The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology
Abstract
:1. Introduction
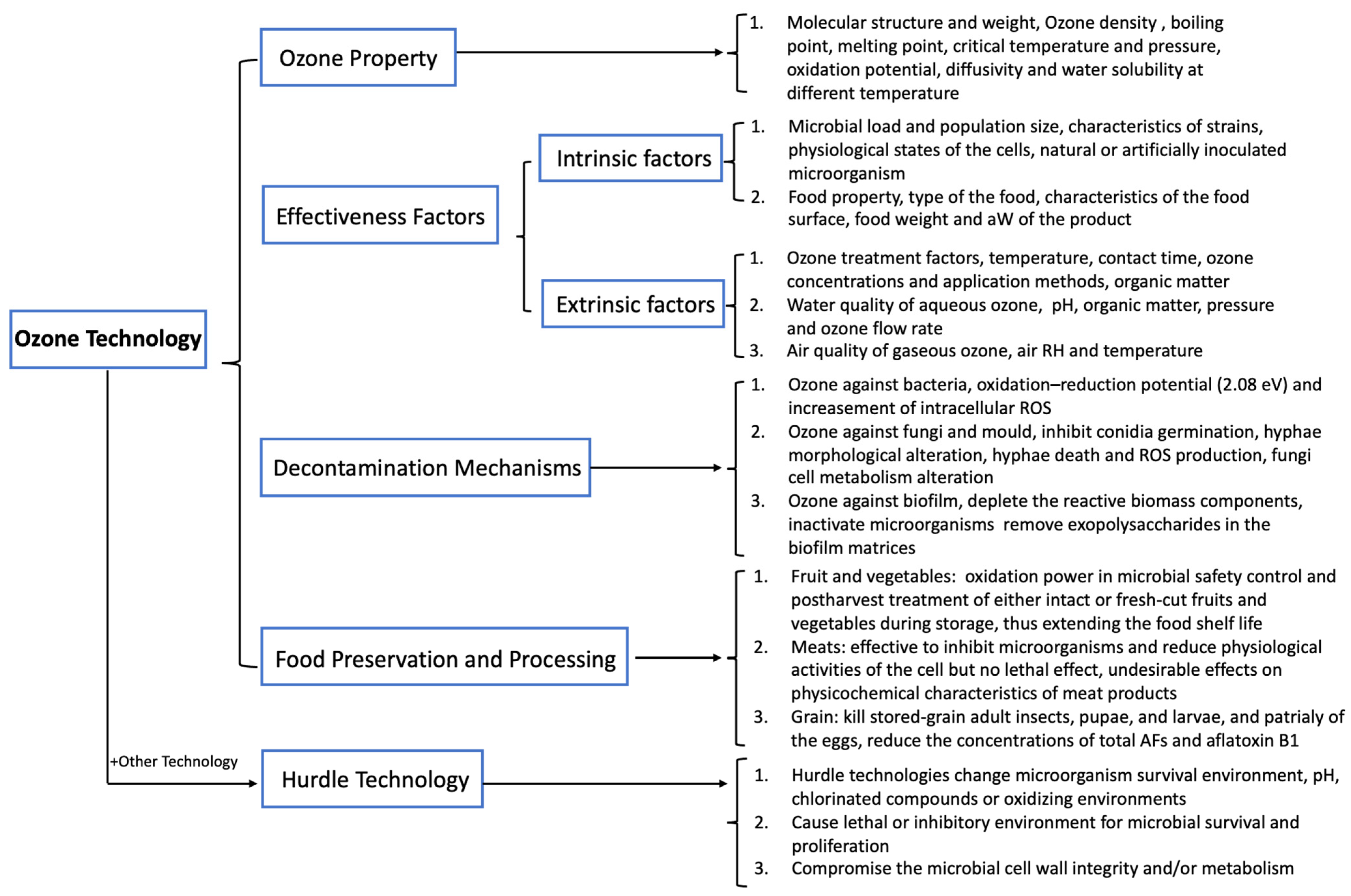
2. Factors Affecting Microorganism Inactivation Efficiency of Ozone Technology
2.1. Intrinsic Factors
2.2. Extrinsic Factors
3. Ozone against Microorganisms
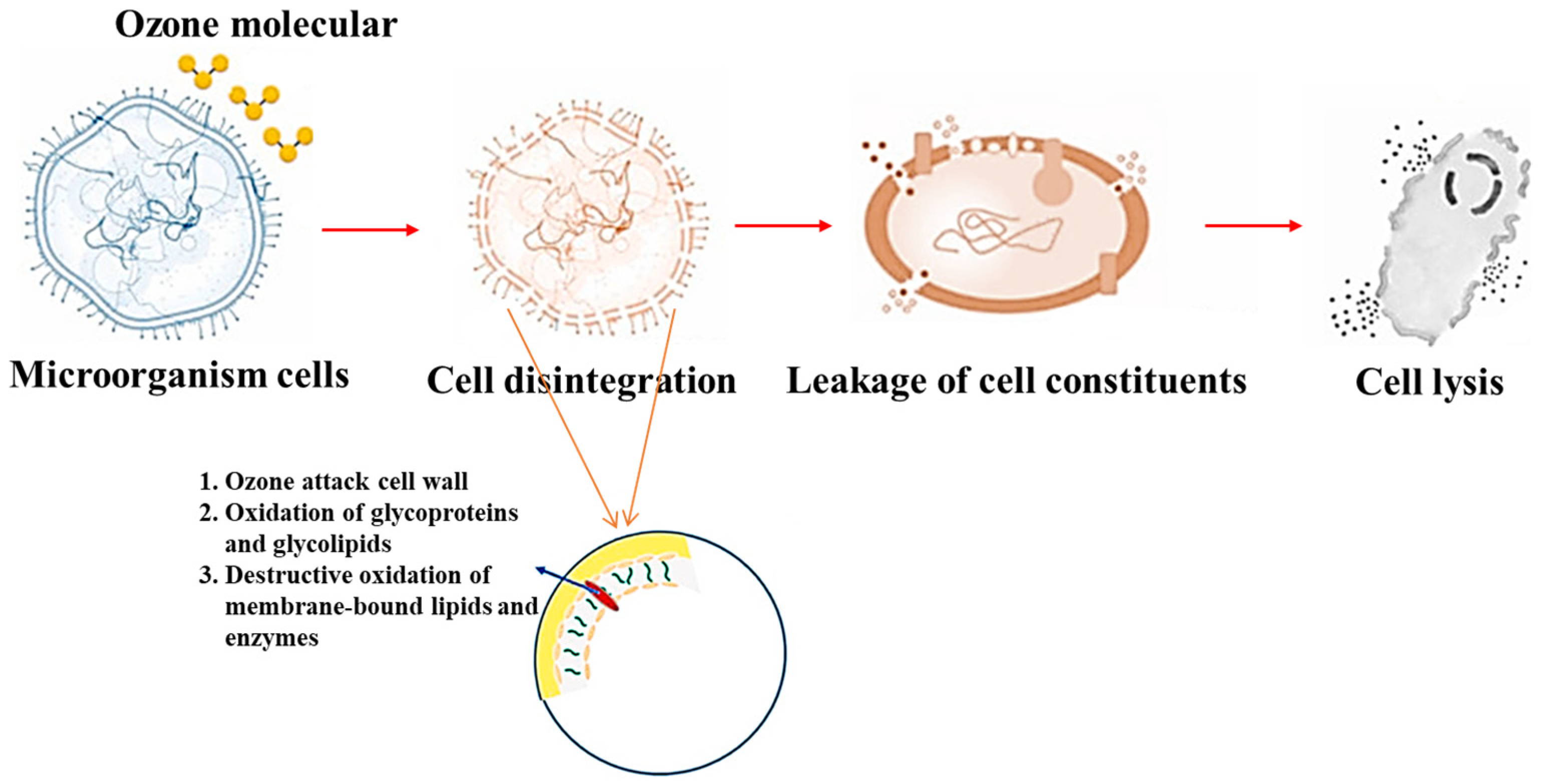
3.1. Mechanisms of Ozone Inactivation of Microorganisms
3.2. Ozone Reaction against Fungi and Mould
3.3. Ozone against Biofilms
4. Use of Ozone in Food Preservation and Processing
4.1. Effects of Ozone in Fruit and Vegetable Processing
4.2. Effects of Ozone in Meat Products’ Processing
4.3. Effects of Ozone in Grain Products’ Processing
5. Combined Applications of Ozone Treatment and Other Technologies in Food Processing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iqbal, J.; Yu, D.; Zubair, M.; Rasheed, M.I.; Khizar, H.M.U.; Imran, M. Health Consciousness, Food Safety Concern, and Consumer Purchase Intentions Toward Organic Food: The Role of Consumer Involvement and Ecological Motives. SAGE Open 2021, 11, 21582440211015727. [Google Scholar] [CrossRef]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review. Foods 2021, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Felter, S.P.; Zhang, X.; Thompson, C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regul. Toxicol. Pharmacol. 2021, 121, 104887. [Google Scholar] [CrossRef] [PubMed]
- Ousji, O.; Sleno, L. Identification of In Vitro Metabolites of Synthetic Phenolic Antioxidants BHT, BHA, and TBHQ by LC-HRMS/MS. Int. J. Mol. Sci. 2020, 21, 9525. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Iatropoulos, M.J.; Whysner, J. Safety Assessment of Butylated Hydroxyanisole and Butylated Hydroxytoluene as Antioxidant Food Additives. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Akhlaghi, A.P.; Alizadeh, A.M.; Dehghan, P.; Maleki, P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review. Toxicol. Rep. 2022, 9, 1066–1075. [Google Scholar] [CrossRef]
- Simpson, A.M.-A.; Mitch, W.A. Chlorine and ozone disinfection and disinfection byproducts in postharvest food processing facilities: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1825–1867. [Google Scholar] [CrossRef]
- Stearns, R.; Freshour, A.; Shen, C. Literature review for applying peroxyacetic acid and/or hydrogen peroxide to control foodborne pathogens on food products. J. Agric. Food Res. 2022, 10, 100442. [Google Scholar] [CrossRef]
- Possas, A.; Pérez-Rodríguez, F.; Tarlak, F.; García-Gimeno, R.M. Quantifying and modelling the inactivation of Listeria monocytogenes by electrolyzed water on food contact surfaces. J. Food Eng. 2021, 290, 110287. [Google Scholar] [CrossRef]
