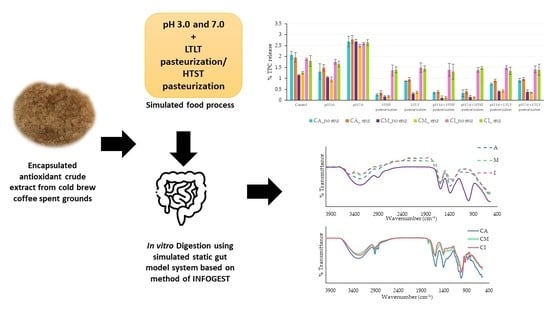
Polyphenol Release and Antioxidant Activity of the Encapsulated Antioxidant Crude Extract from Cold Brew Spent Coffee Grounds under Simulated Food Processes and an In Vitro Static Gastrointestinal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Antioxidant Crude Extracts from CSCG
2.2. Preparation of Alginate, Alginate/Maltodextrin, and Alginate/Inulin Hydrogel Bead Containing Antioxidant Crude Extracts from CSCG
2.3. Microbead Size
2.4. Fourier Transform Infrared (FT-IR) Spectroscopic Analysis
2.5. Encapsulation Efficiency
2.6. Swelling Characteristics of Alginate, Alginate/Maltodextrin, and Alginate/Inulin Hydrogel Beads
2.7. Release Behavior of TPC and Antioxidant Activity (DPPH) from Alginate, Alginate/Maltodextrin, and Alginate/Inulin Hydrogel Beads
2.7.1. Released and Remaining TPC and Antioxidant Activity (DPPH) of Microbeads under Simulated Food Process (pH and Thermal Process)
2.7.2. TPC Release of Microbeads under Simulated Gastrointestinal Fluid (SSF, SGF, and SIF) Conditions
TPC in encapsulated sample) × 100
DPPH in encapsulated sample) × 100
2.7.3. TPC Release of Microbeads under Simulated Gastrointestinal Conditions
in encapsulated sample) × 100
in encapsulated sample) × 100
2.8. Statistical Analysis
3. Results
3.1. Characterization of Microbeads
3.1.1. Average Bead Size of Alginate, Alginate/Maltodextrin, and Alginate/Inulin Hydrogel Beads
3.1.2. Analysis by FT-IR
3.1.3. Encapsulation Efficiency of Alginate, Alginate/Maltodextrin, and Alginate/Inulin Microbeads
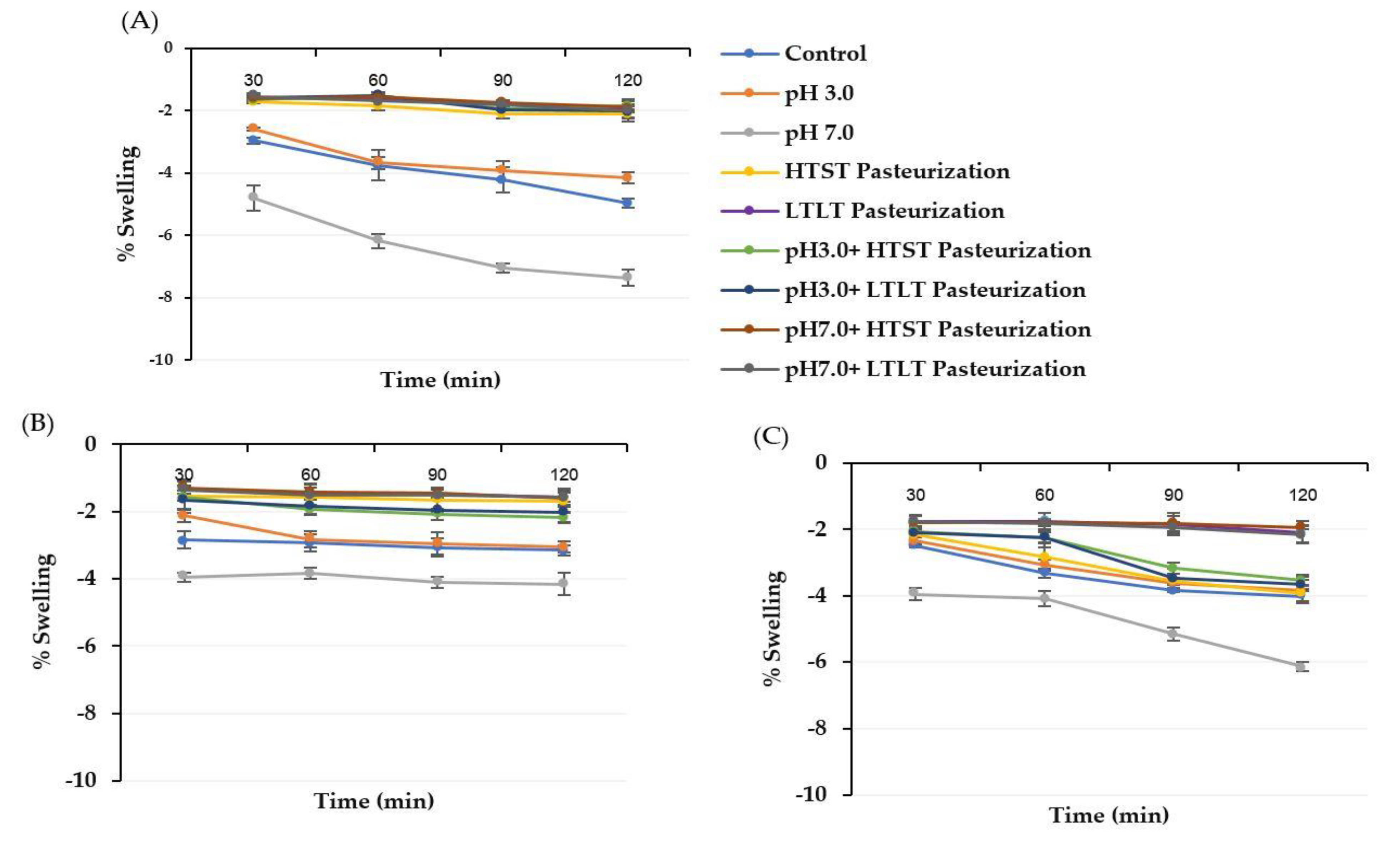
3.2. Swelling Characteristics of Alginate, Alginate/Maltodextrin, and Alginate/Inulin Microbeads
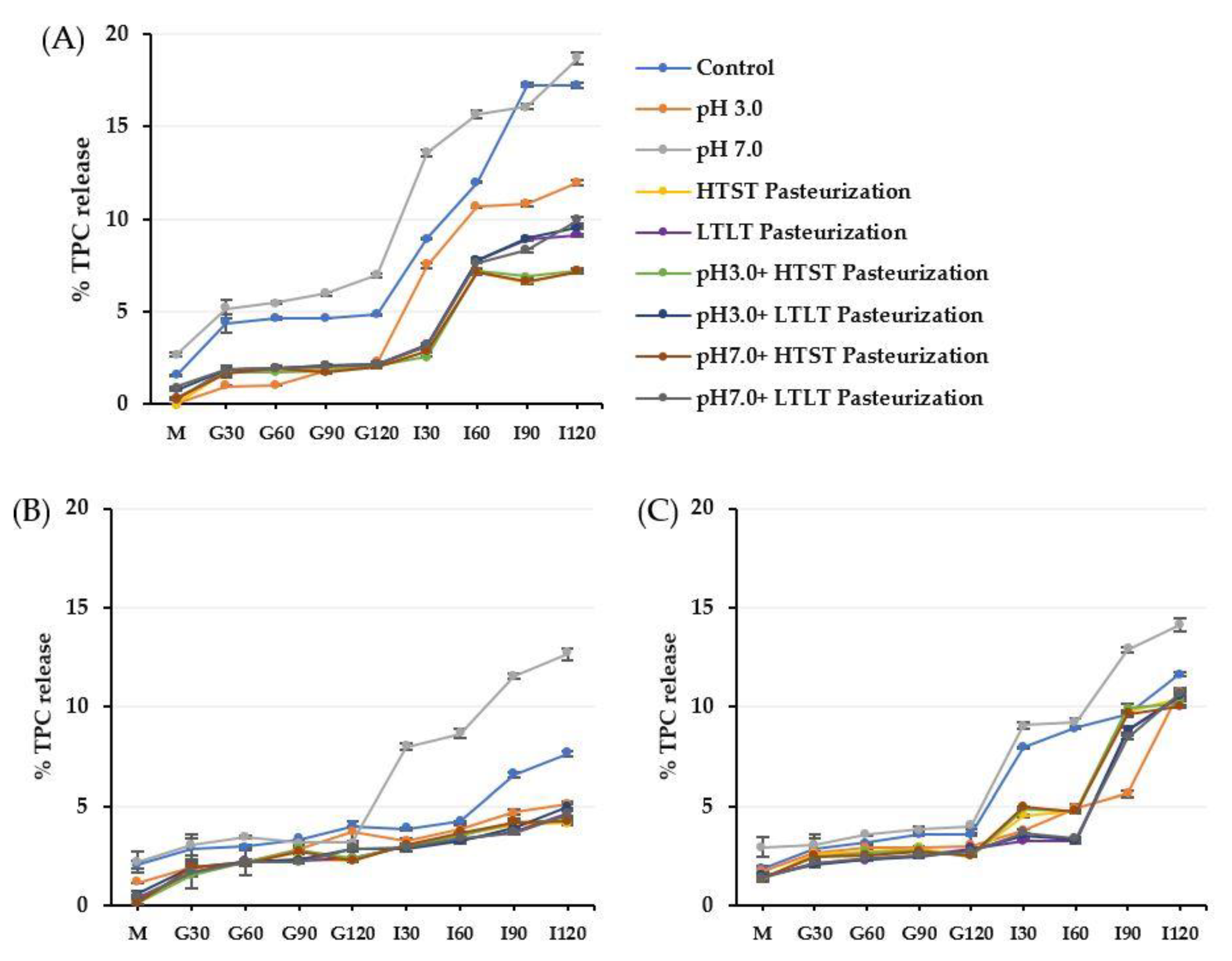
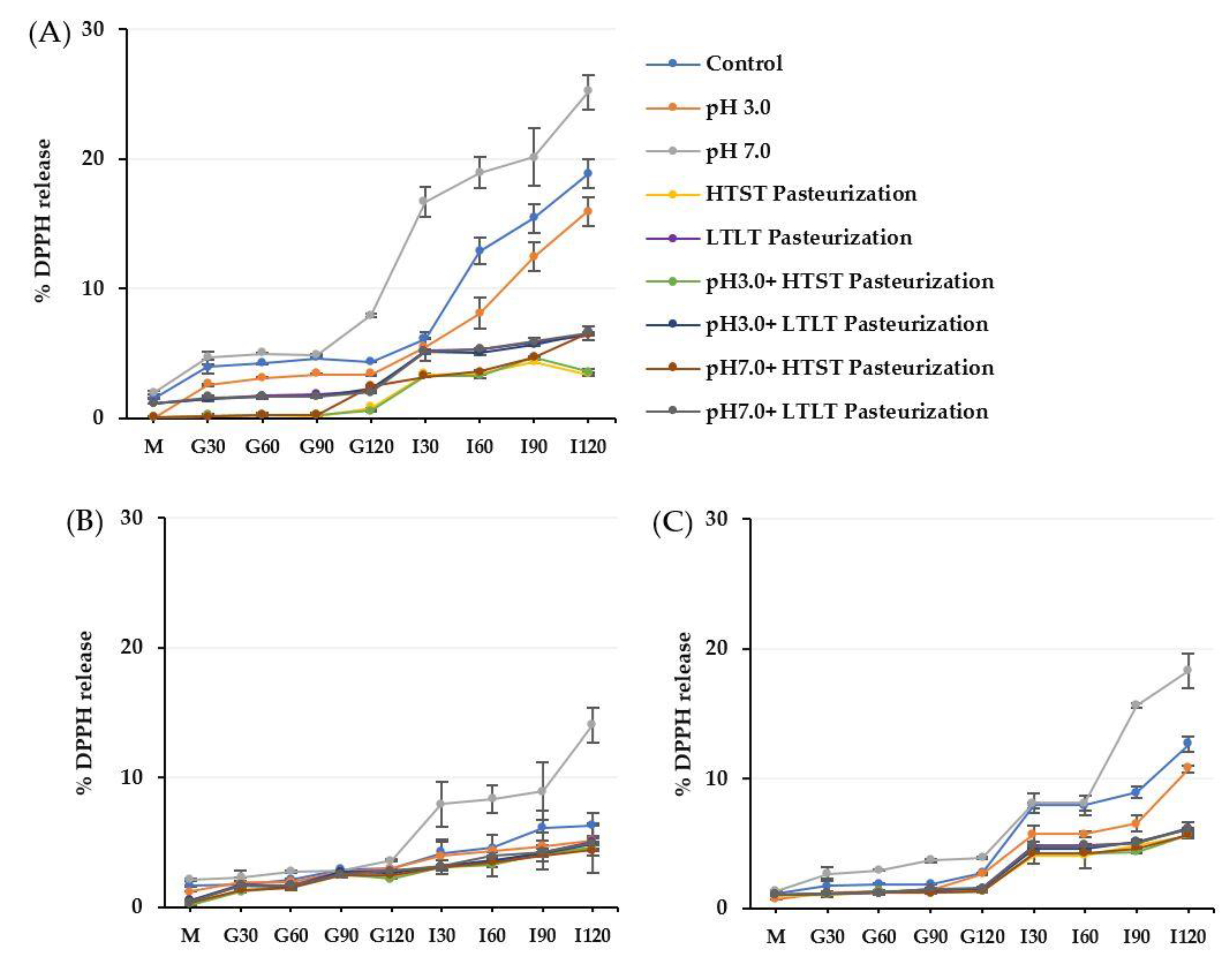
3.3. Release Behavior of TPC and DPPH from Alginate, Alginate/Maltodextrin, and Alginate/Inulin Microbeads
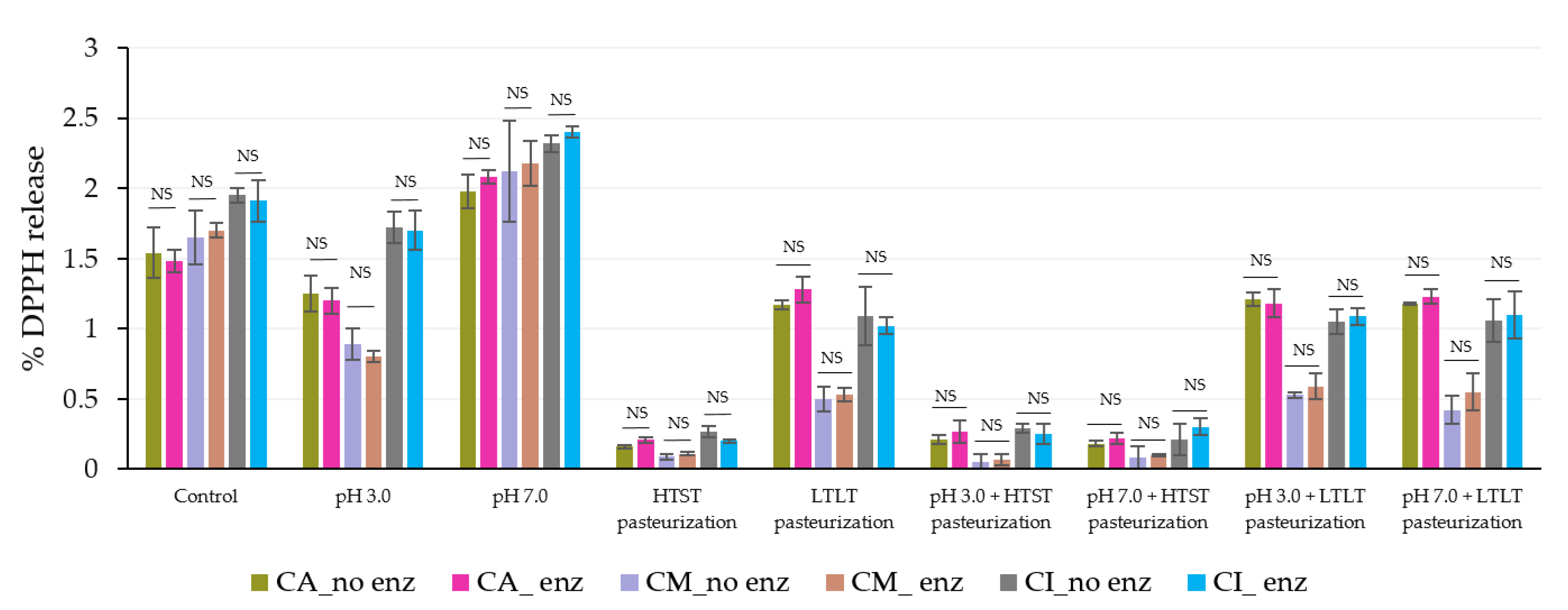
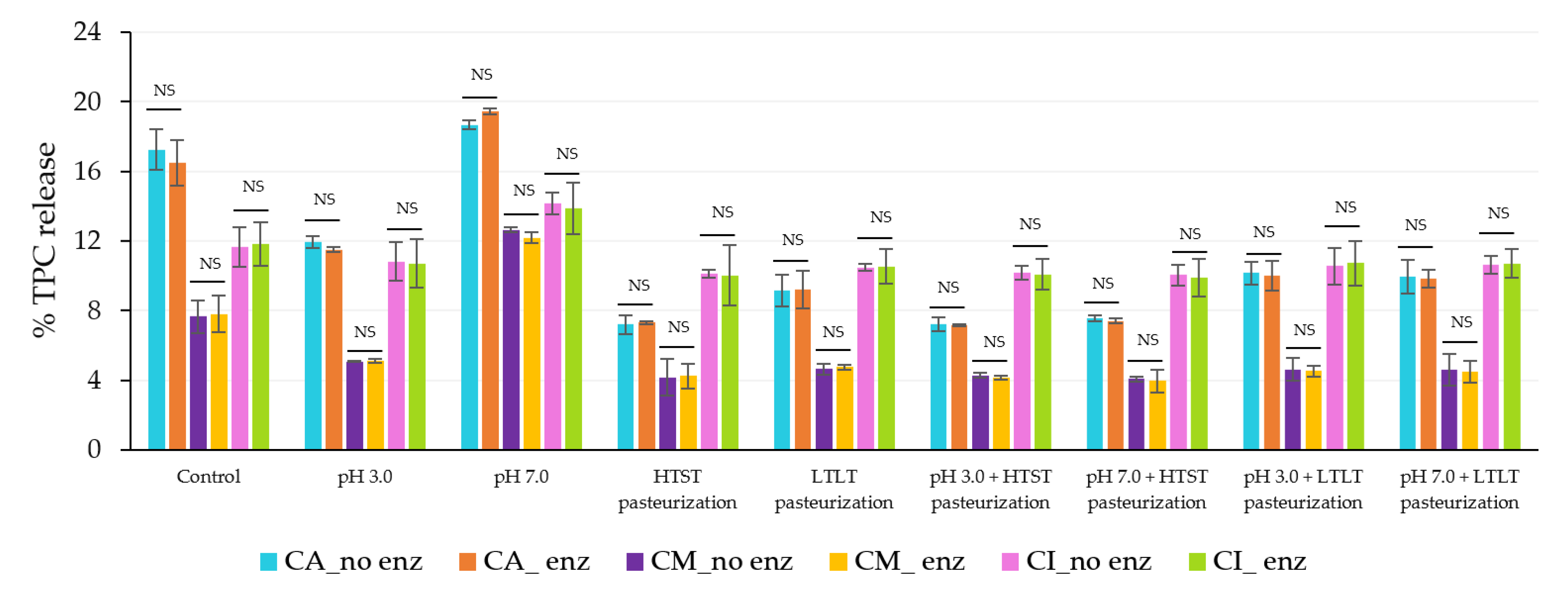
3.3.1. Released and Remaining TPC and Antioxidant Activity (DPPH) from Microbeads under Different pH and Thermal Treatments
3.3.2. Release of TPC and Antioxidant Activity (DPPH) from Microbeads under Simulated Gastrointestinal Fluid Conditions
3.3.3. Release of TPC and Antioxidant Activity (DPPH) from Microbeads under Simulated Gastrointestinal Conditions (In vitro Digestion)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farah, A.; Ferreira dos Santos, T. The coffee plant and beans: An introduction. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 5–10. [Google Scholar]
