Reduction in the Residues of Penthiopyrad in Processed Edible Vegetables by Various Soaking Treatments and Health Hazard Evaluation in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Pretreatment
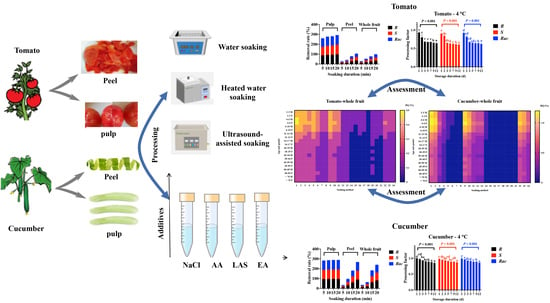
2.4. Processing Experiments
2.4.1. Soaking and Peeling
2.4.2. Saucing (Tomato) and Juicing (Cucumber)
2.5. Data Analysis
3. Results
3.1. Effects of Different Soaking Treatments to Remove Penthiopyrad from Two Edible Vegetables
3.2. Effects of Saucing or Juicing to Remove Penthiopyrad from Two Edible Vegetables and Their Storage Stability
3.3. Potential Enantioselectivity and Health Risk of Penthiopyrad in Tomato and Cucumber after Processing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naman, M.; Masoodi, F.A.; Wani, S.M.; Ahad, T. Changes in concentration of pesticide residues in fruits and vegetables during household processing. Toxicol. Rep. 2022, 9, 1419–1425. [Google Scholar] [CrossRef]
- Lozowicka, B.; Abzeitova, E.; Sagitov, A.; Kaczynski, P.; Toleubayev, K.; Li, A. Studies of pesticide residues in tomatoes and cucumbers from Kazakhstan and the associated health risks. Environ. Monit. Assess. 2015, 187, 609. [Google Scholar] [CrossRef] [Green Version]
- Alenyorege, E.A.; Ma, H.L.; Ayim, I.; Aheto, J.H.; Hong, C.; Zhou, C.S. Effect of multi-frequency multi-mode ultrasound washing treatments on physicochemical, antioxidant potential and microbial quality of tomato. J. Food Meas. Charact. 2019, 13, 677–686. [Google Scholar] [CrossRef]
- Hassan, H.; Elsayed, E.; El-Raouf, A.E.A.; Salman, S.N. Method validation and evaluation of household processing on reduction of pesticide residues in tomato. J. Consum. Prot. Food Saf. 2019, 14, 31–39. [Google Scholar] [CrossRef]
- Hashemi, L.; Golparvar, A.R.; Nasr-Esfahani, M.; Golabadi, M. Expression analysis of defense-related genes in cucumber (Cucumis sativus L.) against Phytophthora melonis. Mol. Biol. Rep. 2020, 47, 4933–4944. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hayashi, K.; Okada, R.; Wari, D.; Ogawara, T. Resistance to succinate dehydrogenase inhibitors in field isolates of Podosphaera xanthii on cucumber: Monitoring, cross-resistance patterns and molecular characterization. Pestic. Biochem. Phys. 2020, 169, 104646. [Google Scholar] [CrossRef]
- Santísima-Trinidad, A.B.L.; Montiel-Rozas, M.D.M.; Diéz-Rojo, M.Á.; Pascual, J.A.; Ros, M. Impact of foliar fungicides on target and non-target soil microbial communities in cucumber crops. Eecotoxicol. Environ. Saf. 2018, 166, 78–85. [Google Scholar] [CrossRef]