- Song, W.J.; Kang, D.H. Inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium in black and red pepper by vacuumed hydrogen peroxide vapour. J. Appl. Microbiol. 2022, 132, 290–297. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, Z.; Wang, S. Microwave processing: Effects and impacts on food components. Crit. Rev. Food Sci. Nutr. 2018, 58, 2476–2489. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting Non-Thermal Food Processing and Preservation Methods—Action Mechanisms, Pros and Cons: A Technological Update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Stadler, D.; Berthiller, F.; Suman, M.; Schuhmacher, R.; Krska, R. Novel analytical methods to study the fate of mycotoxins during thermal food processing. Anal. Bioanal. Chem. 2020, 412, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-H.; Kim, S.-J.; Kwak, S.-J.; Yoon, K.-S. Efficacy of Sodium Hypochlorite and Acidified Sodium Chlorite in Preventing Browning and Microbial Growth on Fresh-Cut Produce. Prev. Nutr. Food Sci. 2012, 17, 210–216. [Google Scholar] [CrossRef]
- Hoenicke, K.; Gatermann, R.; Hartig, L.; Mandix, M.; Otte, S. Formation of semicarbazide (SEM) in food by hypochlorite treatment: Is SEM a specific marker for nitrofurazone abuse? Food Addit. Contam. 2004, 21, 526–537. [Google Scholar] [CrossRef]
- Zengin, N.; Yüzbaşioğlu, D.; Unal, F.; Yilmaz, S.; Aksoy, H. The evaluation of the genotoxicity of two food preservatives: Sodium benzoate and potassium benzoate. Food Chem. Toxicol. 2011, 49, 763–769. [Google Scholar] [CrossRef]
- Kamemura, N. Butylated hydroxytoluene, a food additive, modulates membrane potential and increases the susceptibility of rat thymocytes to oxidative stress. Comput. Toxicol. 2018, 6, 32–38. [Google Scholar] [CrossRef]
- Gopal, K.; Tripathy, S.S.; Bersillon, J.L.; Dubey, S.P. Chlorination byproducts, their toxicodynamics and removal from drinking water. J. Hazard. Mater. 2007, 140, 1–6. [Google Scholar] [CrossRef]
- Diana, M.; Felipe-Sotelo, M.; Bond, T. Disinfection byproducts potentially responsible for the association between chlorinated drinking water and bladder cancer: A review. Water Res. 2019, 162, 492–504. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Priya, E.P.B.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. LWT Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Sivaranjani, S.; Prasath, V.A.; Pandiselvam, R.; Kothakota, A.; Khaneghah, A.M. Recent advances in applications of ozone in the cereal industry. LWT 2021, 146, 111412. [Google Scholar] [CrossRef]
- Hill, A.G.; Rice, R.G. Historical Background, Properties and Applications. In Ozone Technology and Application; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1982; pp. 1–37. [Google Scholar]
- PubChem. Ozone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/24823 (accessed on 1 December 2022).