- Chongsrimsirisakhol, O.; Pirak, T. Total polyphenol content and antioxidant properties of cold brew coffee extracts as affected by ultrasound treatment and their application in low fat pork sausage. Int. J. Food Prop. 2022, 25, 813–826. [Google Scholar] [CrossRef]
- Apoorva, A.; Rameshbabu, A.P.; Dasgupta, S.; Dhara, S.; Padmavati, M. Novel pH-sensitive alginate hydrogel delivery system reinforced with gum tragacanth for intestinal targeting of nutraceuticals. Int. J. Biol. Macromol. 2020, 147, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Arriola, N.D.A.; Chater, P.I.; Wilcox, M.; Luigi, L.; Rocchetti, G.; Da, M. Encapsulation of stevia rebaudiana Bertoni aqueous crude extracts by ionic gelation—Effects of alginate blends and gelling solutions on the polyphenolic profile. Food Chem. 2019, 275, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Wang, L.; Zhou, P.; Li, X.; Gao, L. Development of the pH responsive chitosan-alginate based microgel for encapsulation of Jughans regia L. polyphenols under simulated gastrointestinal digestion in vitro. Carbohydr. Polym. 2020, 250, 116917. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.G.; Borges, C.D.; Zamiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of the phenolic compounds of the blackberry (Rubus fruticosus). LWT Food Sci. Technol. 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Dadwal, V.; Joshi, R.; Gupta, M. Formulation, characterization and in vitro digestion of polysaccharide reinforced Ca-alginate microbeads encapsulating Citrus medica L. phenolics. LWT 2021, 152, 112290. [Google Scholar] [CrossRef]
- Bušić, A.; Belščak-Cvitanović, A.; Cebin, A.; Karlović, S.; Kovač, V.; Špoljarić, I.; Mršić, G.; Komes, D. Structuring new alginate network aimed for delivery of dandelion (Taraxacum officinale L.) polyphenols using ionic gelation and new filler materials. Food Res. Int. 2018, 111, 244–255. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K.; Badwaik, L.S. Physicochemical and release behaviour of phytochemical compounds based on black jamun pulp extracts-filled alginate hydrogel beads through vibration dripping extrusion. Int. J. Biol. Macromol. 2022, 194, 715–725. [Google Scholar] [CrossRef]
