Curcumin Alleviates Aflatoxin B1-Induced Liver Pyroptosis and Fibrosis by Regulating the JAK2/NLRP3 Signaling Pathway in Ducks
Abstract
:1. Introduction
2. Materials and Methods
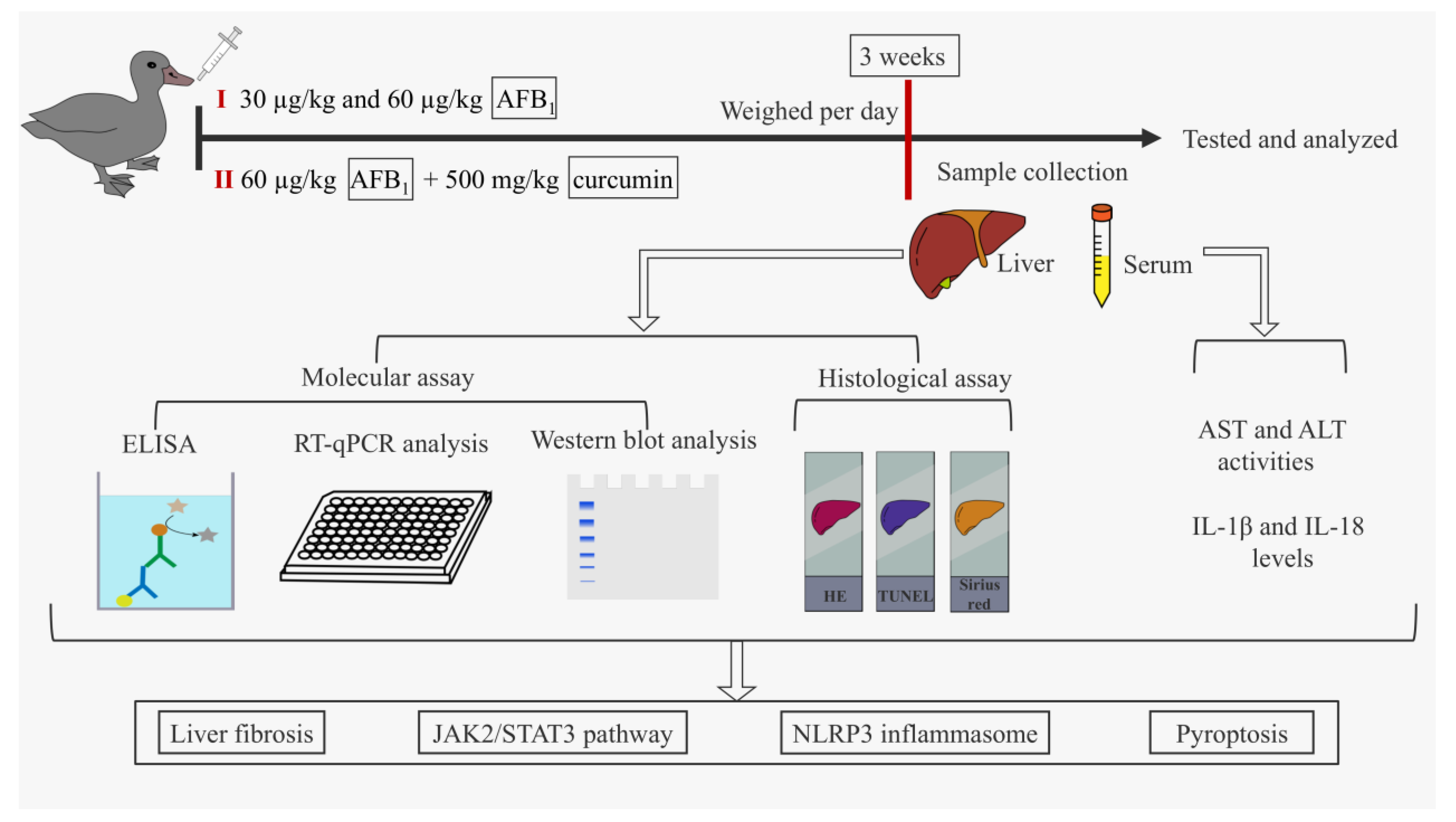
2.1. Schematic Overview of Experimental Program
2.2. Animals and Treatment
2.3. Sample Collection
2.4. Measurement of Serum ALT and AST Activities
2.5. Histopathological Observation
2.6. TUNEL Analysis
2.7. Detection of IL-1β and IL-18 Levels in the Liver and Serum
2.8. RT-qPCR Analysis