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.; Wu, J.; Doan, H. Inactivation of Fungi Associated with Barley Grain by Gaseous Ozone. J. Environ. Sci. Health Part B 2003, 38, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Masaki, H.; Mori, T.; Tsuchiya, T.; Konuma, H.; Hara-Kudo, Y.; Takatori, K. Inactivation Effects of UV Irradiation and Ozone Treatment on the Yeast and the Mold in Mineral Water. J. Food Prot. 2010, 73, 1537–1542. [Google Scholar] [CrossRef]
- Song, W.J.; Shin, J.Y.; Ryu, S.; Kang, D.H. Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes in apple juice at different pH levels by gaseous ozone treatment. J. Appl. Microbiol. 2015, 119, 465–474. [Google Scholar] [CrossRef]
- Alwi, N.A.; Ali, A. Reduction of Escherichia coli O157, Listeria monocytogenes and Salmonella enterica sv. Typhimurium populations on fresh-cut bell pepper using gaseous ozone. Food Control 2014, 46, 304–311. [Google Scholar] [CrossRef]
- Brié, A.; Boudaud, N.; Mssihid, A.; Loutreul, J.; Bertrand, I.; Gantzer, C. Inactivation of murine norovirus and hepatitis A virus on fresh raspberries by gaseous ozone treatment. Food Microbiol. 2018, 70, 1–6. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, K.; Gao, M.; Shi, C.; Ge, C.; Qu, D.; Zhu, J.; Shi, Y.; Han, J. Inactivation of Vibrio parahaemolyticus by Aqueous Ozone. J. Microbiol. Biotechnol. 2018, 28, 1233–1246. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Rice, R.G. Regulatory and Legislative Issues. In Ozone in Food Processing; Wiley-Blackwell: Oxford, UK, 2012; pp. 7–17. [Google Scholar]
- Rice, R.; Graham, D.M.U.S. FDA Regulatory Approval of Ozone as an Antimicrobial Agent–What Is Allowed and What Needs to Be Understood. Ozone News 2001, 29, 22–31. [Google Scholar]
- Secondary Direct Food Additives Permitted in Food for Human Consumption (21 CFR 173.5-173.405).|FAOLEX [Internet]. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC156335/ (accessed on 1 December 2022).
- Brodowska, A.J.; Nowak, A.; Śmigielski, K.B. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, C.; Jiang, A.; Zhang, Y.; Zhao, Q.; Hu, W. Effects of aqueous ozone treatment on microbial growth, quality, and pesticide residue of fresh-cut cabbage. Food Sci. Nutr. 2021, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Premjit, Y.; Sruthi, N.U.; Pandiselvam, R.; Kothakota, A. Aqueous ozone: Chemistry, physiochemical properties, microbial inactivation, factors influencing antimicrobial effectiveness, and application in food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1054–1085. [Google Scholar] [CrossRef]
- Aslam, R.; Alam, M.S.; Singh, S.; Kumar, S. Aqueous ozone sanitization of whole peeled onion: Process optimization and evaluation of keeping quality during refrigerated storage. LWT 2021, 151, 112183. [Google Scholar] [CrossRef]
- Ummat, V.; Singh, A.K.; Sidhu, G.K. Effect of aqueous ozone on quality and shelf life of shredded green bell pepper (Capsicum annuum). J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Stivarius, M.R.; Pohlman, F.W.; McElyea, K.S.; Apple, J.K. Microbial, instrumental color and sensory color and odor characteristics of ground beef produced from beef trimmings treated with ozone or chlorine dioxide. Meat Sci. 2002, 60, 299–305. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K. Ozone in Fruit and Vegetable Processing. In Ozone in Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 55–80. [Google Scholar] [CrossRef]
- Crowe, K.M.; Skonberg, D.; Bushway, A.; Baxter, S. Application of ozone sprays as a strategy to improve the microbial safety and quality of salmon fillets. Food Control 2012, 25, 464–468. [Google Scholar] [CrossRef]
- Shu, X.; Singh, M.; Karampudi, N.B.R.; Bridges, D.F.; Kitazumi, A.; Wu, V.C.H.; Reyes, B.G.D.L. Responses of Escherichia coli and Listeria monocytogenes to ozone treatment on non-host tomato: Efficacy of intervention and evidence of induced acclimation. PLoS ONE 2021, 16, e0256324. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Ozdemir, M. Effect of gaseous ozone on microbial inactivation and sensory of flaked red peppers. Int. J. Food Sci. Technol. 2008, 43, 1657–1662. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, R.; Xue, H.; Bi, Y.; Li, L.; Zhang, Q.; Kouasseu, C.J.; Nan, M.; Prusky, D. Ozone controls potato dry rot development and diacetoxyscirpenol accumulation by targeting the cell membrane and affecting the growth of Fusarium sulphureus. Physiol. Mol. Plant Pathol. 2022, 118, 101785. [Google Scholar] [CrossRef]
- Gibson, K.E.; Almeida, G.; Jones, S.; Wright, K.; Lee, J.A. Inactivation of bacteria on fresh produce by batch wash ozone sanitation. Food Control 2019, 106, 106747. [Google Scholar] [CrossRef]
- Yesil, M.; Kasler, D.R.; Huang, E.; Yousef, A.E. Efficacy of Gaseous Ozone Application during Vacuum Cooling against Escherichia coli O157:H7 on Spinach Leaves as Influenced by Bacterium Population Size. J. Food Prot. 2017, 80, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-G.; Yousef, A.E.; Khadre, M.A. Ozone and its current and future application in the food industry. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2003. [Google Scholar]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- And, M.A.; Yousef, A.E. Efficacy of Ozone Against Escherichia coli O157:H7 on Apples. J. Food Sci. 2001, 66, 1380–1384. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Plessas, S.; Ceciu, S.; Lazar, V.; Mantzourani, I.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Evaluation of ozone efficacy on the reduction of microbial population of fresh cut lettuce (Lactuca sativa) and green bell pepper (Capsicum annuum). Food Control 2013, 30, 491–496. [Google Scholar] [CrossRef]
- Jaksch, D.; Margesin, R.; Mikoviny, T.; Skalny, J.D.; Hartungen, E.; Schinner, F.; Mason, N.; Märk, T. The effect of ozone treatment on the microbial contamination of pork meat measured by detecting the emissions using PTR-MS and by enumeration of microorganisms. Int. J. Mass Spectrom. 2004, 239, 209–214. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (a w) on Microbial Stability as a Hurdle in Food Preservation. In Water Activity in Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar]