- Gorbunova, N.; Bannikova, A.; Evteev, A.; Ivan, E.; Kasapis, S. Alginate-based encapsulation of extracts from beta Vulgaris cv. beet greens: Stability and controlled release under simulated gastrointestinal conditions. LWT 2018, 93, 442–449. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Jarzebski, M.; Pratap-Singh, A.; Guo, Y.; Abd-Manap, Y. Encapsulation of betacyanins from the peel of red dragon fruit (Hylocereus polyrhizus L.) in alginate microbeads. Food Hydrocoll. 2021, 113, 106535. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.A.; Hashem, M.S.; El-Awady, M.K.; Rabie, A.M. pH-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydr. Polym. 2012, 89, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendia, H.; Chavez, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of the weight ratio of alginate-modified tapioca starch on the physicochemical properties and release kinetics of chlorogenic acid containing beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Dey, M.; Ghosh, B.; Giri, T.K. Enhanced intestinal stability and pH sensitive release of quercetin in GIT through gellan gum hydrogels. Colloids Surf. B Biointerfaces 2020, 196, 111341. [Google Scholar] [CrossRef] [PubMed]
- López-Córdoba, A.; Deladino, L.; Martino, M. Release of yerba mate antioxidants from corn starch-alginate capsules as affected by structure. Carbohydr. Polym. 2014, 99, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Balanč, B.; Kalušević, A.; Drvenica, I.; Coelho, M.T.; Djordjević, V.; Alves, V.D.; Sousa, I.; Moldão-Martins, M.; Rakić, V.; Nedović, V.; et al. Calcium-Alginate-Inulin Microbeads as Carriers for Aqueous Carqueja Extract. J. Food Sci. 2015, 81, E65–E75. [Google Scholar] [CrossRef] [PubMed]
- Ćujić, N.; Trifković, K.; Bugarski, B.; Ibrić, S.; Pljevljakušić, D.; Šavikin, K. Chokeberry (Aronia melanocarpa L.) extract loaded in alginate and alginate/inulin system. Ind. Crops Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Ravichandran, K.; Palaniraj, R.; Saw, N.M.; Gabr, A.M.; Ahmed, A.R.; Knorr, D.; Smetanska, I. Effects of different encapsulation agents and drying process on stability of betalains extract. J. Food Sci. Technol. 2014, 51, 2216–2221. [Google Scholar] [CrossRef] [Green Version]
- Pai, D.A.; Vangala, V.R.; Ng, J.; Ng, W.K.; Tan, R.B.H. Resistant maltodextrin as a shell material for encapsulation of naringin: Production and physicochemical characterization. J. Food Eng. 2015, 161, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xiong, H.; Qiu, Y.; Dai, J.; Zhang, Q.; Qin, W. Radiofrequency-assisted low-temperature long-time (LTLT) pasteurization of onion powder. LWT 2022, 170, 114002. [Google Scholar] [CrossRef]
