Three Phenolic Extracts Regulate the Physicochemical Properties and Microbial Community of Refrigerated Channel Catfish Fillets during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Total Viable Counts (TVC)
2.3. High-Throughput Sequencing on the Illumina Platform
2.4. Total Volatile Base Nitrogen (TVB-N)
2.5. Thiobarbituric Acid Reactive Substance (TBARS)
2.6. Shear Force
2.7. Histology
2.8. Centrifugal Loss
2.9. Low-Field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Imaging (MRI)
2.10. ATP-Related Compounds
2.11. Statistical Analysis
3. Results and Discussion
3.1. Microbial Analysis
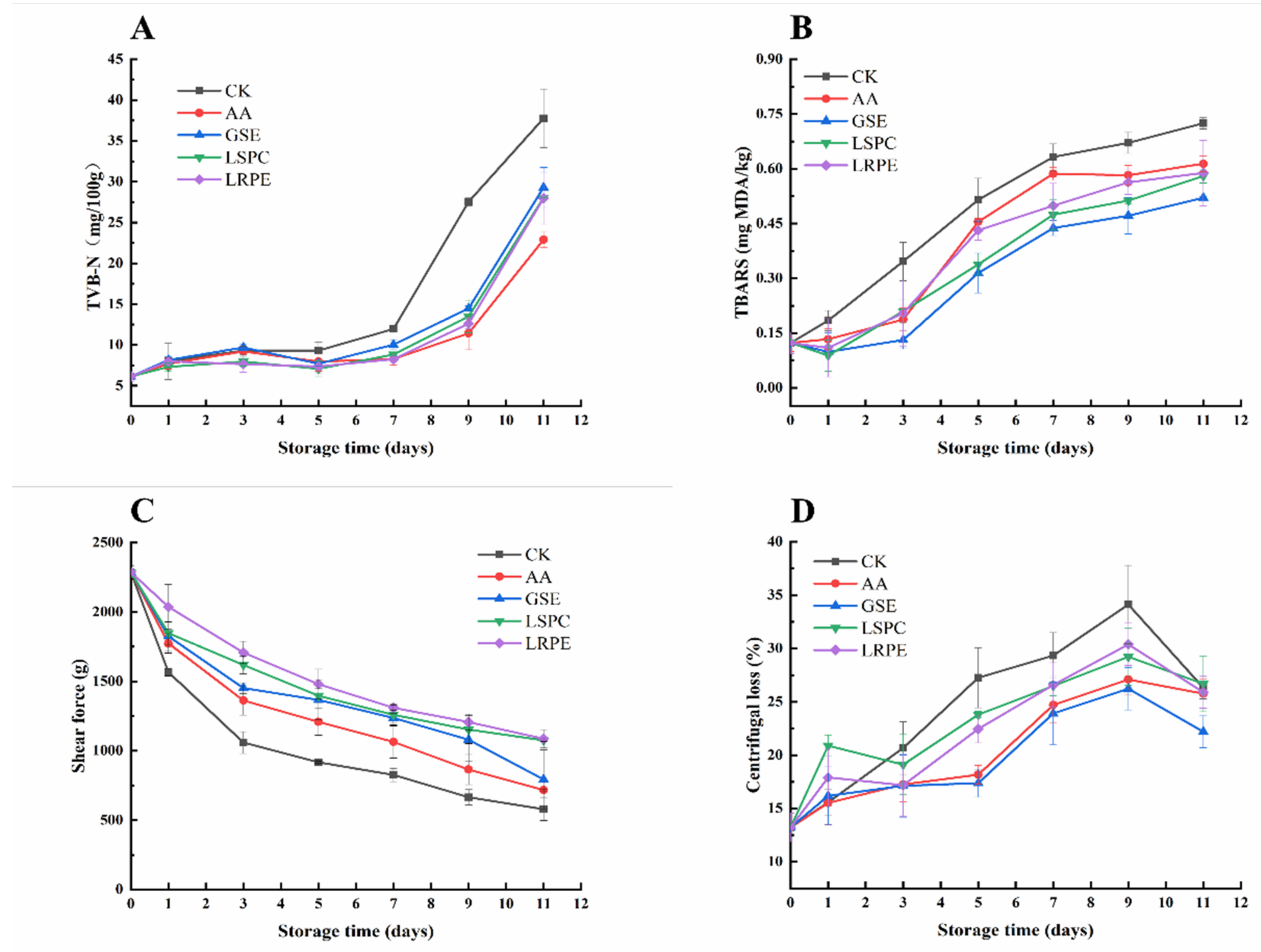
3.2. TVB-N and TBARS
3.3. Shear Force and Histology
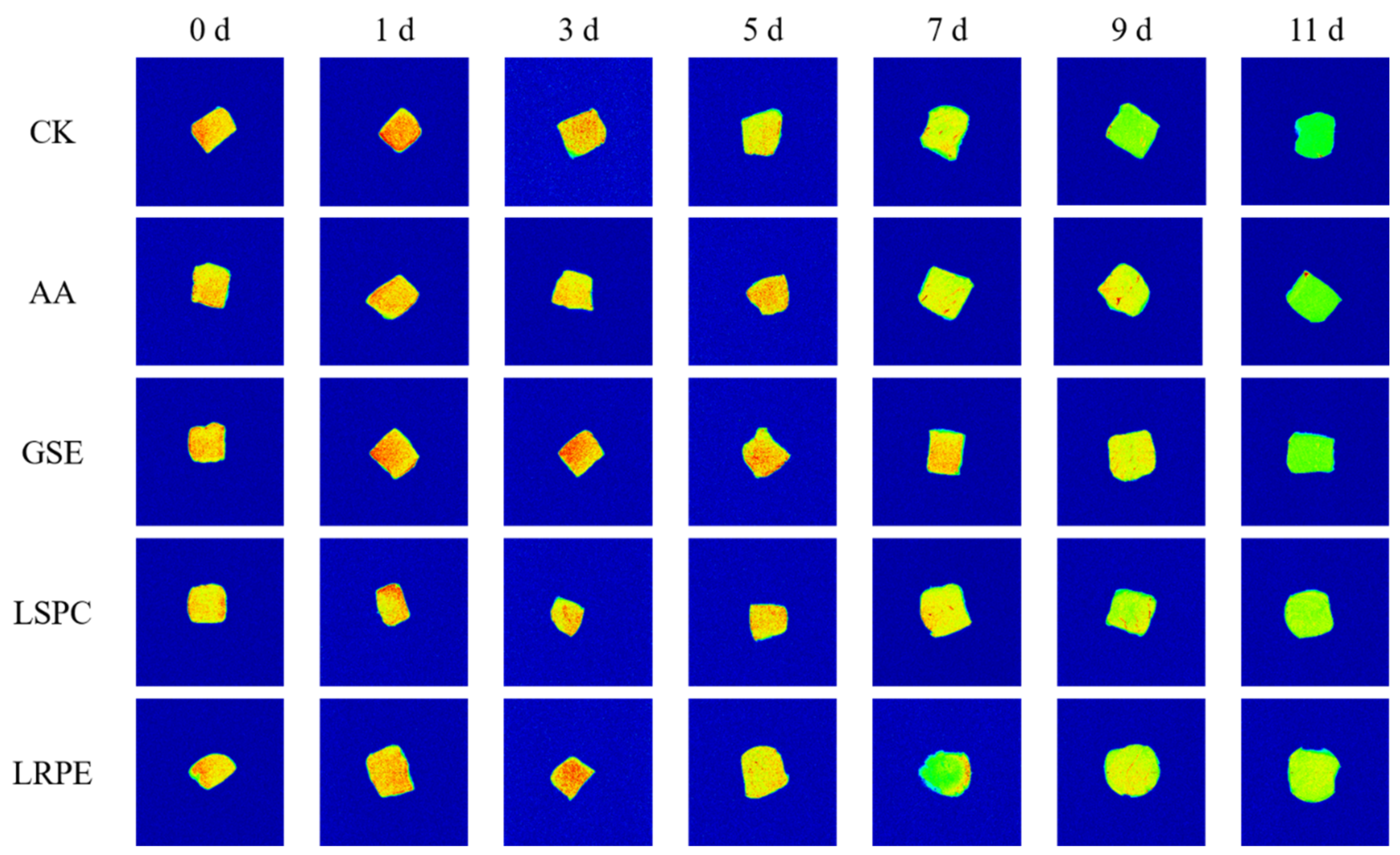
3.4. Centrifugal Loss, Water Molecules Distribution, and MRI Analysis
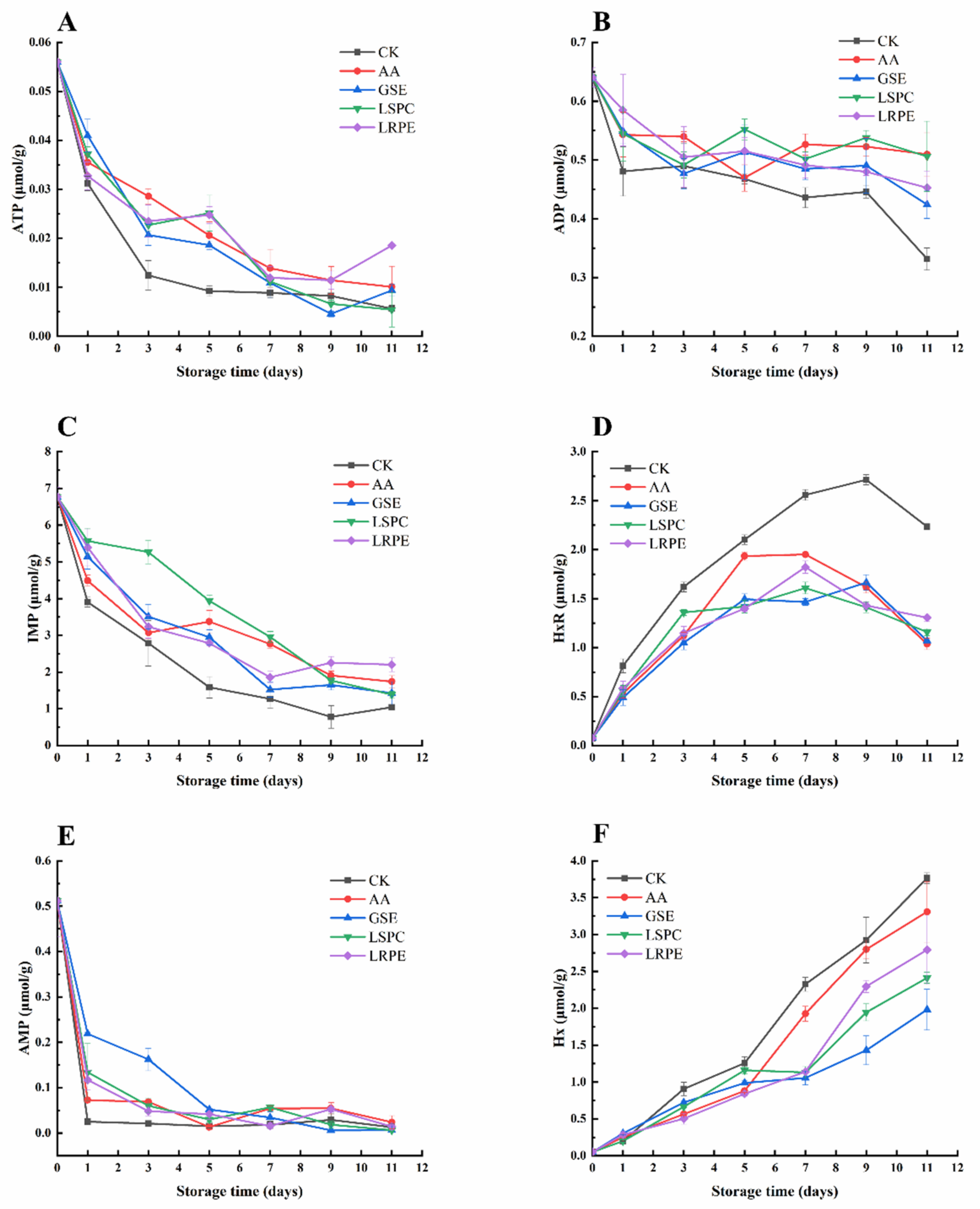
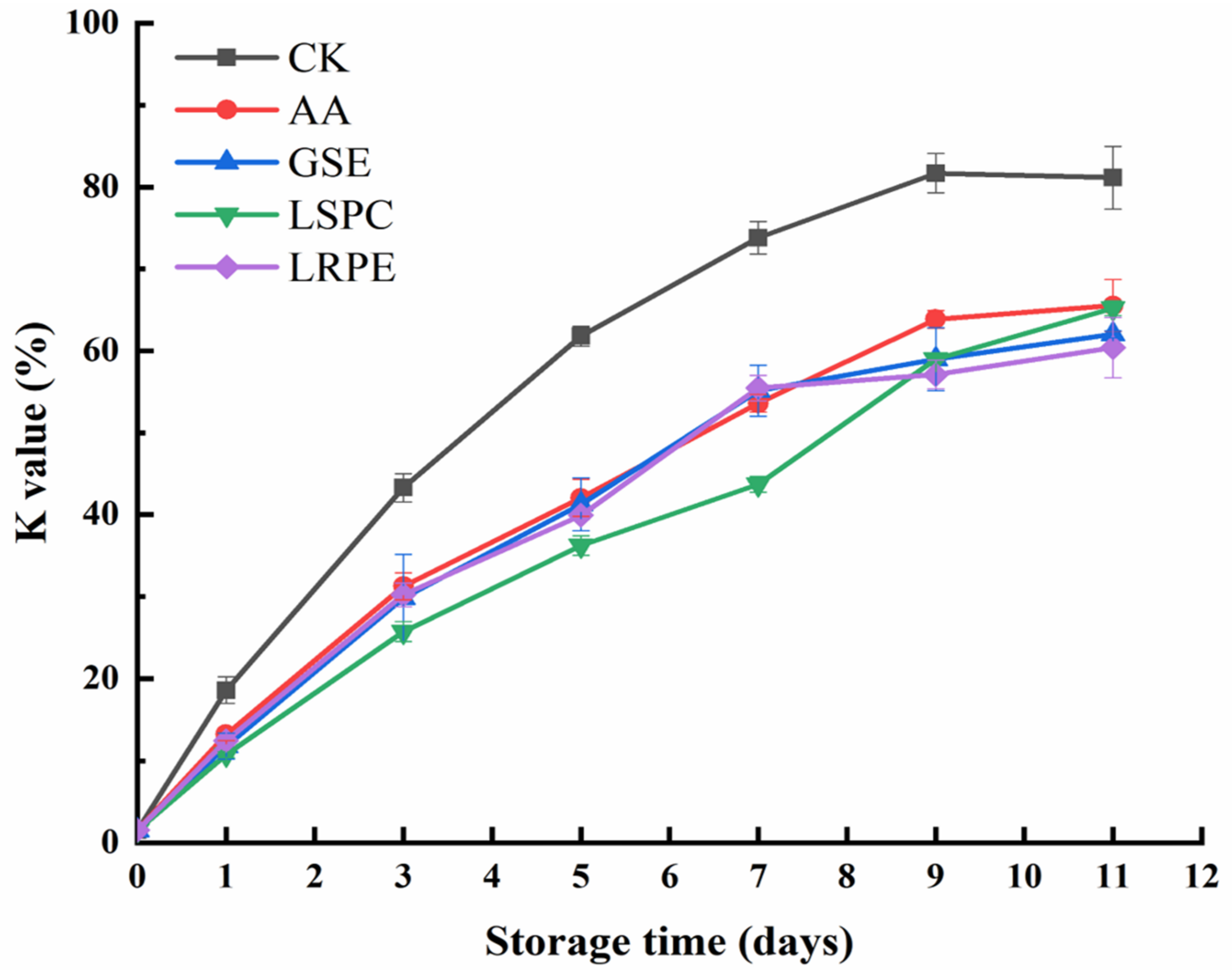
3.5. ATP-Related Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, L.; Song, C.; Chen, X.; Deng, W.; Xiao, Y.; Wang, M.; Qin, Q.; Luan, S.; Kong, J.; Bian, W. Channel catfish in China: Historical aspects, current status, and problems. Aquaculture 2016, 465, 367–373. [Google Scholar]
- Yearbook, C.F.S. China Fishery Statistic Yearbook; China Agriculture Press: Beijing, China, 2018. [Google Scholar]
- Huang, H.; Sun, W.; Xiong, G.; Shi, L.; Jiao, C.; Wu, W.; Li, X.; Qiao, Y.; Liao, L.; Ding, A.; et al. Effects of HVEF treatment on microbial communities and physicochemical properties of catfish fillets during chilled storage. LWT 2020, 131, 109667. [Google Scholar] [CrossRef]
- Shi, L.; Yin, T.; Xiong, G.; Ding, A.; Li, X.; Wu, W.; Qiao, Y.; Liao, L.; Wang, J.; Wang, L. Microstructure and physicochemical properties: Effect of pre-chilling and storage time on the quality of Channel catfish during frozen storage. LWT 2020, 130, 109606. [Google Scholar]
- Guo, A.; Jiang, J.; True, A.D.; Xiong, Y.L. Myofibrillar Protein Cross-Linking and Gelling Behavior Modified by Structurally Relevant Phenolic Compounds. J. Agric. Food Chem. 2021, 69, 1308–1317. [Google Scholar] [CrossRef]