- Wu, J.; Doan, H.; Cuenca, M.A. Investigation of gaseous ozone as an anti-fungal fumigant for stored wheat. J. Chem. Technol. Biotechnol. 2006, 81, 1288–1293. [Google Scholar] [CrossRef]
- Redfern, J.; Verran, J. Effect of humidity and temperature on the survival of Listeria monocytogenes on surfaces. Lett. Appl. Microbiol. 2017, 64, 276–282. [Google Scholar] [CrossRef]
- Bigi, F.; Haghighi, H.; Quartieri, A.; De Leo, R.; Pulvirenti, A. Impact of low-dose gaseous ozone treatment to reduce the growth of in vitro broth cultures of foodborne pathogenic/spoilage bacteria in a food storage cold chamber. J. Food Saf. 2021, 41, e12892. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Zhang, X.; Dong, C.; Xue, W.; Xu, W. The effect of different doses of ozone treatments on the postharvest quality and biodiversity of cantaloupes. Postharvest Biol. Technol. 2020, 163, 111124. [Google Scholar] [CrossRef]
- Onopiuk, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A.; Półtorak, A. Impact of Ozonisation Time and Dose on Health Related and Microbiological Properties of Rapanui Tomatoes. Agriculture 2021, 11, 428. [Google Scholar] [CrossRef]
- Ayranci, U.G.; Ozunlu, O.; Ergezer, H.; Karaca, H. Effects of Ozone Treatment on Microbiological Quality and Physicochemical Properties of Turkey Breast Meat. Ozone Sci. Eng. 2020, 42, 95–103. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.; Zhang, Y.; Wang, J. Use of aqueous ozone rinsing to improve the disinfection efficacy and shorten the processing time of ultrasound-assisted washing of fresh produce. Ultrason. Sonochemistry 2022, 83, 105931. [Google Scholar] [CrossRef] [PubMed]
- El Darra, N.; Xie, F.; Kamble, P.; Khan, Z.; Watson, I. Decontamination of Escherichia coli on dried onion flakes and black pepper using Infra-red, ultraviolet and ozone hurdle technologies. Heliyon 2021, 7, e07259. [Google Scholar] [CrossRef]
- Young, J.C.; Zhu, H.; Zhou, T. Degradation of trichothecene mycotoxins by aqueous ozone. Food Chem. Toxicol. 2006, 44, 417–424. [Google Scholar] [CrossRef]
- Aslam, R.; Alam, M.S.; Pandiselvam, R. Aqueous ozone sanitization system for fresh produce: Design, development, and optimization of process parameters for minimally processed onion. Ozone Sci. Eng. 2022, 44, 3–16. [Google Scholar] [CrossRef]
- Pounraj, S.; Bhilwadikar, T.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Effect of ozone, lactic acid and combination treatments on the control of microbial and pesticide contaminants of fresh vegetables. J. Sci. Food Agric. 2021, 101, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Yoon, J. Disinfection of Water Containing Natural Organic Matter by Using Ozone-Initiated Radical Reactions. Appl. Environ. Microbiol. 2003, 69, 2284–2291. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological Aspects of Ozone Applications in Food: A Review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Gurol, M.D.; Singer, P.C. Kinetics of ozone decomposition: A dynamic approach. Environ. Sci. Technol. 1982, 16, 377–383. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Wilhelm, A.E.; Goulart, I.B.; Tonon, R.V.; Freitas-Silva, O.; Germani, R.; Chávez, D.W.H. Effect of water temperature and pH on the concentration and time of ozone saturation. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Han, Y.; Floros, J.D.; Linton, R.H.; Nielsen, S.S.; Nelson, P.E. Response Surface Modeling for the Inactivation of Escherichia coli O157:H7 on Green Peppers (Capsicum annuum) by Ozone Gas Treatment. J. Food Sci. 2002, 67, 1188–1193. [Google Scholar] [CrossRef]
- Ishizaki, K.; Shinriki, N.; Matsuyama, H. Inactivation of Bacillus spores by gaseous ozone. J. Appl. Bacteriol. 1986, 60, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, A. Alternaria in food products. Curr. Opin. Food Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- de Alencar, E.R.; Faroni, L.R.D.; Soares, N.d.F.F.; da Silva, W.A.; Carvalho, M.C.d.S. Efficacy of ozone as a fungicidal and detoxifying agent of aflatoxins in peanuts. J. Sci. Food Agric. 2012, 92, 899–905. [Google Scholar] [CrossRef]
- Naito, S.; Takahara, H. Ozone Contribution in Food Industry in Japan. Ozone Sci. Eng. 2006, 28, 425–429. [Google Scholar] [CrossRef]
- Quevedo, L.; Oacute, N.; Bast, I.R.; as-Montes, J.M.; Espinoza-Tellez, T. Inactivation of coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation. Sci. Agropecu. 2020, 257–266. [Google Scholar] [CrossRef]
- Greene, A.K.; Güzel-Seydim, Z.B.; Seydim, A.C. Chemical and Physical Properties of Ozone. In Ozone in Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 19–32. [Google Scholar] [CrossRef]
- Casas, D.E.; Vargas, D.A.; Randazzo, E.; Lynn, D.; Echeverry, A.; Brashears, M.M.; Sanchez-Plata, M.X.; Miller, M.F. In-Plant Validation of Novel On-Site Ozone Generation Technology (Bio-Safe) Compared to Lactic Acid Beef Carcasses and Trim Using Natural Microbiota and Salmonella and E. coli O157:H7 Surrogate Enumeration. Foods 2021, 10, 1002. [Google Scholar] [CrossRef]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms 2021, 10, 40. [Google Scholar] [CrossRef]
- Ersoy, Z.G.; Barisci, S.; Dinc, O. Mechanisms of the Escherichia coli and Enterococcus faecalis inactivation by ozone. LWT 2019, 100, 306–313. [Google Scholar] [CrossRef]
- Botta, C.; Ferrocino, I.; Cavallero, M.C.; Riva, S.; Giordano, M.; Cocolin, L. Potentially active spoilage bacteria community during the storage of vacuum packaged beefsteaks treated with aqueous ozone and electrolyzed water. Int. J. Food Microbiol. 2018, 266, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Griffith, C.; Peters, A. Bactericidal properties of ozone and its potential application as a terminal disinfectant. J. Food Prot. 2000, 63, 1100–1106. [Google Scholar] [CrossRef]
- Cullen, P.J.; Valdramidis, V.P.; Tiwari, B.K.; Patil, S.; Bourke, P.; O’Donnell, C.P. Ozone Processing for Food Preservation: An Overview on Fruit Juice Treatments. Ozone Sci. Eng. 2010, 32, 166–179. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Aflatoxigenic fungi and mycotoxins in food: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 709–721. [Google Scholar] [CrossRef]
- Milićević, D.R.; Škrinjar, M.; Baltić, T. Real and Perceived Risks for Mycotoxin Contamination in Foods and Feeds: Challenges for Food Safety Control. Toxins 2010, 2, 572–592. [Google Scholar] [CrossRef]