- Culbreath, A.K.; Brenneman, T.B.; Kemerait, R.C.; Stevenson, K.L.; Henn, A. Effect of DMI and QoI fungicides mixed with the SDHI fungicide penthiopyrad on late leaf spot of peanut. Crop Prot. 2020, 137, 105298. [Google Scholar] [CrossRef]
- Podbielska, M.; Kus-Liśkiewicz, M.; Jagusztyn, B.; Piechowicz, B.; Sadlo, S.; Słowik-Borowiec, M.; Twarużek, M.; Szpyrka, E. Influence of Bacillus subtilis and Trichoderma harzianum on penthiopyrad degradation under laboratory and field studies. Molecules 2020, 25, 1421. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Deng, Y.; Zheng, M.L.; Liu, Y.P.; Wang, Z.K.; Yu, S.M.; Nie, Y.F.; Zhu, W.T.; Zhou, Z.Q.; Diao, J.L. Nano selenium repairs the fruit growth and flavor quality of tomato under the stress of penthiopyrad. Plant Physiol. Biochem. 2022, 184, 126–136. [Google Scholar] [CrossRef]
- Yang, G.Q.; Li, J.M.; Lan, T.T.; Dou, L.; Zhang, K.K. Dissipation, residue, stereoselectivity and dietary risk assessment of penthiopyrad and metabolite PAM on cucumber and tomato in greenhouse and field. Food Chem. 2022, 387, 132875. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Khanashyam, A.C.; Divya, V.; Abdullah, S.K.; Aurum, F.S.; Dakshyani, R.; Kothakota, A.; Ramesh, S.V.; Khaneghah, A.M. Research trends and emerging physical processing technologies in mitigation of pesticide residues on various food products. Environ. Sci. Pollut. Res. 2022, 29, 45131–45149. [Google Scholar] [CrossRef]
- Calderon, R.; García-Hernández, J.; Palma, P.; Leyva-Morales, J.B.; Zambrano-Soria, M.; Bastidas-Bastidas, P.J.; Godoy, M. Assessment of pesticide residues in vegetables commonly consumed in Chile and Mexico: Potential impacts for public health. J. Food Compos. Anal. 2022, 108, 104420. [Google Scholar] [CrossRef]
- Mao, Y.S.; Zhang, Y.; Zhou, M.G.; Duan, Y.B. Occurrence of crown rot disease caused by Fusarium incarnatum on cucumber (Cucumis sativus) in China. Plant Dis. 2019, 104, 593. [Google Scholar] [CrossRef]
- Medina, M.B.; Munitz, M.S.; Resnik, S.L. Effect of household rice cooking on pesticide residues. Food Chem. 2021, 342, 128311. [Google Scholar] [CrossRef]
- Li, Z.J.; Xiong, J. Simulation modeling the effects of peels on pesticide removal from potatoes during household food processing. Environ. Sci. Pollut. Res. 2022, 29, 29841–29853. [Google Scholar] [CrossRef]