- Shen, X.; Su, Y.-C. Application of grape seed extract in depuration for decontaminating Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Food Control. 2017, 73, 601–605. [Google Scholar] [CrossRef]
- Shi, C.; Cui, J.; Yin, X.; Luo, Y.; Zhou, Z. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: Effect on lipid and protein oxidation. Food Control. 2014, 40, 134–139. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Gao, X.; Xie, B.; Sun, Z. Inhibitory effects of lotus seedpod procyanidins against lipid and protein oxidation and spoilage organisms in chilled-storage beef. LWT 2022, 160, 113247. [Google Scholar] [CrossRef]
- Tang, C.; Xie, B.; Sun, Z. Antibacterial activity and mechanism of B-type oligomeric procyanidins from lotus seedpod on enterotoxigenic Escherichia coli. J. Funct. Foods 2017, 38, 454–463. [Google Scholar] [CrossRef]
- Wang, J.; Xie, B.; Sun, Z. The improvement of carboxymethyl β-glucan on the antibacterial activity and intestinal flora regulation ability of lotus seedpod procyanidins. LWT 2021, 137, 110441. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, K.; Chen, Y.; Ouyang, Y.; Feng, Y.; Li, S.; Zhang, L.; Feng, N. Effect of lotus seedpod oligomeric procyanidins on AGEs formation in simulated gastrointestinal tract and cytotoxicity in Caco-2 cells. Food Funct. 2021, 12, 3527–3538. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, S.; He, J.; Thirumdas, R.; Montesano, D.; Barba, F.J. Enzyme-assisted extraction of polyphenol from edible lotus (Nelumbo nucifera) rhizome knot: Ultra-filtration performance and HPLC-MS2 profile. Food Res. Int. 2018, 111, 291–298. [Google Scholar] [CrossRef]
- Hu, M.; Skibsted, L.H. Antioxidative capacity of rhizome extract and rhizome knot extract of edible lotus (Nelumbo nuficera). Food Chem. 2002, 76, 327–333. [Google Scholar] [CrossRef]
- Ceylan, Z.; Sengor, G.F.U.; Yilmaz, M.T. Nanoencapsulation of liquid smoke/thymol combination in chitosan nanofibers to delay microbiological spoilage of sea bass (Dicentrarchus labrax) fillets. J. Food Eng. 2018, 229, 43–49. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Field, J.; Lettinga, G. Toxicity of tannic compounds to microorganisms. In Plant Polyphenols; Hemingway, R., Laks, P., Eds.; Springer: Boston, MA, USA, 1992; pp. 673–692. [Google Scholar]