- Savi, G.D.; Scussel, V.M. Effects of Ozone Gas Exposure on Toxigenic Fungi Species from Fusarium, Aspergillus, and Penicillium Genera. Ozone Sci. Eng. 2014, 36, 144–152. [Google Scholar] [CrossRef]
- Levinskaitė, L.; Vaičekauskytė, V. Control of fungi isolated from cereals: Variations in the susceptibility of fungal species to essential oils, ozone, and UV-C. Int. J. Food Sci. Technol. 2022, 57, 6389–6398. [Google Scholar] [CrossRef]
- Beber-Rodrigues, M.; Savi, G.D.; Scussel, V.M. Ozone Effect on Fungi Proliferation and Genera Susceptibility of Treated Stored Dry Paddy Rice (Oryza sativa L.). J. Food Saf. 2015, 35, 59–65. [Google Scholar] [CrossRef]
- Tachikawa, M.; Yamanaka, K.; Nakamuro, K. Studies on the Disinfection and Removal of Biofilms by Ozone Water Using an Artificial Microbial Biofilm System. Ozone Sci. Eng. 2009, 31, 3–9. [Google Scholar] [CrossRef]
- Marino, M.; Maifreni, M.; Baggio, A.; Innocente, N. Inactivation of Foodborne Bacteria Biofilms by Aqueous and Gaseous Ozone. Front. Microbiol. 2018, 9, 2024. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Afaříková, M.; Šafařík, I. Immunomagnetic separation of Escherichia coli O26, O111 and O157 from vegetables. Lett. Appl. Microbiol. 2001, 33, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Won, G.; Lee, J.H. Salmonella Typhimurium, the major causative agent of foodborne illness inactivated by a phage lysis system provides effective protection against lethal challenge by induction of robust cell-mediated immune responses and activation of dendritic cells. Vet. Res. 2017, 48, 66. [Google Scholar] [CrossRef] [PubMed]
- Macleod, J.; Beeton, M.L.; Blaxland, J. An Exploration of Listeria monocytogenes, Its Influence on the UK Food Industry and Future Public Health Strategies. Foods 2022, 11, 1456. [Google Scholar] [CrossRef]
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef]
- Westrell, T.; Ciampa, N.; Boelaert, F.; Helwigh, B.; Korsgaard, H.; Chríel, M.; Ammon, A.; Mäkelä, P. Zoonotic infections in Europe in 2007: A summary of the EFSA-ECDC annual report. Eurosurveillance 2009, 14, 19100. [Google Scholar] [CrossRef]
- Roy, S.; Choudhury, B.; Johnson, J.; Schindler-Tyka, A. Application of dielectric barrier discharge for improving food shelf life and reducing spoilage. Sci. Rep. 2021, 11, 19200. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Cárdenas, F.C.; Andrés, S.; Giannuzzi, L.; Zaritzky, N. Antimicrobial action and effects on beef quality attributes of a gaseous ozone treatment at refrigeration temperatures. Food Control 2011, 22, 1442–1447. [Google Scholar] [CrossRef]
- Giménez, B.; Graiver, N.; Giannuzzi, L.; Zaritzky, N. Treatment of beef with gaseous ozone: Physicochemical aspects and antimicrobial effects on heterotrophic microflora and listeria monocytogenes. Food Control 2021, 121, 107602. [Google Scholar] [CrossRef]
- Brühl, C.A.; Zaller, J.G. Biodiversity Decline as a Consequence of an Inappropriate Environmental Risk Assessment of Pesticides. Front. Environ. Sci. 2019, 7, 177. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Maleski, A.L.; Balan-Lima, L.; Bernardo, J.T.; Hipolito, L.M.; Seni-Silva, A.C.; Batista-Filho, J.; Falcao, M.A.; Lima, C. Impact of pesticides on human health in the last six years in Brazil. IJERPH 2022, 19, 3198. [Google Scholar] [CrossRef]
- Budnik, L.T.; Kloth, S.; Velasco-Garrido, M.; Baur, X. Prostate cancer and toxicity from critical use exemptions of methyl bromide: Environmental protection helps protect against human health risks. Environ. Health 2012, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef]
- Işikber, A.A.; Öztekin, S. Comparison of susceptibility of two stored-product insects, Ephestia kuehniella Zeller and Tribolium confusum du Val to gaseous ozone. J. Stored Prod. Res. 2009, 45, 159–164. [Google Scholar] [CrossRef]
- White, S.D.; Murphy, P.T.; Leandro, L.F.; Bern, C.J.; Beattie, S.D.; van Leeuwen, J. Mycoflora of high-moisture maize treated with ozone. J. Stored Prod. Res. 2013, 55, 84–89. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Scussel, V.M. Ozone Treatment Efficiency in Aspergillus and Penicillium Growth Inhibition and Mycotoxin Degradation of Stored Wheat Grains (Triticum aestivum L.). J. Food Process. Preserv. 2015, 39, 940–948. [Google Scholar] [CrossRef]
- Porto, Y.D.; Trombete, F.M.; Freitas-Silva, O.; de Castro, I.M.; Direito, G.M.; Ascheri, J.L.R. Gaseous Ozonation to Reduce Aflatoxins Levels and Microbial Contamination in Corn Grits. Microorganisms 2019, 7, 220. [Google Scholar] [CrossRef]
- Tiwari, B.K.W.; Brennan, C.S.; Curran, T.; Gallagher, E.; Cullen, P.J.; O’Donnell, C.P. Application of ozone in grain processing. J. Cereal Sci. 2010, 51, 248–255. [Google Scholar] [CrossRef]
- Isikber, A.A.; Athanassiou, C.G. The use of ozone gas for the control of insects and micro-organisms in stored products. J. Stored Prod. Res. 2015, 64, 139–145. [Google Scholar] [CrossRef]
- Sun, C.; Ji, J.; Wu, S.; Sun, C.; Pi, F.; Zhang, Y.; Tang, L.; Sun, X. Saturated aqueous ozone degradation of deoxynivalenol and its application in contaminated grains. Food Control 2016, 69, 185–190. [Google Scholar] [CrossRef]
- Zhu, F. Effect of ozone treatment on the quality of grain products. Food Chem. 2018, 264, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Pohlman, F.W. Ozone in Meat Processing. Ozone Food Process. 2012, 123–136. [Google Scholar] [CrossRef]
- Pohlman, F.W.; Stivarius, M.R.; McElyea, K.S.; Johnson, Z.B.; Johnson, M.G. The effects of ozone, chlorine dioxide, cetylpyridinium chloride and trisodium phosphate as multiple antimicrobial interventions on microbiological, instrumental color, and sensory color and odor characteristics of ground beef. Meat Sci. 2002, 61, 307–313. [Google Scholar] [CrossRef]
- Lyu, F.; Shen, K.; Ding, Y.; Ma, X. Effect of pretreatment with carbon monoxide and ozone on the quality of vacuum packaged beef meats. Meat Sci. 2016, 117, 137–146. [Google Scholar] [CrossRef]
- Cantalejo, M.J.; Zouaghi, F.; Pérez-Arnedo, I. Combined effects of ozone and freeze-drying on the shelf-life of Broiler chicken meat. LWT Food Sci. Technol. 2016, 68, 400–407. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C.; Wahia, H.; Amanor-Atiemoh, R.; Out, P.; Qudus, A.; Fakayode, O.A.; Ma, H. Sonozonation: Enhancing the antimicrobial efficiency of aqueous ozone washing techniques on cherry tomato. Ultrason. Sonochemistry 2020, 64, 105059. [Google Scholar] [CrossRef]