- Phopin, K.; Wanwimolruk, S.; Norkaew, C.; Buddhaprom, J.; Isarankura-Na-Ayudhya, C. Boiling, blanching, and stir-frying markedly reduce pesticide residues in vegetables. Foods 2022, 11, 1463. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Song, Y.Y.; Chen, X.J.; Ren, Y.J.; Lu, C.L.; Ren, L.; Gen, H.C.; Zhu, J.X.; Yuan, Q.; Li, T.F.; et al. Comparison of removal efficiencies of different household cleaning methods in reducing imidacloprid and triabendazole residues on cherry tomatoes. J. Agric. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, A.A.; Afify, A.S.; Hasaan, I.E.; Mohamed, A. Studying the effect of household-type treatment and processing on the residues of ethion and profenofos pesticides and on the contents of capsaicinoids in green chili pepper using GC-MS/MS and HPLC. Food Anal. Method 2018, 11, 382–393. [Google Scholar] [CrossRef]
- Nematollahi, A.; Rezaei, F.; Afsharian, Z.; Mollakhalili-Meybodi, N. Diazinon reduction in food products: A comprehensive review of conventional and emerging processing methods. Environ. Sci. Pollut. Res. 2022, 29, 40342–40357. [Google Scholar] [CrossRef]
- Hanafi, A.; Elsheshetawy, H.E.; Faied, S.F. Reduction of pesticides residues on okra fruits by different processing treatments. J. Consum. Prot. Food Saf. 2016, 11, 337–343. [Google Scholar] [CrossRef]
- Wang, W.Z.; Gao, Z.Q.; Qiao, C.X.; Liu, F.M.; Peng, Q.R. Residue analysis and removal of procymidone in cucumber after field application. Food Control. 2021, 128, 108168. [Google Scholar] [CrossRef]
- Rasolonjatovo, M.A.; Cemek, M.; Cengiz, M.F.; Ortaç, D.; Konuk, H.B.; Karaman, E.; Kocaman, A.T.; Göneş, S. Reduction of methomyl and acetamiprid residues from tomatoes after various household washing solutions. Int. J. Food Prop. 2017, 20, 2748–2759. [Google Scholar] [CrossRef] [Green Version]
- Cámara, M.A.; Cermeño, S.; Martínez, G.; Oliva, J. Removal residues of pesticides in apricot, peach and orange processed and dietary exposure assessment. Food Chem. 2020, 325, 126936. [Google Scholar] [CrossRef]
- Li, Y.J.; Xu, J.B.; Zhao, X.P.; He, H.M.; Zhang, C.P.; Zhang, Z.H. The dissipation behavior, household processing factor and risk assessment for cyenopyrafen residues in strawberry and mandarin fruits. Food Chem. 2021, 359, 129925. [Google Scholar] [CrossRef]
- Gu, M.Y.; Wang, P.S.; Shi, S.M.; Xue, J. Dietary risk assessment and ranking of multipesticides in Dendrobium officinale. J. Food Qual. 2021, 2021, 9916759. [Google Scholar] [CrossRef]
- Jin, S.G. Survey Report on the Nutrition and Health of Chinese Residents: Data Set on the Status of Nutrition and Health in 2002; People’s Hygiene Press: Beijing, China, 2008. [Google Scholar]
- Guo, J.; Li, M.M.; Liu, Y.G.; Wang, F.Z.; Kong, Z.Q.; Sun, Y.F.; Lu, J.; Jin, N.; Huang, Y.T.; Liu, J.M.; et al. Residue and dietary risk assessment of chiral cyflumetofen in apple. Molecules 2018, 23, 1060. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance penthiopyrad. EFSA J. 2013, 11, 3111. [Google Scholar] [CrossRef]
