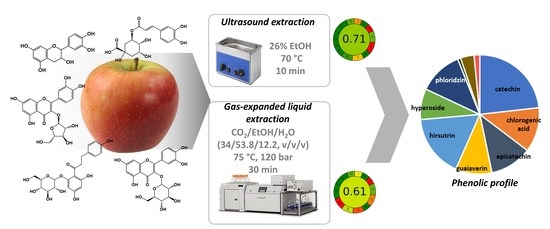
Green Solvents in the Extraction of Bioactive Compounds from Dried Apple Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Standard Solutions
2.3. Dried Apple Samples
2.4. Analysis of the Active Compounds Using UHPLC-DAD
2.5. Design of Experiments
2.6. Extraction Methods
2.6.1. Extraction Using Carbon Dioxide, SFE, and GXLE
2.6.2. Ultrasound Extraction
3. Results and Discussion
3.1. Development of the Extraction Method Using CO2
3.2. Development of Ultrasound Extraction
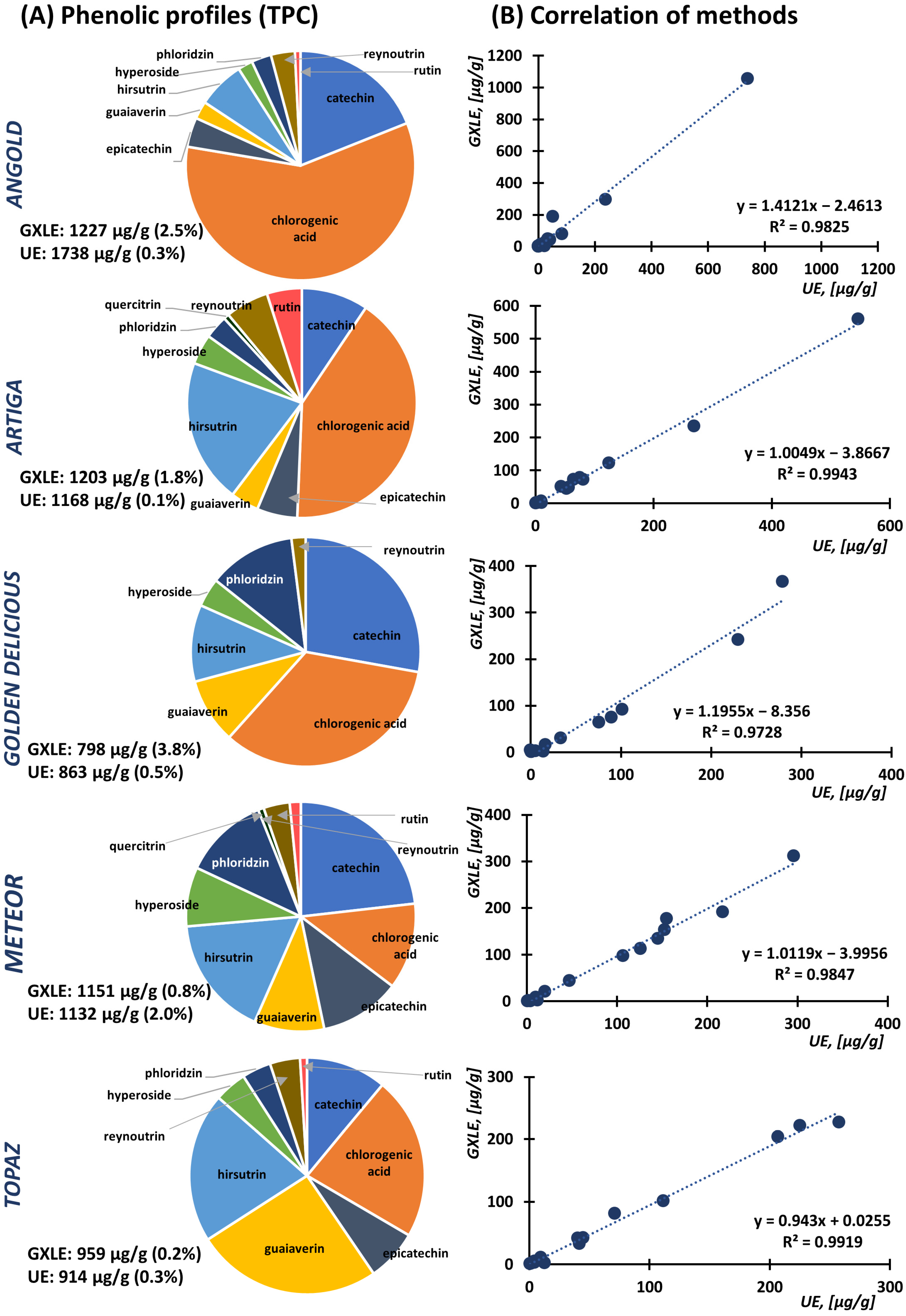
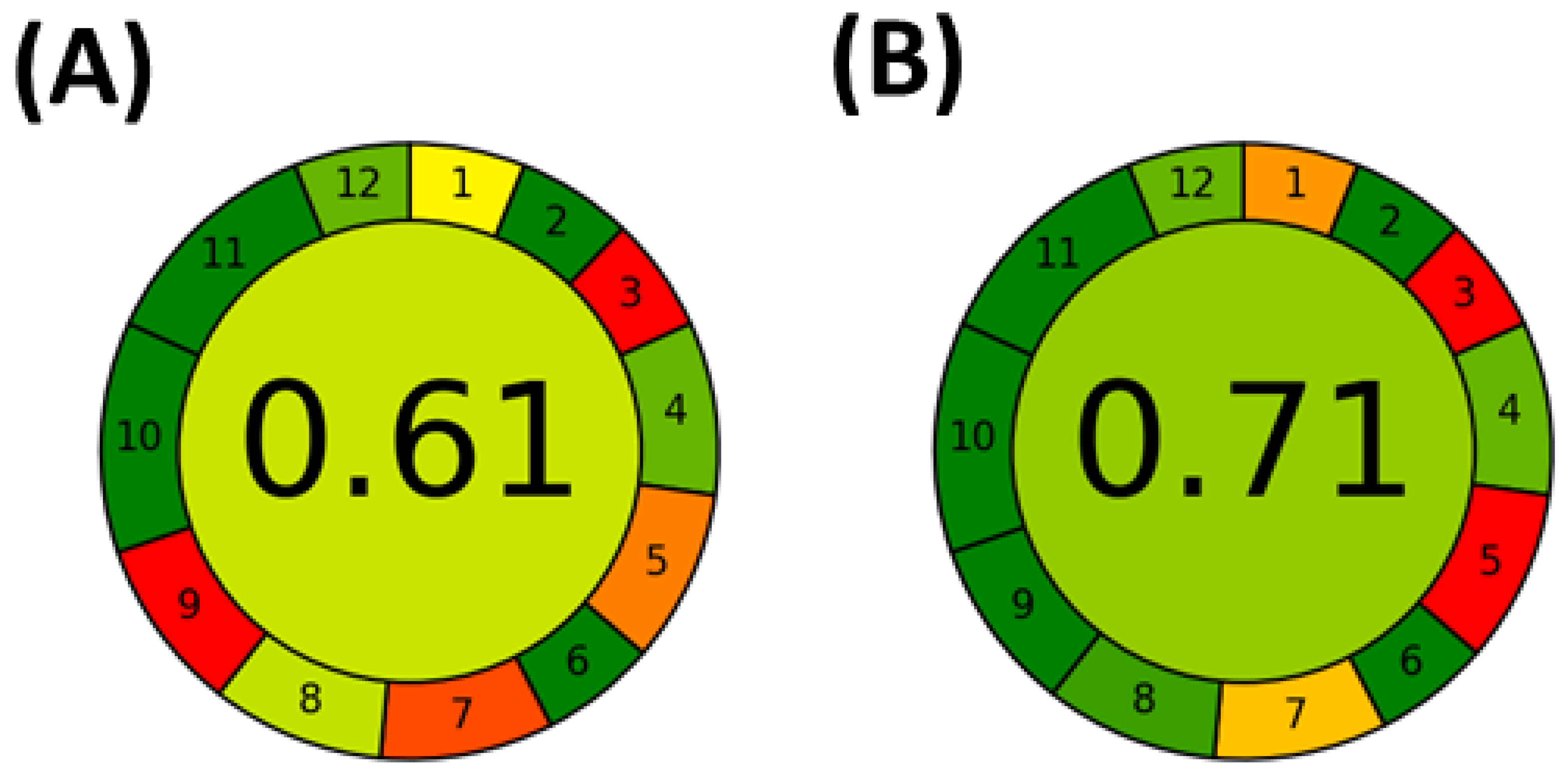
3.3. Comparison of Optimized Approaches and Application to Apple Cultivars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.S.; Nabavi, S.F.; Saeedi, M.; Nabavi, S.M. Recent Advances in Natural Products Analysis, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; p. 878. [Google Scholar]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.; Vigano, J.; de Souza Mesquita, L.M.; Baiao Dias, A.L.; de Souza, M.C.; Sanches, V.L.; Chaves, J.O.; Pizani, R.S.; Contieri, L.S.; Rostagno, M.A. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chem. X 2021, 12, 100133. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Shane, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidovic, S.; Radojčic Redovnikovic, I.; Jokic, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, E.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Dabie, K.; Abassah-Oppong, S.; Peprah-Yamoah, E. Optimization of ultrasound-assisted extraction of phenolic antioxidants from turkey berry (Solanum torvum Sw) fruits using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2022, 30, 100387. [Google Scholar] [CrossRef]