- Fernandez-Avila, C.; Trujillo, A.J. Enhanced stability of emulsions treated by Ultra-High Pressure Homogenization for delivering conjugated linoleic acid in Caco-2 cells. Food Hydrocoll. 2017, 71, 271–281. [Google Scholar] [CrossRef]
- Stojanovic, R.; Belscak-Cvitanovic, A.; Manojlovic, V.; Komes, D.; Nedovic, V.; Bugarski, B. Encapsulation of thyme (Thymus serpyllum L.) aqueous extract in calcium alginate beads. J. Sci. Food Agric. 2012, 92, 685–696. [Google Scholar] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Chan, E.-S.; Yim, Z.-H.; Phan, S.-H.; Mansa, R.F.; Ravindra, P. Encapsulation of herbal aqueous extract through absorption with ca-alginate hydrogel beads. Food Bioprod. Process. 2010, 88, 195–201. [Google Scholar] [CrossRef]
- Vallejo-Castillo, V.; Rodríguez-Stouvenel, A.; Martínez, R.; Bernal, C. Development of alginate-pectin microcapsules by the extrusion for encapsulation and controlled release of polyphenols from papaya (Carica papaya L.). J. Food Biochem. 2020, 44, e13331. [Google Scholar] [CrossRef] [PubMed]
- Belscak-Cvitanovic, A.; Komes, D.; Karlović, S.; Djaković, S.; Spoljarić, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar] [CrossRef]
- López Córdoba, A.; Deladino, L.; Martino, M. Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym. 2013, 95, 315–323. [Google Scholar] [CrossRef]
- da Silva Carvalho, A.G.; da Costa Machado, M.T.; de Freitas Queiroz Barros, H.D.; Cazarin, C.B.; Maróstica Júnior, M.R.; Hubinger, M.D. Anthocyanins from jussara (Euterpe edulis Martius) extract carried by calcium alginate beads pre-prepared using ionic gelation. Powder Technol. 2019, 345, 283–291. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Nardella, M.; Chiarini, M.; Valbonetti, L.; Neri, L.; Difonzo, G.; Pittia, P. Structuring alginate beads with different biopolymers for the development of functional ingredients loaded with olive leaves phenolic extract. Food Hydrocoll. 2020, 108, 105849. [Google Scholar] [CrossRef]
