieri, M. Heavy metals concentrations in some commercially key species from Sicilian coasts (Mediterranean Sea): Potential human health risk estimation. Ecotoxicol. Environ. Saf. 2019, 168, 466–478. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Risk-Based Concentrations Table. 2010. Available online: http://www.epa.gov/reg3hwmd/risk/human/index.htm (accessed on 6 January 2023).
- USEPA (United States Environmental Protection Agency). Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories—Fish Sampling and Analysis, 3rd ed.; EPA823-B-00-007; USEPA: Washington, DC, USA, 2000; Volume 1. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Regional Screening Levels (RSLs)—Generic Tables. 2022. Available online: https://semspub.epa.gov/work/HQ/403628.pdf (accessed on 6 January 2023).
- Bogdanović, T.; Ujević, I.; Sedak, M.; Listeš, E.; Šimat, V.; Petričević, S.; Poljak, V. As, Cd Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem. 2014, 146, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K.; Cheng, W.H.; Karami, A.; Ismail, A. Health risk assessments of heavy metal exposure via consumption of marine mussels collected from anthropogenic sites. Sci. Total Environ. 2016, 553, 285–296. [Google Scholar] [CrossRef] [PubMed]
- European Commission Regulation (EC). No 1881/2006 of the European parliament and the council of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Comm. 2006, 364, 5–24. [Google Scholar]
- European Commission Regulation (EC). No 2015/1005 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs. Off. J. Eur. Union 2015, 161, 9–13. [Google Scholar]
- Li, J.; Huang, Z.Y.; Hu, Y.; Yang, H. Potential risk assessment of heavy metals by consuming shellfish collected from Xiamen, China. Environ. Sci. Pollut. Res. 2013, 20, 2937–2947. [Google Scholar] [CrossRef]
- Naji, A.; Khan, F.R.; Hashemi, S.H. Potential human health risk assessment of trace metals via the consumption of marine fish in Persian Gulf. Mar. Pollut. Bull. 2016, 109, 667–671. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund, Human Health Evaluation Manual (Part A); Interim Final; EPA: Washington, DC, USA, 1989; Volume 1. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluations of the Joint FAO/WHO Expert Committee on Food Additives. 2009. Available online: http://apps.who.int/ipsc//database/evaluations/search.aspx (accessed on 10 July 2022).
- Alamdar, A.; Eqani, S.A.M.A.S.; Hanif, N.; Ali, S.M.; Fasola, M.; Bokhari, H.; Katsoyiannis, I.A.; Shen, H. Human exposure to trace metals and arsenic via consumption of fish from river Chenab, Pakistan and associated health risks. Chemosphere 2017, 168, 1004–1012. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Manera, M.; Zaccaroni, A.; Olivieri, V.; Amorena, M. Heavy metal (As, Cd, Hg, Pb, Cu, Zn, Se) concentrations in muscle and bone of four commercial fish caught in the central Adriatic Sea, Italy. Environ. Monit. Assess. 2013, 186, 2205–2213. [Google Scholar] [CrossRef]
- Conti, G.O.; Copat, C.; Ledda, C.; Fiore, M.; Fallico, R.; Sciacca, S. Evaluation of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in Mullus barbatus from Sicily channel and risk-based consumption limits. Bull. Environ. Contam. Toxicol. 2012, 88, 946–950. [Google Scholar] [CrossRef]
- Acquavita, A.A.; Predonzani, S.; Matassi, G.; Rossin, P.; Tamberlich, F.; Falomo, T.; Valic, I. Heavy metal contents and distribution in coastal sediments of the gulf of trieste (Northern Adriatic Sea, Italy). Water Air Soil Pollut. 2010, 211, 95–111. [Google Scholar] [CrossRef]
- Stancheva, M.; Makedonski, L.; Petrova, E. Determination of heavy metals (Pb, Cd, As and Hg) in Black Sea grey mullet (Mugil cephalus). Bulg. J. Agric. Sci. 2013, 1, 30–34. [Google Scholar]