- Brahmi, F.; Blando, F.; Sellami, R.; Mehdi, S.; De Bellis, L.; Negro, C.; Haddadi-Guemghar, H.; Madani, K.; Makhlouf-Boulekbach, L. Optimization of the conditions for ultrasound-assisted extraction of phenolic compounds from Opuntia ficus-indica [L.] Mill. flowers and comparison with conventional procedures. Ind. Crops Prod. 2022, 184, 114977. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Piagentini, A.M. Sequential ultrasound-assisted extraction of pectin and phenolic compounds for the valorisation of ‘Granny Smith’ apple peel. Food Biosci. 2022, 49, 101958. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasound-assisted intensified aqueous extraction of phenolics from waste Syzygium cumini leaves: Kinetic studies and evaluation of antioxidant, antidiabetic and anticancer potential. Food Biosci. 2022, 46, 101547. [Google Scholar] [CrossRef]
- Yildiz, G.; Yildiz, G.; Khan, M.R.; Aadil, R.M. High-intensity ultrasound treatment to produce and preserve the quality of fresh-cut kiwifruit. J. Food Process Preserv. 2022, 46, e16542. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wand, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Cunico, L.P.; Turner, C. The Application of Green Solvents in Separation Processes. In The Application of Green Solvents in Separation Processes, 1st ed.; Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 155–214. [Google Scholar]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Tyskiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef] [PubMed]
- Pulok, K.M. Extraction and Other Downstream Procedures for Evaluation of Herbal Drugs. In Quality Control and Evaluation of Herbal Drugs, 1st ed.; Pulok, K.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–236. [Google Scholar]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Liu, J.; Ji, F.; Chen, F.; Guo, W.; Yang, M.; Huang, S.; Zhang, F.; Liu, Y. Determination of garlic phenolic compounds using supercritical fluid extraction coupled to supercritical fluid chromatography/tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 159, 513–523. [Google Scholar] [CrossRef]