- Wu, Q.; Chen, H.; Lv, Z.; Li, S.; Hu, B.; Guan, Y.; Xie, B.; Sun, Z. Oligomeric procyanidins of lotus seedpod inhibits the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Chem. 2013, 138, 1493–1502. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, X.; Lei, Y.; Regenstein, J.M.; Luo, Y. Characterization of the microbial composition and quality of lightly salted grass carp (Ctenopharyngodon idellus) fillets with vacuum or modified atmosphere packaging. Int. J. Food Microbiol. 2019, 293, 87–93. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Wu, Q.; Gu, Q.; Chen, M.; Zhang, Y.; Sun, X.; Zhang, J. High-throughput sequencing analysis of bacterial community composition and quality characteristics in refrigerated pork during storage. Food Microbiol. 2019, 83, 86–94. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, F.; Xia, X.; Xu, H.; Kong, B. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019, 51, 281–291. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, H.; Cao, J.; Pan, D. Structural characteristics of Sheldrake meat and secondary structure of myofibrillar protein: Effects of oxidation. Int. J. Food Prop. 2017, 20, 1553–1566. [Google Scholar] [CrossRef]
- Pinheiro, R.S.; Francisco, C.L.; Lino, D.M.; Borba, H. Meat quality of Santa Inês lamb chilled-then-frozen storage up to 12 months. Meat Sci. 2018, 148, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xiong, G.; Ling, J.; Hu, Y.; Shi, L.; Qiao, Y.; Yu, J.; Cui, Y.; Liao, L.; Wu, W.; et al. Effect of ultra-high pressure treatment on shucking and meat properties of red swamp crayfish (Procambarus clarkia). LWT 2018, 87, 234–240. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Y.; Guo, J.; Huang, H.; Wang, L.; Wu, W.; Xiong, G.; Sun, W. Water distribution and textual properties of heat-induced pork myofibrillar protein gel as affected by sarcoplasmic protein. LWT 2019, 103, 308–315. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A study of the antibacterial mechanism of catechins: Isolation and identification of Escherichia coli cell surface proteins that interact with epigallocatechin gallate. Food Control. 