- Sun, X.; Omer, A.M.; Yang, L.Y.; Ouyang, X.K. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, C.; Cho, S.; Kim, S.B. Effects of Thermal Treatment on the Physical Properties of Edible Calcium Alginate Gel Beads: Response Surface Methodological Approach. Foods 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Bannikova, A.; Rasumova, L.; Evteev, A.; Evdokimov, I.; Kasapis, S. Protein-loaded sodium alginate and carboxymethyl cellulose beads for controlled release under simulated gastrointestinal conditions. Int. J. Food Sci. Technol. 2017, 52, 2171–2179. [Google Scholar] [CrossRef]
- Rayment, P.; Wright, P.; Hoad, C.; Ciampi, E.; Haydock, D.; Gowland, P.; Butler, M.F. Investigation of alginate beads for gastro-intestinal functionality, Part 1: In vitro characterisation. Food Hydrocoll. 2009, 23, 816–822. [Google Scholar] [CrossRef]
- Gong, R.; Li, C.; Zhu, S.; Zhang, Y.; Du, Y.; Jiang, J. A novel pH-sensitive hydrogel based on dual crosslinked alginate/N-α-glutaric acid chitosan for oral delivery of protein. Carbohydr. Polym. 2011, 85, 869–874. [Google Scholar] [CrossRef]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [Green Version]
- Alborzi, S.; Lim, L.-T.; Kakuda, Y. Release of folic acid from sodium alginate-pectin-poly(ethylene oxide) electrospun fibers under in vitro conditions. LWT Food Sci. Technol. 2014, 59, 383–388. [Google Scholar] [CrossRef]
- Lopez de Dicastillo, C.; Piña, C.; Garrido, L.; Arancibia, C.; Galotto, M.J. Enhancing Thermal Stability and Bioaccesibility of Açaí Fruit Polyphenols through Electrohydrodynamic Encapsulation into Zein Electrosprayed Particles. Antioxidants 2019, 8, 464. [Google Scholar] [CrossRef] [Green Version]
- Lucinda-Silva, R.M.; Salgado, H.R.N.; Evangelista, R.C. Alginate–chitosan systems: In vitro controlled release of triamcinolone and in vivo gastrointestinal transit. Carbohydr. Polym. 2010, 81, 260–268. [Google Scholar] [CrossRef]
- Szczęsna, W.; Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Balicki, S.; Warszyński, P.; Wilk, K.A. Insight into multilayered alginate/chitosan microparticles for oral administration of large cranberry fruit extract. Eur. Polym. J. 2021, 160, 110776. [Google Scholar] [CrossRef]
- Khorshidian, N.; Mahboubi, A.; Kalantari, N.; Hosseini, H.; Yousefi, M.; Arab, M.; da Cruz, A.G.; Mortazavian, A.M.; Mahdavi, F.S. Chitosan-Coated Alginate Microcapsules Loaded with Herbal galactagogue Extract: Formulation Optimization and Characterization. Iran J. Pharm. Res. 2019, 18, 1180–1195. [Google Scholar] [PubMed]