- Abderrezag, N.; Suárez Montenegro, Z.J.; Louaer, O.; Meniai, A.H.; Cifuentes, A.; Ibánez, E.; Mendiola, J.A. One-step sustainable extraction of Silymarin compounds of wild Algerian milk thistle (Silybum marianum) seeds using Gas Expanded Liquids. J. Chromatogr. A 2022, 1675, 463147. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; Portugal, I.; Silva, C.l.M. Experimental optimization of the supercritical fluid extraction of triterpenoids from Acacia dealbata Link. Leaves. Sep. Purif. Technol. 2023, 306, 122637. [Google Scholar] [CrossRef]
- Pilařová, V.; Kuda, L.; Kočová Vlčková, H.; Nováková, L.; Gupta, S.; Kulkarni, M.; Švec, F.; van Staden, J.; Doležal, K. Carbon dioxide expanded liquid: An effective solvent for the extraction of quercetin from South African medicinal plants. Plant Methods 2022, 18, 87. [Google Scholar] [CrossRef]
- Bermejo, D.V.; Ibánez, E.; Reglero, G.; Fornari, T. Effect of cosolvents (ethyl lactate, ethyl acetate and ethanol) on the supercritical CO2 extraction of caffeine from green tea. J. Supercrit. Fluids 2016, 107, 507–512. [Google Scholar] [CrossRef]
- Pilařová, V.; Al Hamimi, S.; Cunico, L.P.; Nováková, L.; Turner, C. Extending the design space in solvent extraction—From supercritical fluids to pressurized liquids using carbon dioxide, ethanol, ethyl lactate, and water in a wide range of proportions. Green Chem. 2019, 21, 5427–5436. [Google Scholar] [CrossRef]