- Wanwimolruk, S.; Duangsuwan, W.; Phopin, K.; Boonpangrak, S. Food safety in Thailand 5: The effect of washing pesticide residues found in cabbages and tomatoes. J. Consum. Prot. Food Saf. 2017, 12, 209–221. [Google Scholar] [CrossRef]
- Kaushik, E.; Dubey, J.K.; Patyal, S.K.; Katna, S.; Chauhan, A.; Devi, N. Persistence of tetraniliprole and reduction in its residues by various culinary practices in tomato in India. Environ. Sci. Pollut. Res. 2019, 26, 22464–22471. [Google Scholar] [CrossRef]
- Heshmati, A.; Nazemi, F. Dichlorvos (DDVP) residue removal from tomato by washing with tap and ozone water, a commercial detergent solution and ultrasonic cleaner. Food Sci. Technol. 2018, 38, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Yigit, N.; Velioglu, Y.S. Effects of processing and storage on pesticide residues in foods. Crit. Rev. Food Sci. 2020, 60, 3622–3641. [Google Scholar] [CrossRef]
- Rodrigues, A.A.Z.; de Queiroz, M.E.L.R.; Neves, A.A.; de Oliveira, A.F.; Prates, L.H.F.; de Freitas, J.F.; Heleno, F.F.; Faroni, L.R.D. Use of ozone and detergent for removal of pesticides and improving storage quality of tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef]
- Xue, J.Y.; Pan, D.D.; Li, M.; Wu, X.W.; Wang, X.G.; Hua, R.M. Degradation of amisulbrom and its metabolite IT-4 in cucumber under field conditions and processing. Int. J. Environ. Anal. Chem. 2018, 98, 67–81. [Google Scholar] [CrossRef]
- Acoglu, B.; Omeroglu, P.Y. Effectiveness of different type of washing agents on reduction of pesticide residues in orange (Citrus sinensis). LWT-Food Sci. Technol. 2021, 147, 111690. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, T.; Xu, D.X.; Wang, S.J.; Yuan, Y.M.; He, S.D.; Cao, Y.P. The removal of pesticide residues from pakchoi (Brassica rape L. ssp. chinensis) by ultrasonic treatment. Food Control. 2019, 95, 176–180. [Google Scholar] [CrossRef]
- Prakash, A.; Prabhudev, S.H.; Vijayalaskshmi, M.R.; Prakash, M.; Baskaran, R. Implication of processing and differential blending on quality characteristics in nutritionally enriched ketchup (Nutri-Ketchup) from acerola and tomato. J. Food Sci. Technol. 2015, 53, 3175–3185. [Google Scholar] [CrossRef] [Green Version]




| Treatment | Duration (min) | Additive Content | PF (Average ± SD, n = 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulp | Peel | Whole Fruit | ||||||||||||
| R | p | S | p | R | p | S | p | R | p | S | p | |||
| A | 5 | / | 0.13 ± 0 d | <0.001 | 0.14 ± 0.01 d | <0.001 | 0.91 ± 0.03 b | <0.001 | 0.91 ± 0.03 c | <0.001 | 0.90 ± 0.02 c | <0.001 | 0.91 ± 0.03 d | <0.001 |