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. LWT 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Trošt, K.; Klančnik, A.; Vodopivec, B.M.; Lemut, M.S.; Novšak, K.J.; Raspor, P.; Možina, S.S. Polyphenol, antioxidant and antimicrobial potential of six different white and red wine grape processing leftovers. J. Sci. Food Agric. 2016, 96, 4809–4820. [Google Scholar] [CrossRef]
- ICMSF. Sampling for microbiological analysis: Principles and specific applications. In Microorganisms in Foods 2; International Commission on Microbiological Specifications for Foods, University of Toronto Press: Toronto, ON, Canada, 1986; pp. 181–196. [Google Scholar]
- Li, Y.; Zhuang, S.; Liu, Y.; Zhang, L.; Liu, X.; Cheng, H.; Liu, J.; Shu, R.; Luo, Y. Effect of grape seed extract on quality and microbiota community of container-cultured snakehead (Channa argus) fillets during chilled storage. Food Microbiol. 2020, 91, 103492. [Google Scholar] [CrossRef]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control. 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Luo, Y. Changes in microbial communities and quality attributes of white muscle and dark muscle from common carp ( Cyprinus carpio ) during chilled and freeze-chilled storage. Food Microbiol. 2018, 73, 237–244. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Yu, D.; Gao, P.; Jiang, Q.; Yang, F. Dynamics and diversity of microbial community succession during fermentation of Suan yu, a Chinese traditional fermented fish, determined by high throughput sequencing. Food Res. Int. 2018, 111, 565–573. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Jia, S.; Hong, H.; Liu, Y.; Luo, Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019, 82, 445–454. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 degrees C. Food Chem. 2013, 140, 105–114. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Zang, J.; Jiang, Q.; Xia, W.; Xu, Y. Inhibition of microbial spoilage of grass carp (Ctenopharyngodon idellus) fillets with a chitosan-based coating during refrigerated storage. Int. J. Food Microbiol. 2018, 285, 61–68. [Google Scholar] [CrossRef]