- Naeem, U.; Arshad, M.U.; Saeed, F.; Imran, A. Extraction and characterization of polyphenols from fruits and vegetable waste through green extraction technologies with special reference to antioxidant profile. J. Food Process Preserv. 2022, 46, 16807. [Google Scholar] [CrossRef]
- Sklenářová, H.; Bílková, A.; Pechová, M.; Chocholouš, P. Determination of major phenolic compounds in apples: Part I—Optimization of high-performance liquid chromatography separation with diode array detection. J. Sep. Sci. 2018, 41, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Bílková, A.; Baďurová, K.; Svobodová, P.; Vávra, R.; Jakubec, P.; Chocholouš, P.; Švec, F.; Sklenářová, H. Content of major phenolic compounds in apples: Benefits of ultra-low oxygen conditions in long-term storage. J. Food Comp. Anal. 2020, 92, 103587. [Google Scholar] [CrossRef]
- Hollá, M.; Bílková, A.; Jakubec, P.; Košková, S.; Kočová Vlčková, H.; Šatínský, D.; Švec, F.; Sklenářová, H. Benefits and Pitfalls of HPLC Coupled to Diode-Array, Charged Aerosol, and Coulometric Detections: Effect of Detection on Screening of Bioactive Compounds in Apples. Molecules 2021, 26, 3246. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, J.J.; Chun, H.S. Phase equilibria for carbon dioxide-ethanol-water system at elevated pressures. J. Supercrit. Fluids 1994, 7, 219–230. [Google Scholar] [CrossRef]
- Abrahamsson, V. Fundamental Research on Supercritical Fluid Extraction Kinetics: From On-Line Measurements to Inverse Modeling. Ph.D. Thesis, Lund University, Lund, Sweden, 2016. [Google Scholar]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical greenness metric for sample preparation. Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- AGREE Analytical Greenness Calculator. Available online: https://cdn.mostwiedzy.pl/f1/43/4d/a3/0_202006101932080735751_FME/agree-sfx.exe (accessed on 16 January 2023).