- Šlejkovec, Z.; Stajnko, A.; Falnoga, I.; Lipej, L.; Mazej, D.; Horvat, M.; Faganeli, J. Bioaccumulation of Arsenic Species in Rays from the Northern Adriatic Sea. Int. J. Mol. Sci. 2014, 15, 22073–22091. [Google Scholar] [CrossRef]
- Saha, N.; Mollah, M.Z.I.; Alam, M.F.; Safiur Rahman, M. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control 2016, 70, 110–118. [Google Scholar] [CrossRef]
- Julshamn, K.; Nilsen, B.; Frantzen, S.; Valdersnes, S.; Maage, A.; Nedreaas, K.; Sloth, J.J. Total and inorganic arsenic in fish samples from Norwegian waters. Food Addit. Contam. Part B Surveill. 2012, 5, 229–235. [Google Scholar] [CrossRef]
- Fattorini, D.; Notti, A.; Di Mento, R.; Cicero, A.M.; Gabellini, M.; Russo, A. Seasonal, spatial and inter-annual variations of trace metals in mussels from the Adriatic Sea: A regional gradient for arsenic and implications for monitoring the impact of off-shore activities. Chemosphere 2008, 72, 1524–1533. [Google Scholar] [CrossRef]
- Falcó, G.; Llobet, J.M.; Bocio, A.; Domingo, J.L. Daily intake of arsenic, camdium, mercury and lead by consumption of edible marine species. J. Agric. Food Chem. 2006, 54, 6106–6112. [Google Scholar] [CrossRef]
- Višnjić-Jeftić, Z.; Jarić, I.; Jovanović, L.; Skorić, S.; Smederevac-Lalic, M.; Nikcević, M.; Lenhardt, M. Heavy metal and trace element accumulation in muscle, liver and gills of the Pontic shad (Alosa immaculata Bennet 1835) from the Danube River (Serbia). Microchem. J. 2010, 95, 341–344. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G. Toxic metals (Hg, Pb, and Cd) in commercially important demersal fish from Mediterranean Sea: Contamination levels and dietary exposure assessment. J. Food Sci. 2013, 78, 362–366. [Google Scholar] [CrossRef]
- Storelli, M.M. Intake of essential minerals and metals via consumption of seafood from the Mediterranean Sea. J. Food Prot. 2009, 72, 1116–1120. [Google Scholar] [CrossRef]
- Storelli, M.; Storelli, A.; Giacominelli-Stuffler, R.; Marcotrigiano, G. Mercury speciation in the muscle of two commercially important fish, hake (Merluccius merluccius) and striped mullet (Mullus barbatus) from the Mediterranean sea: Estimated weekly intake. Food Chem. 2005, 89, 295–300. [Google Scholar] [CrossRef]
- Copat, C.; Grasso, A.; Fiore, M.; Cristaldi, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. Trace elements in seafood from the Mediterranean sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Al-Kazaghly, R.F.; Hamid, M.; Ighwela, K.A. Bioaccumulation of some heavy metals in red mullet (Mullus barbatus) and common pandora (Pagellus erythrinus) in Zliten Coast, Libya. J. Ilm. Perikan Kelaut. 2021, 13, 81–86. [Google Scholar]
- Martínez-Gómez, C.; Fernández, B.; Benedicto, J.; Valdés, J.; Campillo, J.A.; León, V.M.; Vethaak, A.D. Health status of red mullets from polluted areas of the Spanish Mediterranean coast, with special reference to Portmán (SE Spain). Mar. Environ. Res. 2012, 77, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kragulj, T.; Purić, M.; Bursić, V.; Vuković, G.; Đukić, M. Lead contamination of fish and water from costal sea of Bar region (Montenegro). J. Agron. Technol. Eng. Manag. 2018, 1, 124–129. [Google Scholar]
- Antović, I.; Šuković, D.; Andjelić, S.; Svrkota, N. Heavy metals and radionuclides in muscles of fish species in the South Adriatic—Montenegro. RAP Conf. Proc. 2019, 4, 96–102. [Google Scholar]
- Brkić, D.; Bošnir, J.; Gross Bošković, A.; Miloš, S.; Šabarić, J.; Lasić, D.; Jurak, G.; Cvetković, B.; Racz, A.; Mojsović Ćuić, A. Determination of heavy metals in different fish species sampled from markets in Croatia and possible health effects. Med. Jadertina 2017, 47, 89–105. [Google Scholar]
- Türkmen, M.; Türkmen, A.; Tepe, Y.; Töre, Y.; Ateş, A. Determination of metals in fish species from Aegean and Mediterranean seas. Food Chem. 2009, 113, 233–237. [Google Scholar] [CrossRef]