| 10 | / | 0.08 ± 0 c | 0.08 ± 0 c | 0.87 ± 0.03 b | 0.87 ± 0.05 bc | 0.82 ± 0.02 c | 0.83 ± 0.03 c | |||||||
| 15 | / | 0.07 ± 0.01 b | 0.06 ± 0.01 b | 0.74 ± 0.03 a | 0.75 ± 0.02 ab | 0.73 ± 0.01 b | 0.75 ± 0.01 b | |||||||
| 20 | / | 0.02 ± 0 a | 0.02 ± 0 a | 0.66 ± 0.07 a | 0.65 ± 0.07 a | 0.68 ± 0.05 a | 0.67 ± 0.02 a | |||||||
| B | 5 | / | 0.42 ± 0.01 d | <0.001 | 0.39 ± 0.01 c | <0.001 | 0.68 ± 0.03 c | <0.001 | 0.67 ± 0.03 c | <0.001 | 0.78 ± 0.05 c | <0.001 | 0.76 ± 0.07 c | <0.001 |
| 10 | / | 0.37 ± 0.01 c | 0.38 ± 0.02 b | 0.57 ± 0.01 b | 0.57 ± 0 b | 0.60 ± 0.10 c | 0.58 ± 0.11 bc | |||||||
| 15 | / | 0.32 ± 0.01 b | 0.30 ± 0 a | 0.53 ± 0.02 ab | 0.53 ± 0.02 ab | 0.43 ± 0.01 b | 0.42 ± 0 b | |||||||
| 20 | / | 0.23 ± 0.03 a | 0.23 ± 0.02 a | 0.40 ± 0.07 a | 0.39 ± 0.08 a | 0.37 ± 0.02 a | 0.37 ± 0.02 a | |||||||
| C | 4 | / | 0.73 ± 0.01 d | <0.001 | 0.73 ± 0.01 d | <0.001 | 0.71 ± 0.03 c | <0.001 | 0.71 ± 0.04 c | <0.001 | 0.79 ± 0.05 c | <0.001 | 0.78 ± 0.06 c | <0.001 |
| 6 | / | 0.58 ± 0.02 c | 0.60 ± 0.05 c | 0.55 ± 0.03 b | 0.55 ± 0.03 b | 0.68 ± 0.02 b | 0.68 ± 0.02 b | |||||||
| 8 | / | 0.49 ± 0.01 b | 0.49 ± 0.02 b | 0.50 ± 0.03 b | 0.51 ± 0.03 b | 0.57 ± 0.04 ab | 0.56 ± 0.05 ab | |||||||
| 10 | / | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.39 ± 0.03 a | 0.39 ± 0.03 a | 0.27 ± 0.05 a | 0.26 ± 0.05 a | |||||||
| D | 15 | 0.2% | 0.03 ± 0 b | <0.001 | 0.03 ± 0.01 a | 0.026 | 0.54 ± 0.06 c | <0.001 | 0.52 ± 0.05 c | <0.001 | 0.27 ± 0.02 b | <0.001 | 0.25 ± 0.02 b | <0.001 |
| 15 | 0.5% | 0.02 ± 0 b | 0.02 ± 0 a | 0.43 ± 0.02 b | 0.41 ± 0.02 b | 0.14 ± 0 b | 0.13 ± 0 b | |||||||
| 15 | 1% | 0.02 ± 0 a | 0.02 ± 0 a | 0.30 ± 0.01 a | 0.28 ± 0.02 a | 0.11 ± 0 a | 0.10 ± 0 a | |||||||
| E | 15 | 0.2% | 0.03 ± 0 b | 0.001 | 0.03 ± 0 b | 0.002 | 0.31 ± 0.01 c | <0.001 | 0.30 ± 0.02 c | <0.001 | 0.15 ± 0 b | <0.001 | 0.15 ± 0.01 b | <0.001 |
| 15 | 0.5% | 0.03 ± 0 a | 0.03 ± 0 ab | 0.16 ± 0 b | 0.16 ± 0 b | 0.13 ± 0.01 a | 0.13 ± 0.01 a | |||||||
| 15 | 1% | 0.02 ± 0 a | 0.02 ± 0 a | 0.06 ± 0.0 a1 | 0.05 ± 0.0 a1 | 0.08 ± 0.01 a | 0.08 ± 0.01 a | |||||||
| F | 15 | 0.2% | 0.06 ± 0 b | 0.002 | 0.06 ± 0 b | 0.003 | 0.14 ± 0 a | 0.052 | 0.14 ± 0 b | 0.022 | 0.21 ± 0.01 b | <0.001 | 0.21 ± 0.02 b | 0.002 |
| 15 | 0.5% | 0.05 ± 0 b | 0.05 ± 0 b | 0.13 ± 0.01 a | 0.13 ± 0 ab | 0.17 ± 0 b | 0.16 ± 0.01 b | |||||||
| 15 | 1% | 0.05 ± 0 a | 0.05 ± 0 a | 0.12 ± 0.01 a | 0.11 ± 0.02 a | 0.15 ± 0 a | 0.15 ± 0 a | |||||||
| G | 15 | 0.2% | 0.75 ± 0.02 c | <0.001 | 0.64 ± 0.03 c | <0.001 | 0.85 ± 0.02 c | <0.001 | 0.81 ± 0.02 c | <0.001 | 0.51 ± 0.05 b | 0.003 | 0.46 ± 0.04 b | 0.002 |