- Perez, S.L.; Chianfrone, D.J.; Bagnato, V.S.; Blanco, K.C. Optical technologies for antibacterial control of fresh meat on display. LWT 2022, 160, 113213. [Google Scholar] [CrossRef]
- Szeto, W.; Yam, W.C.; Huang, H.; Leung, D.Y.C. The efficacy of vacuum-ultraviolet light disinfection of some common environmental pathogens. BMC Infect. Dis. 2020, 20, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Value |
|---|---|
| Molecular formula | O3 |
| Molecular structure | 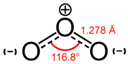 |
| Molecular weight (g/mol) | 48 |
| Density (g/L, 1 atm) | 2.14 |
| Boiling point (°C, 1 atm) | −111.9 |
| Melting point (°C, 1 atm) | −192.6 |
| Critical temperature (°C, 1 atm) | −12.1 |
| Critical pressure (atm) | 54.6 |
| Oxidation potential (V) | −2.07 |
| Diffusivity (20 °C) | 1.79 × 10−9 m2/s (liquid form), 1.46 × 10−5 (gaseous form) |
| Solubility in water at 0 °C (L/L) | 0.640 |
| Solubility in water at 15 °C (L/L) | 0.456 |
| Solubility in water at 27 °C (L/L) | 0.270 |
| Solubility in water at 40 °C (L/L) | 0.112 |
| Solubility in water at 60 °C (L/L) | 0.000 |
| Oxidising Agent | Oxidising Potential (V) |
|---|---|
| Fluorine | 3.06 |
| Ozone | 2.07 |
| Hydrogen peroxide | 1.78 |
| Permanganate | 1.67 |
| Chlorine dioxide | 1.50 |
| Hypochlorous acid | 1.49 |
| Chlorine gas | 1.36 |
| Oxygen | 1.23 |
| Ozone Application and Conservation Conditions | Produce and Targets | Ozone Treatment Effects in Microbiology | Ozone Treatment Effects on Physical, Chemical, and Nutritional Qualities | References |
|---|---|---|---|---|
| Gaseous ozone at 0 (control), 1, 3, 5, 7, and 9 ppm; 0.5, 3, 6, and 24 h; 18–20 °C; 95% RH. | Bacterial population change after ozone treatment on fresh-cut bell pepper. | Ozone at 9 ppm, for 6 h, reduced colony counts by 2.89, 2.56, and 3.06 log for E. coli O157, S. Typhimurium, and L. monocytogenes, respectively. | / | [29] |
| Gaseous ozone at 6.432, 10.720, and 15.008 mg/m3; 1 h; weekly occurred. Samples were put on ice by air and were processed immediately at 4 °C after arrival for 42 days. | Microbial safety and postharvest quality of cantaloupes. | Ozone failed to reduce the microbial populations at low concentrations; 15.008 mg/m3 ozone effectively reduces the microbial populations and can inhibit most of the bacteria and fungi growth. | The respiration rate and ethylene production rate were significantly lower after 15.008 mg/m3 treatment when compared with control and other groups; other factors, e.g., firmness, pectin content, titratable acidity, sarcocarp, and exocarp were significantly higher. | [57] |
| Gaseous ozone at 0.9 and 2.5 mg/L; 30- and 120 min; 95% RH; up to 15 days; 12 ± 1 °C. | Microbiological properties and health-related properties of Rapanui tomatoes. | Ozonised samples showed lower total amount of yeasts and moulds at 0. Ozone caused a significant reduction in yeast and mould content at day 5, 10, and 15. Ozone at 2.5 mg/L for 120 min was the most effective in bacteria inactivation. | Treatment with ozone increased the content of total soluble solids and reduced titratable acidity and maintained the total flavonoid, lycopene, total antioxidant activity, and total carotenoid content. | [58] |
| Gaseous ozone at 126–136 ppm; 3 min and 15 min. Ozone was produced by the dielectric barrier discharge generator. | Combinations of spoiled green beans, grape tomatoes, lettuce, and strawberries and Salmonella enterica. | Ozone exposure (126–136 ppm, 3 min and 15 min) results in 1 and 4 log reduction, respectively, in food pathogens. Periodic ozone exposure (3 min per day) result in a >5 log reduction of both bacteria and mould species. | / | [95] |
| Gaseous ozone at 1, 2, and 3 μg/g; 1, 2, and 3 h. Fruit samples were placed in sterile plastic bags and incubatedovernight at 4 °C. | E. coli and L. monocytogenes survival on tomato. | Ozone insignificantly reduced E. coli on tomato; ozone at 3 μg/g caused significant bacteria reduction in a time-dependent manner. For L. monocytogenes, 2 μg/g ozone caused significant bacterial reduction with short-duration exposure (1 h). | / | [43] |
| Aqueous ozone at 1, 1.4, 2, 2.4, and 3 mg/L; 1, 3, and 5 min. Samples were stored at 5 ± 2 °C; 85% ± 5% RH, without any initial gas injection for 16 days. | Physicochemical characteristics, microbiological qualities, and overall acceptability of shredded green bell pepper. | Ozone (>2.4 mg/L) treatments with higher durations significantly reduced the microbial load. | Ozone treatment led to better retention of ascorbic acid, firmness, colour, and overall acceptability as compared to the control samples. The shelf life was 14 days when treated with 2.4 mg/L ozone for 5 min at 5 ± 0.5 °C. | [39] |
| Aqueous ozone at 1.4 mg/L; 1, 5, and 10 min. Samples were stored at 4 °C for 12 days. | Pesticide residue on fresh-cut cabbage and the growth rates of aerobic bacteria, coliforms, and yeasts. | Approximately 1.2, 1.5, and 1.6 log reduction of aerobic bacteria; 0.2, 0.5, and 0.8 log reductions of coliforms; 1.1–1.4 log reduction of yeasts and a significant reduction in mould in the 1, 5, and 10 min aqueous ozone groups on day 12. | Ozone stimulated initial respiratory metabolism, reduced ethylene production, and improved the overall quality of the samples. Ozone treatment greatly removes trichlorfon, chlorpyrifos, methomyl, dichlorvos, and omethoate. | [36] |
| Aqueous ozone concentration at 1–5 mg/L; 2–8 min; aqueous pH 3–5. | Microbial reductions, pyruvate content, colour change, and overall acceptability of peeled onion. | Aqueous ozone at 4.51 mg/L exposed to the onions for 8 min at a pH of 3 provided the optimal microbial load reductions (3.74 logs). | The values of pyruvate content ranged from 0.107 (1 mg/L aqueous ozone for 2 min, pH 4) to 0.131 (3 mg/L aqueous ozone for 8 min, pH 3) μM/mL. Non-significant effect of ozone doses on the colour of the samples. | [38] |
| Ozone Application and Conservation Conditions | Produce and Targets | Ozone Treatment Effects in Microbiology | Ozone Treatment Effects on Physical, Chemical, and Nutritional Qualities | References |
|---|---|---|---|---|