- Lin, H.T.; Chen, S.W.; Shen, C.J.; Chu, C. Arsenic speciation in fish on the market. J. Food Drug Anal. 2008, 16, 70–75. [Google Scholar] [CrossRef]
- Caumette, G.; Koch, I.; Reimer, K.J. Arsenobetaine formation in plankton: A review of studies at the base of the aquatic food chain. J. Environ. Monit. Assess. 2012, 14, 2841–2853. [Google Scholar] [CrossRef]
- Miklavcic, A.; Casetta, A.; Snoj Tratnik, J.; Mazej, D.; Krsnik, M.; Mariuz, M.; Sofianou, K.; Spiric, Z.; Barbone, F.; Horvat, M. Mercury, arsenic and selenium exposure levels in relation to fish consumption in the Mediterranean area. Environ. Res. 2013, 120, 7–17. [Google Scholar] [CrossRef]
- Brandon, E.F.; Janssen, P.J.; de Wit-Bos, L. Arsenic: Bio- accessibility from seaweed and rice, dietary exposure calculations and risk assessment. Food Addit. Contam. 2014, 31, 1993–2003. [Google Scholar] [CrossRef]
- ATSDR (Agency for Toxic Substance and Disease Registry). Toxicological Profile for Arsenic; ATSDR (Agency for Toxic Substance and Disease Registry): Atlanta, GA, USA, 2007. [Google Scholar]
- Hong, S.; Khim, J.S.; Park, J.; Son, H.S.; Choi, S.D.; Choi, K.; Ryu, J.; Kim, C.Y. Species- and tissue-specific bioaccumulation of arsenicals in various aquatic organisms from a highly industrialized area in the Pohang City, Korea. Environ. Pollut. 2014, 192, 27–35. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L.; Wang, W.-X. Biotransformation and detoxification of inorganic arsenic in a marine juvenile fish Terapon jarbua after waterborne and diet borne exposure. J. Hazard. Mater. 2012, 221, 162–169. [Google Scholar] [CrossRef]
- Dolenec, T.; Faganeli, J.; Pirc, S. Major, minor and trace elements in surficial sediments from the open Adriatic Sea: A regional geochemical study. Geol. Croat. 1998, 51, 59–73. [Google Scholar]
- Joksimović, D.; Castelli, A.; Pestorić, B.; Perošević, A. An assessment of trace metal contamination in surface sediments of the montenegrin coast by using pollution indexes and statistical analysis. Fresenius Environ. Bull. 2019, 28, 738–743. [Google Scholar]
- Joksimović, D.; Perošević, A.; Castelli, A.; Pestorić, B.; Šuković, D.; Đurović, D. Assessment of heavy metal pollution in surface sediments of the Montenegrin coast: A 10-year review. J. Soils Sediments. 2020, 20, 2598–2607. [Google Scholar] [CrossRef]
- Al-Busaidi, M.; Yesudhason, P.; Al-Mughairi, S.; Al-Rahbi, W.A.K.; Al-Harthy, K.S.; Al-Mazrooei, N.A.; Al-Habsi, S.H. Toxic metals in commercial marine fish in Oman with reference to national and international standards. Chemosphere 2011, 85, 67–73. [Google Scholar] [CrossRef]

| Trace Elements | Certified | Measured | Recovery (%) | Certified | Measured | Recovery (%) |
|---|---|---|---|---|---|---|
| IAEA 407 | IAEA 436 | |||||
| Hg | 0.222 | 0.223 | 100.4 | / | / | / |
| Pb | 0.12 | 0.12 | 100.0 | / | / | / |
| As | 12.6 | 12.5 | 99.2 | 1.98 | 1.97 | 99.5 |
| Cd | 0.189 | 0.187 | 98.9 | 0.052 | 0.052 | 100.0 |
| Species | Sampling Period | Sampling Area | Concentration of Trace Elements | |||
|---|---|---|---|---|---|---|
| As | Hg | Pb | Cd | |||
| M. barbatus | Spring 19 | Boka Kotorska Bay | 12.489 | 0.744 | 0.037 | <0.02 |
| Coastal part of the open sea | 23.520 | 0.181 | 0.033 | <0.02 | ||
| Autumn 19 | Boka Kotorska Bay | 8.572 | 0.548 | 0.053 | <0.02 | |
| Coastal part of the open sea | 21.606 | 0.547 | 0.034 | <0.02 | ||
| Spring 20 | Boka Kotorska Bay | 13.021 | 0.801 | 0.023 | <0.02 | |
| Coastal part of the open sea | 19.113 | 0.252 | <0.02 | <0.02 | ||
| Autumn 20 | Coastal part of the open sea | 39.501 | 0.408 | <0.02 | <0.02 | |
| Mean value | 19.689 ± 10.25 | 0.497 ± 0.233 | 0.031 ± 0.01 | <0.02 | ||
| M. merluccius | Spring 19 | Coastal part of the open sea | 9.522 | 0.176 | 0.034 | <0.02 |