| 15 | 0.5% | 0.59 ± 0.05 b | 0.42 ± 0.07 b | 0.73 ± 0.02 b | 0.69 ± 0.02 b | 0.41 ± 0.01 b | 0.37 ± 0.01 b | |||||||
| 15 | 1% | 0.17 ± 0 a | 0.10 ± 0.01 a | 0.60 ± 0.02 a | 0.56 ± 0.04 a | 0.37 ± 0 a | 0.33 ± 0 a | |||||||
| Treatment | Duration (min) | Additive Content | PF (Average ± SD, n = 3) | |||||
|---|---|---|---|---|---|---|---|---|
| Tomato | ||||||||
| Pulp | p | Peel | p | Whole Fruit | p | |||
| A | 5 | / | 0.14 ± 0.01 d | <0.001 | 0.91 ± 0.03 b | <0.001 | 0.91 ± 0.02 d | <0.001 |
| 10 | / | 0.08 ± 0 c | 0.87 ± 0.04 b | 0.83 ± 0.02 c | ||||
| 15 | / | 0.07 ± 0.01 b | 0.74 ± 0.03 a | 0.74 ± 0.01 b | ||||
| 20 | / | 0.02 ± 0 a | 0.65 ± 0.07 a | 0.68 ± 0.03 a | ||||
| B | 5 | / | 0.41 ± 0.01 c | <0.001 | 0.68 ± 0.03 c | <0.001 | 0.77 ± 0.06 c | <0.001 |
| 10 | / | 0.37 ± 0.02 b | 0.57 ± 0.01 b | 0.59 ± 0.11 bc | ||||
| 15 | / | 0.31 ± 0.01 a | 0.53 ± 0.02 ab | 0.43 ± 0 b | ||||
| 20 | / | 0.23 ± 0.02 a | 0.39 ± 0.07 a | 0.37 ± 0.02 a | ||||
| C | 4 | / | 0.73 ± 0.01 d | <0.001 | 0.71 ± 0.03 c | <0.001 | 0.78 ± 0.06 c | <0.001 |
| 6 | / | 0.59 ± 0.03 c | 0.55 ± 0.03 b | 0.68 ± 0.02 b | ||||
| 8 | / | 0.49 ± 0.02 b | 0.50 ± 0.03 b | 0.56 ± 0.04 ab | ||||
| 10 | / | 0.06 ± 0.01 a | 0.39 ± 0.03 a | 0.27 ± 0.05 a | ||||
| D | 15 | 0.2% | 0.03 ± 0.01 b | 0.006 | 0.53 ± 0.05 c | <0.001 | 0.26 ± 0.02 b | <0.001 |
| 15 | 0.5% | 0.02 ± 0 ab | 0.42 ± 0.02 b | 0.14 ± 0 b | ||||
| 15 | 1% | 0.02 ± 0 a | 0.29 ± 0.01 a | 0.11 ± 0 a | ||||
| E | 15 | 0.2% | 0.03 ± 0 b | 0.001 | 0.30 ± 0.01 c | 0.002 | 0.15 ± 0.01 b | <0.001 |
| 15 | 0.5% | 0.03 ± 0 a | 0.16 ± 0 b | 0.13 ± 0 a | ||||
| 15 | 1% | 0.02 ± 0 a | 0.06 ± 0.01 a | 0.08 ± 0.01 a | ||||
| F | 15 | 0.2% | 0.06 ± 0 b | 0.001 | 0.14 ± 0 a | 0.033 | 0.21 ± 0.02 b | 0.001 |
| 15 | 0.5% | 0.05 ± 0 b | 0.13 ± 0.01 a | 0.17 ± 0 b | ||||
| 15 | 1% | 0.05 ± 0 a | 0.11 ± 0.02 a | 0.15 ± 0 a | ||||
| G | 15 | 0.2% | 0.69 ± 0.02 c | <0.001 | 0.83 ± 0.02 c | <0.001 | 0.49 ± 0.05 b | <0.001 |
| 15 | 0.5% | 0.50 ± 0.06 b | 0.71 ± 0.02 b | 0.39 ± 0.01 b | ||||
| 15 | 1% | 0.13 ± 0 a | 0.58 ± 0.02 a | 0.35 ± 0 a | ||||
| Treatment | Duration (min) | Additive Content | PF (Average ± SD, n = 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulp | Peel | Whole Fruit | ||||||||||||
| R | p | S | p | R | p | S | p | R | p | S | p | |||
| A | 5 | / | 0.06 ± 0 c | <0.001 | 0.06 ± 0 c | <0.001 | 0.89 ± 0.03 d | <0.001 | 0.89 ± 0.04 d | <0.001 | 0.87 ± 0.02 d | <0.001 | 0.90 ± 0.02 c | <0.001 |
| 10 | / | 0.05 ± 0 c | 0.05 ± 0 c | 0.68 ± 0.06 c | 0.68 ± 0.05 c | 0.79 ± 0.01 c | 0.79 ± 0.02 d | |||||||