- Fan, W.; Chi, Y.; Zhang, S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008, 108, 148–153. [Google Scholar] [CrossRef]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Mirhosseini, S.H.; Hosseini, S.M.H. Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT 2015, 64, 898–904. [Google Scholar] [CrossRef]
- Shirahigue, L.D.; Plataoviedo, M.; de Alencar, S.M.; D’Arce, M.A.B.R.; Ferreira de Souza Vieira, T.M.; Oldoni, T.; Contreras-Castillo, C.J. Wine industry residue as antioxidant in cooked chicken meat. Int. J. Food Sci. Technol. 2010, 45, 863–870. [Google Scholar] [CrossRef]
- Jiang, Q.; Jia, R.; Nakazawa, N.; Hu, Y.; Osako, K.; Okazaki, E. Changes in protein properties and tissue histology of tuna meat as affected by salting and subsequent freezing. Food Chem. 2018, 271, 550–560. [Google Scholar] [CrossRef]
- Wang, X.; Geng, L.; Xie, J.; Qian, Y.-F. Relationship Between Water Migration and Quality Changes of Yellowfin Tuna (Thunnus albacares) During Storage at 0 °C and 4 °C by LF-NMR. J. Aquat. Food Prod. Technol. 2017, 27, 35–47. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Li, W.; Li, H.K.; Chen, X.H.; Jiang, M.; Dong, M.S. Low-field nuclear magnetic resonance for online determination of water content during sausage fermentation. J. Food Eng. 2017, 212, 291–297. [Google Scholar] [CrossRef]
- Andersen, C.M.; Rinnan, Å. Distribution of water in fresh cod. LWT 2002, 35, 687–696. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The Importance of ATP-related Compounds for the Freshness and Flavor of Post-mortem Fish and Shellfish Muscle: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Özoğul, Y.; Özoğul, F. Degradation products of adenine nucleotide in rainbow trout (Oncorhynchus mykiss) stored in ice and in modified atmosphere packaging. Turk. J. Zool. 2002, 26, 127–130. [Google Scholar]
- Zhao, X.; Wu, J.; Chen, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on metabolite profiles of tilapia (Oreochromis niloticus) fillets during storage. Food Chem. 2019, 293, 418–428. [Google Scholar] [CrossRef]


| Samples | Sequences | OTUs | Chao | Good’s Coverage | Shannon | Simpson |