| Gaseous ozone at 100 ppm and 1000 ppm; 10 min. The samples were then stored at 25 °C; 46–49 h. | Microbial control of ozone treatment on pork meat. | Ozone treatment greatly suppressed microbial activity. However, ozone treatment failed to effectively reduce the number of microorganisms over the 46–49 h incubation period. | / | [52] |
| Gaseous ozone at 154 × 10−6 kg/m3 (72 ppm); 3 and 24 h; 0 and 4 °C. | Ozone effects on AMHM and E.coli counts in culture media and in beef samples. Ozone effects on beef quality properties. | Gaseous-ozone-treated E. coli media culture after 3 or 24 h, at 0 °C and 4 °C caused a total inactivation of E. coli. The highest microbial inhibition was at 0 °C, 24 h exposure, producing a log decrease of 0.7 and 2.0 in E. coli and total AMHM counts, respectively. | Ozone treatment for 3 h and at both 0 °C and 4 °C reduced AMHM and E. coli counts, without changing the colour or producing rancidity in beef; 24 h treatments failed to significantly reduce microbial counts without affecting beef surface colour and rancidity. | [98] |
| Gaseous ozone at 0.01 kg/m3; up to 8 h, samples were withdrawn at 2 h intervals; 22.0 ± 0.8 °C; 21.6 ± 0.5% RH. | Ozone effects on AMB and Enterobacteriaceae counts, and on physicochemical properties of turkey breast muscle. | Gaseous ozone treatment for 6 and 8 h, reduced up to 3 logs of AMB counts. Ozone reduced around 1.0–1.5 log (2 and 4 h) and 2.3 and 2.0 log (6 and 8 h) Enterobacteriaceae counts. The yeast-mould count reductions were 0.9 log (2 h) and 1.7 log (4 h). Longer time treatments showed no further inactivation of yeasts and moulds. | Ozone increased carbonyl contents and thiobarbituric acid reactive substances. Ozone caused significant colour and pH value change in the samples. Both water holding capacity and cooking yield of treated samples increased significantly. | [59] |
| Gaseous ozone 218 mg/m3; A: 2 min ozone pulses + 30 min no ozone intervals, for 3 h in total; B: 2 min ozone pulses + 30 min intervals no ozone, for 5 h in total; C: Repeated sample B after 24 h; D: Gaseous ozone 276–283 mg/m3. pulses were 5, 10, 20, and 40 min + 30 min no ozone intervals, for 5 h in total. Treatment D (5 min ozone pulse; D5) samples were stored at 4 ± 0.5 °C. D5 samples had repeat inoculation with L. monocytogenes; 4 ± 0.5 °C and 10 ± 0.5°C. | Ozone effects on the physicochemical characteristics and food safety of beef. | In A, B, and C, heterotrophic microbial count reductions were between 0.5 and 2 logs. In D, all microorganisms > 1 log reduction. Ozonation intensity showed a significant effect in reducing the counts of mesophilic bacteria, LAB, enterobacteria, moulds, and yeasts. At 4 °C storage, control beef samples (4-day shelf life) showed higher microbial counts than D5 samples (8 day shelf life). D5 showed an immediate around 1 log reduction in L. monocytogenes counts. During both 4 °C and 10 °C storages, up to 16 days, L. monocytogenes counts in ozonated beef were significantly lower than in control samples. | During refrigerated storage at 4 °C the colour parameters presented no significant differences (p > 0.05) when compared with fresh and ozonated beef samples. | [99] |
| Aqueous ozone at 1% and water bath; 7 and 15 min; 7.2 °C. | Antimicrobial, colour, and odour effects of ozone on ground beef. | Aqueous ozone (15 min) reduced coliforms, S. typhimurium, and aerobic plate counts; 7 min treatment effectively reduced S. typhimurium and aerobic plate counts. | Aqueous-ozone-treated ground beef became lighter. Minimal effects on colour or odour characteristics by aqueous ozone treatment. | [40] |
| Aqueous ozone at 6.00 ± 0.25 mg/L. The samples were packed singly in linear low-density polyethylene and vacuum packed and stored at 4 °C. | Ozone effects on the complexity and dynamics of the potential active microbiota of beefsteaks, and their associated volatilome. | Aqueous ozone was not able to reduce the initial microbial counts of the beefsteak samples. | Aqueous ozone was incapable of modifying the microbiota composition, dynamics and the related volatilome to any great extent during chilled vacuum packaging storage. | [79] |
| Ozone Treatment and Conservation Conditions | Produce and Targets | Ozone Treatment Effects on Microbiology, Insect Species and Detoxifying | References |
|---|---|---|---|
| Treatment A: gaseous ozone in a fumigation chamber (3 L) at 13.88 mg/L; 2 h; treatment B: gaseous ozone (13.9 mg/L) flush treatment of 2 kg wheat in 3 L chamber at 30 min intervals with 10 pulses for 5 h in total. | Effectiveness of ozone on the mortality of stored-product insects’ (Ephestia kuehniella and Tribolium confusum) larvae, pupae, eggs, and adults. | Empty space ozone treatment caused complete mortality of E. kuehniella adults, pupae, and larvae, 62.5% of the eggs were killed. Ozone treatment caused low mortality of T. confusum adults, pupae, and eggs, ranging from 4.2 to 14.1%, only larvae had a high mortality (74%). Ozone flush treatment caused almost complete mortality of all life stages of E. kuehniella placed in the top position of 2 kg wheat, whereas eggs of E. kuehniella placed in the bottom position were hard to kill. T. confusum, larvae placed in the bottom position were easily killed, eggs, pupae, and adults survived. | [105] |
| Gaseous ozone at concentrations of 13 and 21 mg/L; 0, 24, 48, 72, and 96 h. | The fungicidal and detoxifying effects of ozone on AFs in peanut kernels. | Ozone at 13 and 21 mg/L effectively controlled the potential aflatoxin-producing species A. flavus and A. parasiticus. Ozone at 21 mg/L for 96 h effectively controlled total fungi and potentially aflatoxigenic species in peanuts, with a > 3 log (CFU/g) reduction. Ozone also caused a reduction in the percentage of peanuts with internal fungal populations. Ozone-treated kernels at 21 mg/L for 96 h caused a reduction in the concentrations of total AFs and aflatoxin B1. | [72] |
| Gaseous ozone at rates of 0, 50, 500, 1000, and 15,000 ppm in factorial with moisture contents of 18, 22, and 26% for 1 h, at 0.5 L/min flow rate. | Ozone treatment efficacy of high-moisture maize to reduce the occurrence of fungal infections within kernels during storage | Ozone concentration at 500 and 1000 ppm effectively reduced the presence of Aspergillus, Fusarium and Mucor. Penicillium infections decreased with ozone at 1000 and 15,000 ppm. Ozone at 15,000 ppm was necessary to reduce Rhizopus infection. Ozone can penetrate the surface of maize kernels to reduce fungal infections during storage. | [106] |