- Li, Y.; Fan, R.; Xing, H.; Fei, Y.; Cheng, J.; Lu, L. Study on swelling and drug releasing behaviors of ibuprofen-loaded bimetallic alginate aerogel beads with pH-responsive performance. Colloids Surf. B Biointerfaces 2021, 205, 111895. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Hogan, S.A.; López-Rubio, A.; Brodkorb, A. Nano- and microstructural evolution of alginate beads in simulated gastrointestinal fluids. Impact of M/G ratio, molecular weight and pH. Carbohydr. Polym. 2019, 223, 115121. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, C.; Zhou, W.; Luan, Q.; Li, W.; Deng, Q.; Dong, X.; Tang, H.; Huang, F. A pH-Responsive Gel Macrosphere Based on Sodium Alginate and Cellulose Nanofiber for Potential Intestinal Delivery of Probiotics. ACS Sustain. Chem. Eng. 2018, 6, 13924–13931. [Google Scholar] [CrossRef]
- Mulia, K.; Halimah, N.; Krisanti, E. Effect of alginate composition on profile release and characteristics of chitosan-alginate microparticles loaded with mangosteen extract. AIP Conf. Proc. 2016, 1823, 020010. [Google Scholar]
- Valenzuela, C.; Hernández, V.; Morales, M.S.; Pizarro, F. Heme Iron Release from Alginate Beads at In Vitro Simulated Gastrointestinal Conditions. Biol. Trace Elem. Res. 2016, 172, 251–257. [Google Scholar] [CrossRef]
- Anbinder, P.S.; Deladino, L.; Navarro, A.S.d.R.; Amalvy, J.; Martino, M.N. Yerba Mate Extract Encapsulation with Alginate and Chitosan Systems: Interactions between Active Compound Encapsulation Polymers. J. Encapsulation Adsorpt. Sci. 2011, 1, 14011. [Google Scholar] [CrossRef] [Green Version]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-L.; Yung-Sheng, L. Effects of pH on the Shape of Alginate Particles and Its Release Behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef] [Green Version]
- Ziar, H.; Gérard, P.; Riazi, A. Calcium alginate-resistant starch mixed gel improved the survival of Bifidobacterium animalis subsp. lactis Bb12 and Lactobacillus rhamnosus LBRE-LSAS in yogurt and simulated gastrointestinal conditions. Int. J. Food Sci. Technol. 2012, 47, 1421–1429. [Google Scholar] [CrossRef]






| Antioxidant Crude Extract | Encapsulated Materials | Abbreviation | ||
|---|---|---|---|---|
| Alginate (% w/v) | Maltodextrin (% w/v) | Inulin (% w/v) | ||
| CSCG | 2 | - | - | CA |
| 2 | 2 | - | CM | |
| 2 | - | 5 | CI | |
| Encapsulated Sample | Size (mm) |
|---|---|
| CA | 1.95 ns ± 0.97 |
| CM | 1.87 ns ± 0.66 |
| CI | 1.93 ns ± 0.74 |
| Encapsulated Sample | % Encapsulation Efficiency Based on TPC |