|---|---|---|---|---|---|---|
| CK-0 d | 96,878 | 287 | 374 | 0.9993 | 2.81 | 0.73 |
| AA-0 d | 105,787 | 252 | 350 | 0.9990 | 2.92 | 0.68 |
| GSE-0 d | 110,338 | 275 | 371 | 0.9992 | 3.25 | 0.77 |
| LSPC-0 d | 117,002 | 256 | 391 | 0.9991 | 3.14 | 0.74 |
| LRPE-0 d | 96,209 | 267 | 377 | 0.9991 | 2.60 | 0.63 |
| CK-7 d | 112,132 | 185 | 289 | 0.9991 | 3.35 | 0.83 |
| AA-7 d | 104,106 | 192 | 294 | 0.9992 | 3.49 | 0.83 |
| GSE-7 d | 126,224 | 169 | 299 | 0.9993 | 3.46 | 0.79 |
| LSPC-7 d | 129,438 | 176 | 312 | 0.9991 | 2.83 | 0.64 |
| LRPE-7 d | 116,472 | 187 | 234 | 0.9994 | 1.53 | 0.33 |
| CK-11 d | 127,863 | 165 | 250 | 0.9995 | 2.73 | 0.69 |
| AA-11 d | 128,558 | 151 | 237 | 0.9994 | 2.30 | 0.52 |
| GSE-11 d | 124,207 | 98 | 152 | 0.9997 | 1.76 | 0.41 |
| LSPC-11 d | 107,973 | 104 | 132 | 0.9998 | 1.81 | 0.42 |
| LRPE-11 d | 126,801 | 83 | 135 | 0.9998 | 1.93 | 0.45 |
| Relaxation Time | Samples | Storage Time (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 9 | 11 | ||
| T21 (ms) | CK | 0.89 ± 0.08 Ad | 1.02 ± 0.11 Ad | 1.26 ± 0.09 Ac | 1.31 ± 0.06 Ac | 1.38 ± 0.08 Abc | 1.51 ± 0.11 Ab | 2.25 ± 0.14 Aa |
| AA | 0.89 ± 0.08 Ae | 1.09 ± 0.08 Acd | 0.98 ± 0.02 Bde | 1.17 ± 0.12 Abc | 1.24 ± 0.08 Aabc | 1.28 ± 0.11 Cab | 1.34 ± 0.10 Ba | |
| GSE | 0.89 ± 0.08 Ac | 1.13 ± 0.13 Ab | 1.20 ± 0.04 Aab | 1.18 ± 0.12 Aab | 1.29 ± 0.07 Aab | 1.27 ± 0.06 Cab | 1.34 ± 0.05 Ba | |
| LSPC | 0.89 ± 0.08 Ac | 1.04 ± 0.13 Ac | 1.08 ± 0.04 Bbc | 1.11 ± 0.19 Abc | 1.30 ± 0.19 Aab | 1.74 ± 0.04 ABa | 1.73 ± 0.03 Ba | |
| LRPE | 0.89 ± 0.08 Ac | 1.05 ± 0.10 Ab | 1.31 ± 0.07 Aa | 1.32 ± 0.12 Aa | 1.36 ± 0.08 Aa | 1.32 ± 0.04 BCa | 1.42 ± 0.10 Ba | |
| T22 (ms) | CK | 53.54 ± 0.05 Ad | 61.10 ± 0.33 Ac | 63.29 ± 5.30 Abc | 63.29 ± 4.52 Abc | 64.87 ± 0.41 Abc | 66.46 ± 2.04 Ab | 78.21 ± 0.08 Aa |
| AA | 53.54 ± 0.05 Ab | 61.20 ± 0.33 Aa | 61.57 ± 2.22 Aa | 57.17 ± 2.60 Bab | 63.16 ± 6.34 Aa | 63.13 ± 2.59 Ba | 63.17 ± 4.11 Ba | |
| GSE | 53.54 ± 0.05 Ac | 60.61 ± 0.41 Ab | 60.31 ± 0.37 Ab | 63.50 ± 2.68 Aab | 63.95 ± 3.28 Aa | 61.46 ± 1.00 Bab | 60.51 ± 0.73 Bb | |