| 15 | / | 0.04 ± 0 b | 0.04 ± 0 b | 0.40 ± 0.03 b | 0.40 ± 0.04 b | 0.39 ± 0.03 b | 0.40 ± 0.03 b | |||||||
| 20 | / | 0.04 ± 0 a | 0.04 ± 0 a | 0.11 ± 0 a | 0.11 ± 0 a | 0.20 ± 0.01 a | 0.21 ± 0.02 a | |||||||
| B | 5 | / | 0.17 ± 0 d | <0.001 | 0.17 ± 0.01 d | <0.001 | 0.31 ± 0 c | <0.001 | 0.31 ± 0 c | <0.001 | 0.06 ± 0.01 c | <0.001 | 0.06 ± 0.01 c | <0.001 |
| 10 | / | 0.11 ± 0 c | 0.11 ± 0 c | 0.28 ± 0.01 b | 0.28 ± 0.05 b | 0.04 ± 0 bc | 0.04 ± 0 bc | |||||||
| 15 | / | 0.09 ± 0.01 b | 0.09 ± 0 b | 0.22 ± 0.03 a | 0.23 ± 0.01 ab | 0.03 ± 0 b | 0.03 ± 0 b | |||||||
| 20 | / | 0.03 ± 0 a | 0.03 ± 0 a | 0.15 ± 0 a | 0.18 ± 0.01 a | 0.02 ± 0 a | 0.02 ± 0 a | |||||||
| C | 4 | / | 0.45 ± 0.23 b | 0.014 | 0.41 ± 0.19 c | 0.007 | 0.81 ± 0.04 d | <0.001 | 0.80 ± 0.04 d | <0.001 | 0.83 ± 0.03 d | <0.001 | 0.84 ± 0.03 d | <0.001 |
| 6 | / | 0.35 ± 0.03 ab | 0.35 ± 0.04 bc | 0.60 ± 0.01 c | 0.62 ± 0.01 c | 0.71 ± 0.03 c | 0.71 ± 0.03 c | |||||||
| 8 | / | 0.12 ± 0.08 ab | 0.11 ± 0.06 ab | 0.47 ± 0.01 b | 0.47 ± 0.01 b | 0.47 ± 0.02 b | 0.47 ± 0.03 b | |||||||
| 10 | / | 0.06 ± 0.01 a | 0.06 ± 0 a | 0.31 ± 0.03 a | 0.32 ± 0.03 a | 0.40 ± 0.01 a | 0.39 ± 0.01 a | |||||||
| D | 15 | 0.2% | 0.03 ± 0 b | <0.001 | 0.03 ± 0 b | <0.001 | 0.42 ± 0.02 b | <0.001 | 0.39 ± 0.02 b | <0.001 | 0.41 ± 0.03 b | <0.001 | 0.39 ± 0.03 b | <0.001 |
| 15 | 0.5% | 0.02 ± 0 a | 0.02 ± 0 a | 0.19 ± 0.01 b | 0.19 ± 0.01 b | 0.06 ± 0.01 b | 0.06 ± 0.01 b | |||||||
| 15 | 1% | 0.02 ± 0 a | 0.02 ± 0 a | 0.16 ± 0 a | 0.15 ± 0 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | |||||||
| E | 15 | 0.2% | 0.03 ± 0 b | <0.001 | 0.03 ± 0 b | <0.001 | 0.30 ± 0.01 c | <0.001 | 0.29 ± 0 c | <0.001 | 0.02 ± 0 b | <0.001 | 0.02 ± 0 b | <0.001 |
| 15 | 0.5% | 0.02 ± 0 b | 0.02 ± 0 b | 0.27 ± 0.01 b | 0.26 ± 0.01 b | 0.02 ± 0 b | 0.02 ± 0 a | |||||||
| 15 | 1% | 0.02 ± 0 a | 0.02 ± 0 a | 0.21 ± 0 a | 0.20 ± 0 a | 0.02 ± 0 a | 0.01 ± 0 a | |||||||
| F | 15 | 0.2% | 0.05 ± 0 c | <0.001 | 0.05 ± 0 b | <0.001 | 0.18 ± 0 b | <0.001 | 0.18 ± 0 c | <0.001 | 0.03 ± 0 b | <0.001 | 0.03 ± 0 c | <0.001 |
| 15 | 0.5% | 0.05 ± 0 b | 0.05 ± 0 a | 0.18 ± 0 a | 0.16 ± 0 b | 0.02 ± 0 a | 0.02 ± 0 b | |||||||
| 15 | 1% | 0.03 ± 0 a | 0.03 ± 0 a | 0.01 ± 0 a | 0.01 ± 0 a | 0.01 ± 0 a | 0.01 ± 0 a | |||||||
| G | 15 | 0.2% | 0.31 ± 0.01 b | <0.001 | 0.31 ± 0.01 b | <0.001 | 0.75 ± 0.03 c | <0.001 | 0.74 ± 0.03 c | <0.001 | 0.76 ± 0.01 b | 0.001 | 0.77 ± 0.02 b | 0.001 |
| 15 | 0.5% | 0.28 ± 0.01 a | 0.27 ± 0.02 a | 0.65 ± 0 b | 0.63 ± 0.02 b | 0.66 ± 0.06 a | 0.67 ± 0.07 a | |||||||
| 15 | 1% | 0.09 ± 0.02 a | 0.09 ± 0.02 a | 0.59 ± 0.02 a | 0.57 ± 0.01 a | 0.47 ± 0.04 a | 0.46 ± 0.05 a | |||||||