|---|---|
| CA | 83.64 a ± 1.20 |
| CM | 89.76 c ± 1.90 |
| CI | 85.78 b ± 0.78 |
| Encapsulated Sample | % Swelling | ||
|---|---|---|---|
| CA | CM | CI | |
| Control | ND | ND | ND |
| pH 3.0 | −0.56 cA ± 0.03 | −0.18 cB ± 0.02 | −0.21 bB ± 0.05 |
| pH 7.0 | 4.98 dB ± 0.10 | 3.11 dA ± 0.05 | 3.18 cA ± 0.15 |
| HTST Pasteurization | −8.85 aA ± 0.05 | −5.55 aB ± 0.03 | −2.72 aC ± 0.21 |
| LTLT Pasteurization | −6.19 bA ± 0.11 | −3.01 bB ± 0.21 | −2.16 aC ± 0.98 |
| pH 3.0 + HTST Pasteurization | −8.46 aA ± 0.05 | −5.88 aB ± 0.07 | −2.54 aC ± 0.13 |
| pH 7.0 + HTST Pasteurization | −8.58 aA ±0.06 | −5.84 aB ± 0.03 | −2.29 aC ± 0.01 |
| pH 3.0 + LTLT Pasteurization | −6.58 bA ± 0.47 | −2.98 bB ± 0.11 | −2.01 aC ± 0.14 |
| pH 7.0 + LTLT Pasteurization | −6.80 bA ± 0.04 | −3.14 bB ± 0.35 | −2.12 aC ± 0.57 |
| Encapsulated Sample | % TPC Released | ||
|---|---|---|---|
| CA | CM | CI | |
| Control | ND | ND | ND |
| pH 3.0 | 0.08 aA ± 0.04 | 0.02 aA ± 0.07 | 0.05 aA ± 0.06 |
| pH 7.0 | 2.72 bC ± 0.13 | 1.95 bB ±0.05 | 1.18 bA ± 0.09 |
| HTST Pasteurization | 8.28 cB ± 0.08 | 6.43 cA ± 0.04 | 6.72 cA ± 0.15 |
| LTLT Pasteurization | 25.18 eB ± 0.26 | 20.95 dA ± 0.39 | 20.74 dA ± 0.47 |
| pH 3.0 + HTST Pasteurization | 8.89 dB ± 0.10 | 6.42 cA ± 0.02 | 6.54 cA ± 0.19 |
| pH 7.0 + HTST Pasteurization | 8.92 dB ± 0.08 | 6.58 cA ± 0.82 | 6.29 cA ± 0.11 |
| pH 3.0 + LTLT Pasteurization | 24.96 eB ± 0.57 | 20.81 dA ± 0.16 | 20.68 dA ± 0.51 |
| pH 7.0 + LTLT Pasteurization | 25.09 eB ± 0.32 | 19.97 dA ± 0.98 | 20.81 dA ± 0.99 |
| Encapsulated Samples | % DPPH Released | ||
|---|---|---|---|
| CA | CM | CI | |
| Control | ND | ND | ND |
| pH 3.0 | 1.58 aC ± 0.11 | 1.09 aB ± 0.08 | 0.87 aA ± 0.01 |
| pH 7.0 | 1.66 aB ± 0.08 | 1.25 aA ± 0.04 | 1.16 bA ± 0.05 |
| HTST Pasteurization | 5.91 bC ± 0.15 | 4.14 bB ± 0.23 | 3.85 cA ± 0.17 |
| LTLT Pasteurization | 11.95 cC ± 1.03 | 9.85 cA ± 0.64 | 10.15 dB ± 2.05 |
| pH 3.0 + HTST Pasteurization | 6.05 bB ± 0.94 | 3.87 bA ± 0.21 | 3.91 cA ± 0.55 |
| pH 7.0 + HTST Pasteurization | 5.98 bB ± 0.32 | 3.95 bA ± 0.67 | 4.05 cA ± 0.14 |
| pH 3.0 + LTLT Pasteurization | 11.84 cB ± 1.05 | 9.75 cA ± 1.22 | 9.94 dA ± 1.95 |
| pH 7.0 + LTLT Pasteurization | 12.00 cB ± 1.32 | 9.97 cA ± 0.98 | 10.07 dA ± 1.65 |
| Encapsulated Sample | % TPC Remaining | ||
|---|---|---|---|
| CA | CM | CI | |
| Control | 99.85 dA ± 0.38 | 99.12 dA ± 0.38 | 99.45 cA ± 0.38 |