| Gaseous ozone at 40 and 60 μmol/mol; 30, 60, 120, and 180 min; 25 ± 0.5 °C. | The effectiveness of ozone treatment against A. flavus and P. citrinum strains’ growth as well as AFs and CTR degradation in wheat grains. | Ozone at 40 and 60 μmol/mol >30 min significantly reduced A. flavus and P. citrinum. Ozone at 60 μmol/mol, for 180 min, showed 100% growth inhibition of A. flavus and P. citrinum and significantly reduced AFB1 and AFB2 levels. Ozone at 40 and 60 μmol/mol for 180 min significantly reduced CTR levels. | [107] |
| Gaseous ozone concentration at 20 to 60 mg/L; 120 to 480 min. | The effects of ozonation to corn grits, including the levels of AFs (B1, B2, G1, and G2), fungal contamination, and total mesophilic count. | Ozone at highest concentration 60 mg/L and 480 min exposure time and 1 kg of corn grits, reached log reductions of 2.04 (Aspergillus spp.) and 2.77 (Fusarium spp.) in corn grits (CFU/g), total mesophilic counts were reduced to non-detectable levels. After above ozone detoxification, observed greatest reductions were for AFG1, AFB1, AFG2, and AFB2. | [108] |
| Ozone Treatment Conditions | Combination Technologies | Produce and Targets | Ozone Treatment Effects | Combination Effects | References | ||
|---|---|---|---|---|---|---|---|
| Microbiology | Other Qualities | Microbiology | Other Qualities | ||||
| Aqueous ozone at 1%; water bath sample at 7.2 °C for 15 min. | 5% acetic acid/0.5% cetylpyridinium chloride. | Antimicrobial, colour, and odour effects of ground beef. | Ozone treatment reduced coliforms, S. typhimurium, and aerobic plate count. | Samples became lighter; similar redness, percentage discolouration, odour, and off odour intensities as the control | Ozone with 5% acetic and 0.5% cetylpyridinium chloride reduced all bacterial types. | Combination treatments showed little effects on sample colour and odour. | [114] |
| Gaseous ozone at 2%, 5%, and 10%. | CO modified atmosphere package. | Combination effects on the microbiological, chemical, physical, and sensory characteristics of beef. | Ozone at 5% and 10%, caused the largest reduction in total viable counts on day 0. | The drip loss, metmyoglobin, thiobarbituric acid reactive substances, total volatile basic nitrogen, and pH were significantly lower in >2% ozone-treated samples. | The total viable counts of >2% ozone groups were reduced significantly when compared with the CO only groups. | The combination treatment significantly reduced the drip loss, metmyoglobin, thiobarbituric acid reactive substances, total volatile basic nitrogen, and pH. | [115] |
| Gaseous ozone at 0.4, 0.6, and 0.72 ppm; 10, 30, 60, and 12 min; 4 ± 0.5 °C; 90 ± 1% RH. | Slow freezing, 20.5 h of primary drying (12 h at 0 °C and 8.5 h at 10 °C) at 30 Pa. | Combination effects on the microbiological load, sensory characteristics, and shelf life of chicken. | Ozone (>0.4 ppm) significantly reduced total aerobic mesphilic bacteria counts, lactic acid bacteria counts throughout 8 months. | Ozone (0.4 ppm, >30 min) increased the aW and humidity; decreased the rehydration of the samples. | The combination reduced the total AMB, the mesophilic, and lactic acid bacteria counts. | The combination significantly reduced the pH values, the aW, and humidity;. increased the maximum force value. | [116] |
| Aqueous ozone at 0.85 ± 0.2 mg/L; 5, 10, and 15 min. | Ultrasound: mono-mode frequency irradiation, dual-mode frequency irradiation. | Microbial safety and nutritional quality, firmness, bioactive compounds, and antioxidants of cherry tomato. | Ozone reduced the mesophilic bacteria (0.40–0.71 logs) and moulds/yeasts (0.29–0.49 logs). | Ozone slows down the loss in firmness (23.07–24.58%) after 21 days storage. Ozone-treated samples had the lowest electrolyte leakage, less loss in bioactive compound, and increased antioxidant activity. | The dual-mode frequency irradiation with ozone reduced mesophilic bacteria (2.09–3.42 logs) and moulds/yeasts (2.30–3.72 log). | The combinations slowed the maturity process; maintained the bioactive compounds, total soluble solids content, titratable acidity, and pH values; increased the antioxidant activity. | [117] |
| Ozone (3–9 mg/L) passed into a covered beaker and with sterile water through a sparger. | Lactic acid solution | The removal of microbial and chemical contaminants from fresh vegetables. | Ozonated water at 9 mg/L for 10 min reduced 0.9–2.4 logs of natural microbes and 1.3–2.1 logs of E. coli from vegetable samples. | / | Combinations Reduced natural mesophilic bacteria and E. coli from tomato, cucumber, carrot, and lettuce. | The combinations showed no effects on the sensory quality of fresh vegetables. | [64] |
| Aqueous ozone at 0.9 ppm in cycles; a total of 10 sprays for 30 s with an interval of 1 h, total 10 h. | A: UV-C (15 s of) +30 s ozone spraying; 10 cycles; 10 h. B: UV-C alternately applied; 10 cycles; total 10 h. | The effects of treatments in the microorganisms and in preservation of beef meat characteristics. | A significant microbial reduction (p < 0.05) was not observed concerning the initial control sample. | / | A: significant reductions in all 10 cycles. Cycles 5 and 8, a 0.7 log reduction of E. coli. Initial microbial load was maintained in other cycles. B: significant reduction in microbial load for cycles 2–10. | The action of the combined treatments on the meat showed no effects in the pH, lipid oxidisation, and total protein amount. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. https://doi.org/10.3390/foods12040814
Xue W, Macleod J, Blaxland J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods. 2023; 12(4):814. https://doi.org/10.3390/foods12040814
Chicago/Turabian StyleXue, Wenya, Joshua Macleod, and James Blaxland. 2023. "The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology" Foods 12, no. 4: 814. https://doi.org/10.3390/foods12040814
APA StyleXue, W., Macleod, J., & Blaxland, J. (2023). The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods, 12(4), 814. https://doi.org/10.3390/foods12040814