| Autumn 19 | Coastal part of the open sea | 9.016 | 0.156 | 0.025 | <0.02 | |
| Spring 20 | Boka Kotorska Bay | 2.593 | 0.055 | <0.02 | <0.02 | |
| Coastal part of the open sea | 9,901 | 0.222 | <0.02 | <0.02 | ||
| Autumn 20 | Coastal part of the open sea | 10.746 | 0.146 | 0.025 | <0.02 | |
| Mean value | 8.356 ± 3.28 | 0.153 ± 0.06 | 0.025 ± 0.01 | <0.02 | ||
| Species | Trace Elements | THQ | ||
|---|---|---|---|---|
| Boka Kotorska Bay | Coastal Part of the Open Sea | Montenegrin Coast | ||
| M. barbatus | As | 9.472 | 21.61 | 16.84 |
| Hg | 1.744 | 0.867 | 1.240 | |
| Pb | 0.038 | 0.018 | 0.020 | |
| Cd | 0.050 | 0.050 | 0.050 | |
| HI | 11.304 | 22.545 | 18.15 | |
| M. merluccius | As | 2.142 | 8.167 | 7.086 |
| Hg | 0.137 | 0.437 | 0.382 | |
| Pb | 0.010 | 0.017 | 0.016 | |
| Cd | 0.050 | 0.050 | 0.050 | |
| HI | 2.339 | 8.671 | 7.534 | |
| Species | Trace Elements | EDI | Recommended Daily Dietary Allowance of JECFA [36] | ||
|---|---|---|---|---|---|
| Boka Kotorska Bay | Coastal Part of the Open Sea | Montenegrin Coast | |||
| M. barbatus | As | 2.842 | 6.482 | 5.053 | 0.13 |
| Hg | 0.174 | 0.087 | 0.124 | 0.03 | |
| Pb | 0.009 | 0.007 | 0.008 | 0.21 | |
| Cd | 0.005 | 0.005 | 0.005 | 0.06 | |
| M. merluccius | As | 0.643 | 2.450 | 2.126 | 0.13 |
| Hg | 0.014 | 0.044 | 0.038 | 0.03 | |
| Pb | 0.005 | 0.006 | 0.006 | 0.21 | |
| Cd | 0.005 | 0.005 | 0.005 | 0.06 | |
| Species | Sampling Site | TEs | Reference | |||
|---|---|---|---|---|---|---|
| As | Hg | Pb | Cd | |||
| M. barbatus | Montenegro (Adriatic Sea) | 19.689 | 0.497 | 0.031 | <0.02 | Present study |
| Croatia (Adriatic Sea) | 5.91 | 0.06 | 0.02 | 0.002 | [18] | |
| Italy (Adriatic Sea) | 59.91 | 0.48 | 0.05 | 0.07 | [38] | |
| Italy (Adriatic Sea) | / | 0.43 | 0.10 | 0.08 | [48] | |
| Italy (Adriatic Sea) | / | 0.55 | 0.06 | 0.02 | [49] | |
| Italy (Adriatic Sea) | / | 0.49 | / | / | [50] | |
| Italy (Mediterranean Sea) | 9.477 | 0.054 | 0.006 | 0.002 | [51] | |
| Italy (Mediterranean Sea) | 12.09 | 0.18 | 0.05 | <0.001 | [23] | |
| Italy (Mediterranean Sea) | 27.01 | 0.027 | 0.04 | 0.001 | [39] | |
| Libya (Mediterranean Sea) | / | 0.07 | 0.19 | 0.6 | [52] | |
| Spain (Mediterranean Sea) | 15.05 | 0.09 | 0.05 | 0.001 | [53] | |
| M. merluccius | Montenegro (Adriatic Sea) | 8.356 | 0.153 | 0.02 | <0.02 | Present study |
| Montenegro (Adriatic Sea) | / | / | 0.25 | / | [54] | |
| Montenegro (Adriatic Sea) | / | / | <0.1 | <0.2 | [55] | |
| Croatia (Adriatic Sea) | 4.38 | 0.295 | <0.05 | <0.01 | [56] | |
| Italy (Adriatic Sea) | 38.70 | 0.59 | 0.03 | 0.06 | [38] | |
| Italy (Adriatic Sea) | / | 0.12 | 0.04 | 0.30 | [49] | |
| Italy (Adriatic Sea) | / | 0.18 | / | / | [50] | |
| Italy (Mediterranean Sea) | 8.75 | 0.15 | 0.03 | <0.001 | [23] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bošković, N.; Joksimović, D.; Bajt, O. Content of Trace Elements and Human Health Risk Assessment via Consumption of Commercially Important Fishes from Montenegrin Coast. Foods 2023, 12, 762. https://doi.org/10.3390/foods12040762
Bošković N, Joksimović D, Bajt O. Content of Trace Elements and Human Health Risk Assessment via Consumption of Commercially Important Fishes from Montenegrin Coast. Foods. 2023; 12(4):762. https://doi.org/10.3390/foods12040762
Chicago/Turabian StyleBošković, Neda, Danijela Joksimović, and Oliver Bajt. 2023. "Content of Trace Elements and Human Health Risk Assessment via Consumption of Commercially Important Fishes from Montenegrin Coast" Foods 12, no. 4: 762. https://doi.org/10.3390/foods12040762
APA StyleBošković, N., Joksimović, D., & Bajt, O. (2023). Content of Trace Elements and Human Health Risk Assessment via Consumption of Commercially Important Fishes from Montenegrin Coast. Foods, 12(4), 762. https://doi.org/10.3390/foods12040762