| pH 3.0 | 98.45 dA ± 0.38 | 99.51 dB ± 0.18 | 99.28 cB ± 0.54 |
| pH 7.0 | 96.41 cA ± 0.25 | 97.74 cB ± 0.26 | 97.65 cB ± 0.14 |
| HTST Pasteurization | 84.94 bA ± 0.14 | 90.28 bB ± 0.30 | 90.05 bB ± 0.28 |
| LTLT Pasteurization | 58.77 aA ± 1.21 | 65.68 aB ± 1.87 | 66.84 aB ± 1.95 |
| pH 3.0 + HTST Pasteurization | 85.02 bA ± 0.57 | 90.11 bB ± 0.27 | 90.20 bB ± 0.71 |
| pH 7.0 + HTST Pasteurization | 84.45 bA ± 0.65 | 89.92 bB ± 0.26 | 90.17 bB ± 1.25 |
| pH 3.0 + LTLT Pasteurization | 59.02 aA ± 1.38 | 65.77 aB ± 1.02 | 66.61 aB ± 0.64 |
| pH 7.0 + LTLT Pasteurization | 58.87 aA ± 0.99 | 65.48 aB ± 1.57 | 66.97 aB ± 0.84 |
| Encapsulated Sample | % DPPH Remaining | ||
|---|---|---|---|
| CA | CM | CI | |
| Control | 99.05 cA ± 0.85 | 99.61 cA ± 0.05 | 99.27 cA ± 0.67 |
| pH 3.0 | 98.75 cA ± 2.05 | 99.05 cA ± 1.26 | 99.65 cA ± 0.51 |
| pH 7.0 | 98.67 cA ± 1.95 | 99.17 cA ± 1.49 | 99.37 cA ± 1.98 |
| HTST Pasteurization | 90.15 bA ± 1.06 | 92.65 bB ± 1.06 | 93.65 bC ± 1.25 |
| LTLT Pasteurization | 75.80 aA ± 0.15 | 80.47 aB ± 1.11 | 81.47 aB ± 1.44 |
| pH 3.0 + HTST Pasteurization | 89.97 bA ± 1.47 | 92.18 bB ± 0.52 | 93.84 bC ± 0.65 |
| pH 7.0 + HTST Pasteurization | 90.22 bA ± 2.32 | 91.84 bA ± 1.36 | 93.55 bA ± 2.06 |
| pH 3.0 + LTLT Pasteurization | 75.17 aA ± 0.85 | 79.97 aB ± 1.65 | 80.95 aB ± 1.00 |
| pH 7.0 + LTLT Pasteurization | 75.46 aA ± 1.66 | 80.15 aB ± 1.20 | 81.67 aB ± 1.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chongsrimsirisakhol, O.; Pirak, T. Polyphenol Release and Antioxidant Activity of the Encapsulated Antioxidant Crude Extract from Cold Brew Spent Coffee Grounds under Simulated Food Processes and an In Vitro Static Gastrointestinal Model. Foods 2023, 12, 1000. https://doi.org/10.3390/foods12051000
Chongsrimsirisakhol O, Pirak T. Polyphenol Release and Antioxidant Activity of the Encapsulated Antioxidant Crude Extract from Cold Brew Spent Coffee Grounds under Simulated Food Processes and an In Vitro Static Gastrointestinal Model. Foods. 2023; 12(5):1000. https://doi.org/10.3390/foods12051000
Chicago/Turabian StyleChongsrimsirisakhol, Onamon, and Tantawan Pirak. 2023. "Polyphenol Release and Antioxidant Activity of the Encapsulated Antioxidant Crude Extract from Cold Brew Spent Coffee Grounds under Simulated Food Processes and an In Vitro Static Gastrointestinal Model" Foods 12, no. 5: 1000. https://doi.org/10.3390/foods12051000