| Experiment | Run Order | CO2 [vol. %] | H2O [vol. %] | T [°C] | P [bar] | Extracted Amount [µg/g] * |
|---|---|---|---|---|---|---|
| N1 | 8 | 10 | 5 | 55 | 200 | 932 |
| N2 | 14 | 70 | 5 | 55 | 200 | 427 |
| N3 | 5 | 10 | 20 | 55 | 200 | 1495 |
| N4 | 19 | 70 | 20 | 55 | 200 | 603 |
| N5 | 3 | 40 | 12.5 | 30 | 100 | 987 |
| N6 | 7 | 40 | 12.5 | 80 | 100 | 1254 |
| N7 | 10 | 40 | 12.5 | 30 | 300 | 1085 |
| N8 | 4 | 40 | 12.5 | 80 | 300 | 1306 |
| N9 | 9 | 10 | 12.5 | 55 | 100 | 598 |
| N10 | 20 | 70 | 12.5 | 55 | 100 | 734 |
| N11 | 13 | 10 | 12.5 | 55 | 300 | 1531 |
| N12 | 22 | 70 | 12.5 | 55 | 300 | 429 |
| N13 | 2 | 40 | 5 | 30 | 200 | 270 |
| N14 | 25 | 40 | 20 | 30 | 200 | 711 |
| N15 | 26 | 40 | 5 | 80 | 200 | 1413 |
| N16 | 12 | 40 | 20 | 80 | 200 | 1600 |
| N17 | 23 | 10 | 12.5 | 30 | 200 | 608 |
| N18 | 17 | 70 | 12.5 | 30 | 200 | 626 |
| N19 | 27 | 10 | 12.5 | 80 | 200 | 887 |
| N20 | 15 | 70 | 12.5 | 80 | 200 | 629 |
| N21 | 16 | 40 | 5 | 55 | 100 | 771 |
| N22 | 18 | 40 | 20 | 55 | 100 | 1437 |
| N23 | 24 | 40 | 5 | 55 | 300 | 828 |
| N24 | 6 | 40 | 20 | 55 | 300 | 635 |
| N25 | 21 | 40 | 12.5 | 55 | 200 | 1143 |
| N26 | 11 | 40 | 12.5 | 55 | 200 | 1349 |
| N27 | 1 | 40 | 12.5 | 55 | 200 | 1302 |
| Experiment | Run Order | EtOH in Water [vol. %] | T [°C] | Extracted Amount [µg/g] * |
|---|---|---|---|---|
| N1 | 9 | 0 | 30 | 1668 |
| N2 | 3 | 100 | 30 | 616 |
| N3 | 8 | 0 | 70 | 2011 |
| N4 | 4 | 100 | 70 | 1366 |
| N5 | 5 | 0 | 57 | 1885 |
| N6 | 1 | 100 | 43 | 810 |
| N7 | 7 | 100 | 57 | 928 |
| N8 | 10 | 66.6 | 30 | 1747 |
| N9 | 12 | 33.3 | 70 | 2237 |
| N10 | 11 | 66.6 | 70 | 1759 |
| N11 | 15 | 50 | 50 | 1836 |
| N12 | 6 | 50 | 50 | 2096 |
| N13 | 13 | 50 | 50 | 1855 |
| N14 | 2 | 50 | 50 | 1883 |
| N15 | 14 | 0 | 30 | 1650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollá, M.; Pilařová, V.; Švec, F.; Sklenářová, H. Green Solvents in the Extraction of Bioactive Compounds from Dried Apple Cultivars. Foods 2023, 12, 893. https://doi.org/10.3390/foods12040893
Hollá M, Pilařová V, Švec F, Sklenářová H. Green Solvents in the Extraction of Bioactive Compounds from Dried Apple Cultivars. Foods. 2023; 12(4):893. https://doi.org/10.3390/foods12040893
Chicago/Turabian StyleHollá, Marcela, Veronika Pilařová, František Švec, and Hana Sklenářová. 2023. "Green Solvents in the Extraction of Bioactive Compounds from Dried Apple Cultivars" Foods 12, no. 4: 893. https://doi.org/10.3390/foods12040893
APA StyleHollá, M., Pilařová, V., Švec, F., & Sklenářová, H. (2023). Green Solvents in the Extraction of Bioactive Compounds from Dried Apple Cultivars. Foods, 12(4), 893. https://doi.org/10.3390/foods12040893