| LSPC | 53.54 ± 0.05 Ad | 56.25 ± 1.13 Bcd | 63.16 ± 0.90 Aab | 59.27 ± 1.41 ABbc | 60.42 ± 3.99 Abc | 63.34 ± 0.47 Bab | 66.48 ± 4.93 Ba | |
| LRPE | 53.54 ± 0.05 Ad | 55.86 ± 2.07 Bcd | 60.11 ± 0.89 Aab | 57.24 ± 2.26 Bbc | 63.13 ± 1.87 Aa | 62.84 ± 1.10 Ba | 62.84 ± 3.22 Ba | |
| T23 (ms) | CK | 550.73 ± 12.14 Ae | 691.73 ± 2.01 Ad | 695.99 ± 19.12 Ad | 709.62 ± 12.95 Ad | 815.47 ± 25.18 Ac | 926.52 ± 21.49 Ab | 1259.95 ± 21.71 Aa |
| AA | 550.73 ± 12.14 Ae | 635.71 ± 17.08 Bd | 654.40 ± 8.66 Bd | 699.58 ± 6.25 Ac | 718.55 ± 16.19 Bc | 827.21 ± 14.68 Bb | 860.71 ± 10.86 Ba | |
| GSE | 550.73 ± 12.14 Ad | 592.51 ± 6.66 Cc | 610.87 ± 20.60 Cbc | 618.77 ± 8.97 Bb | 629.26 ± 13.57 Db | 724.20 ± 16.21 Ca | 740.49 ± 5.78 CDa | |
| LSPC | 550.73 ± 12.14 Ae | 551.27 ± 17.50 De | 582.79 ± 18.47 Cd | 628.44 ± 9.16 Bc | 654.74 ± 2.88 CDb | 705.71 ± 12.19 CDa | 726.23 ± 15.47 Da | |
| LRPE | 550.73 ± 12.14 Af | 595.52 ± 5.68 Ce | 607.93 ± 6.30 Cde | 617.33 ± 3.70 Bd | 662.58 ± 15.62 Cc | 683.92 ± 3.34 Db | 757.46 ± 17.12 Ca | |
| Water Distribution | Samples | Storage Time (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 9 | 11 | ||
| P21 (%) | CK | 1.01 ± 0.44 Ac | 1.65 ± 0.26 BCc | 2.74 ± 0.48 Ab | 2.76 ± 0.29 Ab | 2.52 ± 0.37 Ab | 3.04 ± 0.25 Aab | 3.60 ± 0.72 Aa |
| AA | 1.01 ± 0.44 Ac | 1.00 ± 0.25 Cc | 1.93 ± 0.16 Ab | 2.15 ± 0.86 Aab | 2.64 ± 0.42 Aab | 2.86 ± 0.20 Aa | 2.78 ± 0.25 ABa | |
| GSE | 1.01 ± 0.44 Ac | 1.84 ± 0.79 Bbc | 2.30 ± 0.77 Aab | 2.37 ± 0.34 Aab | 2.52 ± 0.21 Aab | 2.87 ± 0.26 Aa | 2.47 ± 0.36 Bab | |
| LSPC | 1.01 ± 0.44 Ac | 1.61 ± 0.09 BCbc | 1.99 ± 0.66 Aabc | 3.19 ± 1.64 Aa | 3.02 ± 0.89 Aa | 2.83 ± 0.42 Aab | 2.56 ± 0.32 Bab | |
| LRPE | 1.01 ± 0.44 Ab | 2.67 ± 0.27 Aa | 2.67 ± 0.23 Aa | 2.97 ± 0.64 Aa | 2.60 ± 0.34 Aa | 2.50 ± 0.49 Aa | 1.63 ± 0.48 Cb | |
| P22 (%) | CK | 98.70 ± 0.47 Aa | 97.94 ± 0.06 ABa | 96.82 ± 0.68 Ab | 96.66 ± 0.56 Ab | 95.95 ± 0.29 Cbc | 96.03 ± 0.20 Abc | 95.29 ± 0.72 Bc |
| AA | 98.70 ± 0.47 Aa | 98.74 ± 0.33 Aa | 97.95 ± 0.21 Aa | 97.13 ± 0.73 Ab | 96.96 ± 0.61 ABb | 96.72 ± 0.16 Abc | 96.02 ± 0.35 ABc | |
| GSE | 98.70 ± 0.47 Aa | 97.49 ± 1.15 Bb | 97.00 ± 0.61 Abc | 96.58 ± 0.39 Abcd | 96.53 ± 0.45 ABCbcd | 96.22 ± 0.49 Acd | 95.54 ± 0.50 ABd | |