| Treatment | Duration (min) | Additive Content | PF (Average ± SD, n = 3) | |||||
|---|---|---|---|---|---|---|---|---|
| Cucumber | ||||||||
| Pulp | p | Peel | p | Whole Fruit | p | |||
| A | 5 | / | 0.06 ± 0 c | <0.001 | 0.89 ± 0.03 d | <0.001 | 0.89 ± 0.02 d | <0.001 |
| 10 | / | 0.05 ± 0 c | 0.68 ± 0.06 c | 0.79 ± 0.01 c | ||||
| 15 | / | 0.04 ± 0 b | 0.40 ± 0.03 b | 0.39 ± 0.03 b | ||||
| 20 | / | 0.04 ± 0 a | 0.11 ± 0 a | 0.20 ± 0.02 a | ||||
| B | 5 | / | 0.17 ± 0.01 d | <0.001 | 0.31 ± 0 c | <0.001 | 0.06 ± 0.01 c | <0.001 |
| 10 | / | 0.11 ± 0 c | 0.28 ± 0.01 b | 0.04 ± 0 bc | ||||
| 15 | / | 0.09 ± 0.01 b | 0.22 ± 0.04 a | 0.03 ± 0 b | ||||
| 20 | / | 0.03 ± 0 a | 0.15 ± 0.01 a | 0.02 ± 0 a | ||||
| C | 4 | / | 0.43 ± 0.21 b | 0.001 | 0.81 ± 0.04 d | <0.001 | 0.84 ± 0.03 d | <0.001 |
| 6 | / | 0.35 ± 0.04 ab | 0.61 ± 0.01 c | 0.71 ± 0.02 c | ||||
| 8 | / | 0.12 ± 0.07 ab | 0.47 ± 0.01 b | 0.47 ± 0.02 b | ||||
| 10 | / | 0.06 ± 0 a | 0.31 ± 0.03 a | 0.39 ± 0.01 a | ||||
| D | 15 | 0.2% | 0.03 ± 0 b | <0.001 | 0.40 ± 0.02 b | <0.001 | 0.40 ± 0.03 b | <0.001 |
| 15 | 0.5% | 0.02 ± 0 a | 0.19 ± 0.0 ab | 0.06 ± 0.01 b | ||||
| 15 | 1% | 0.02 ± 0 a | 0.15 ± 0 a | 0.03 ± 0.01 a | ||||
| E | 15 | 0.2% | 0.03 ± 0 b | <0.001 | 0.30 ± 0 c | <0.001 | 0.02 ± 0 c | <0.001 |
| 15 | 0.5% | 0.02 ± 0 b | 0.27 ± 0.01 b | 0.02 ± 0 b | ||||
| 15 | 1% | 0.02 ± 0 a | 0.21 ± 0 a | 0.01 ± 0 a | ||||
| F | 15 | 0.2% | 0.05 ± 0 c | <0.001 | 0.18 ± 0 c | <0.001 | 0.03 ± 0 c | <0.001 |
| 15 | 0.5% | 0.05 ± 0 b | 0.17 ± 0 b | 0.02 ± 0 b | ||||
| 15 | 1% | 0.03 ± 0 a | 0.01 ± 0 a | 0.01 ± 0 a | ||||
| G | 15 | 0.2% | 0.31 ± 0.01 b | <0.001 | 0.74 ± 0.03 c | <0.001 | 0.77 ± 0.02 b | <0.001 |
| 15 | 0.5% | 0.28 ± 0.01 a | 0.64 ± 0.01 b | 0.67 ± 0.07 a | ||||
| 15 | 1% | 0.09 ± 0.02 a | 0.58 ± 0.01 a | 0.47 ± 0.04 a | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Dou, L.; Ye, Y.; Zhang, K. Reduction in the Residues of Penthiopyrad in Processed Edible Vegetables by Various Soaking Treatments and Health Hazard Evaluation in China. Foods 2023, 12, 892. https://doi.org/10.3390/foods12040892
Chang J, Dou L, Ye Y, Zhang K. Reduction in the Residues of Penthiopyrad in Processed Edible Vegetables by Various Soaking Treatments and Health Hazard Evaluation in China. Foods. 2023; 12(4):892. https://doi.org/10.3390/foods12040892
Chicago/Turabian StyleChang, Jinming, Li Dou, Yu Ye, and Kankan Zhang. 2023. "Reduction in the Residues of Penthiopyrad in Processed Edible Vegetables by Various Soaking Treatments and Health Hazard Evaluation in China" Foods 12, no. 4: 892. https://doi.org/10.3390/foods12040892