| LSPC | 98.70 ± 0.47 Aa | 97.92 ± 0.21 ABab | 97.07 ± 1.08 Abc | 96.21 ± 1.41 Ac | 96.03 ± 0.70 BCc | 96.40 ± 0.43 Ac | 96.49 ± 0.43 Ac | |
| LRPE | 98.70 ± 0.47 Aa | 96.94 ± 0.19 Bb | 97.09 ± 0.23 Ab | 96.13 ± 0.66 Ab | 97.07 ± 0.34 Ab | 96.47 ± 0.84 Ab | 96.37 ± 0.38 Ab | |
| P23 (%) | CK | 0.28 ± 0.08 Ad | 0.40 ± 0.29 Acd | 0.44 ± 0.20 ABcd | 0.58 ± 0.28 Abcd | 1.53 ± 0.63 Aa | 0.96 ± 0.01 Abc | 1.11 ± 0.04 Bab |
| AA | 0.28 ± 0.08 Acd | 0.27 ± 0.10 Acd | 0.12 ± 0.12 Bd | 0.72 ± 0.15 Ab | 0.40 ± 0.24 Bc | 0.42 ± 0.06 Bc | 1.20 ± 0.10 Ba | |
| GSE | 0.28 ± 0.08 Ac | 0.33 ± 0.11 Ac | 0.91 ± 0.52 Ab | 1.06 ± 0.38 Ab | 0.95 ± 0.28 ABb | 0.90 ± 0.27 ABb | 1.99 ± 0.18 Aa | |
| LSPC | 0.28 ± 0.08 Ac | 0.50 ± 0.09 Aabc | 0.94 ± 0.46 Aa | 0.46 ± 0.03 Abc | 0.95 ± 0.22 ABa | 0.77 ± 0.01 ABab | 0.96 ± 0.28 Ba | |
| LRPE | 0.28 ± 0.08 Ac | 0.39 ± 0.08 Ac | 0.24 ± 0.01 Bc | 0.90 ± 0.49 Ab | 0.31 ± 0.03 Bc | 1.03 ± 0.53 Ab | 2.00 ± 0.16 Aa | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wang, L.; Zhu, Z.; Zhang, Y.; Xiong, G.; Li, S. Three Phenolic Extracts Regulate the Physicochemical Properties and Microbial Community of Refrigerated Channel Catfish Fillets during Storage. Foods 2023, 12, 765. https://doi.org/10.3390/foods12040765
Huang J, Wang L, Zhu Z, Zhang Y, Xiong G, Li S. Three Phenolic Extracts Regulate the Physicochemical Properties and Microbial Community of Refrigerated Channel Catfish Fillets during Storage. Foods. 2023; 12(4):765. https://doi.org/10.3390/foods12040765
Chicago/Turabian StyleHuang, Jian, Lan Wang, Zhenzhou Zhu, Yun Zhang, Guangquan Xiong, and Shuyi Li. 2023. "Three Phenolic Extracts Regulate the Physicochemical Properties and Microbial Community of Refrigerated Channel Catfish Fillets during Storage" Foods 12, no. 4: 765. https://doi.org/10.3390/foods12040765
APA StyleHuang, J., Wang, L., Zhu, Z., Zhang, Y., Xiong, G., & Li, S. (2023). Three Phenolic Extracts Regulate the Physicochemical Properties and Microbial Community of Refrigerated Channel Catfish Fillets during Storage. Foods, 12(4), 765. https://doi.org/10.3390/foods12040765