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. AFB1 Exposure Caused Liver Damage in Ducks
3.2. AFB1 Exposure Activated JAK2/NLRP3-Mediated Pyroptosis in Duck Livers
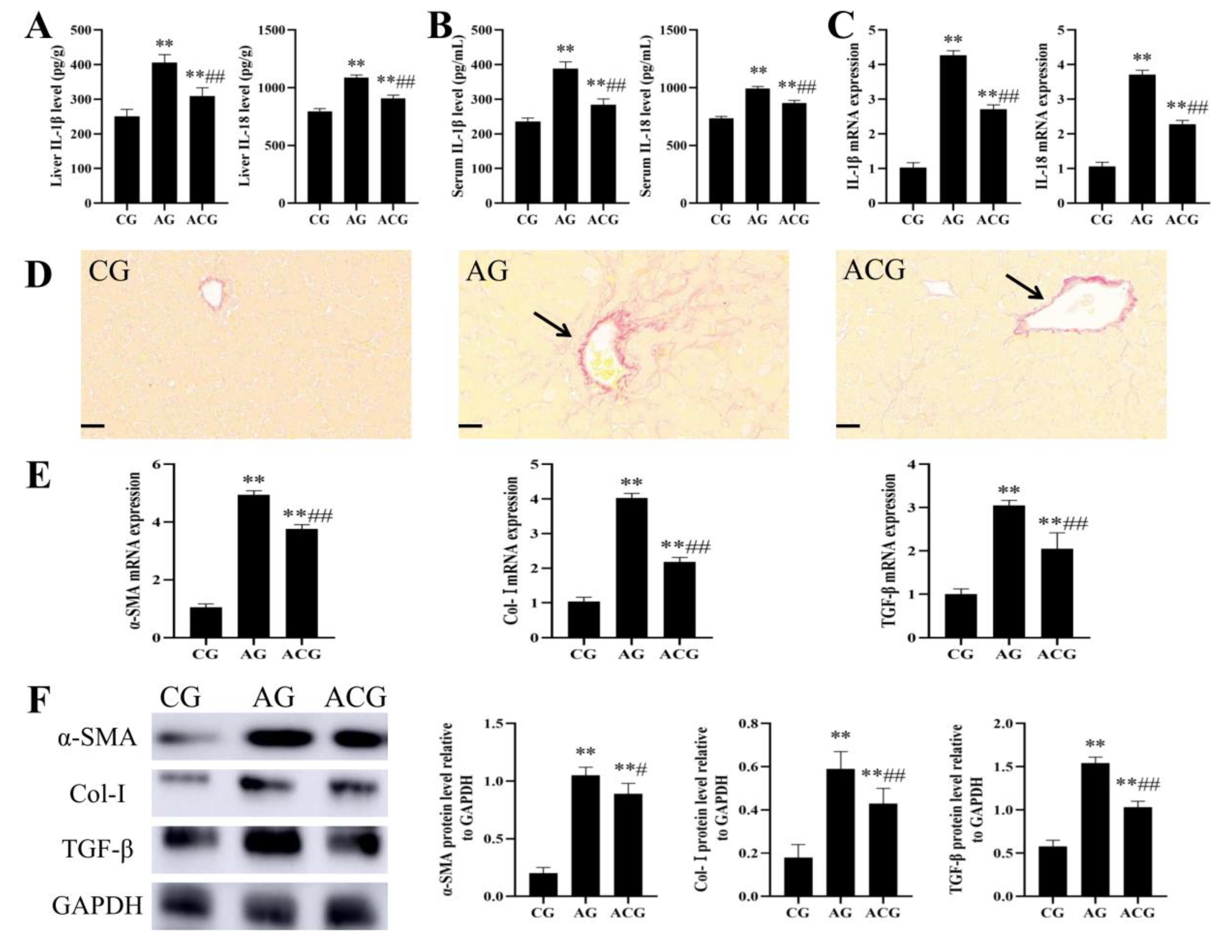
3.3. AFB1 Exposure Caused Liver Fibrosis in Ducks
3.4. Curcumin Alleviated Liver Damage in Ducks Caused by AFB1 Exposure
3.5. Curcumin Alleviated JAK2/NLRP3-Mediated Pyroptosis in the Liver of Ducks Exposed to AFB1
3.6. Curcumin Alleviated Liver Fibrosis in Ducks Caused by AFB1 Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Jia, F.B.; Guo, C.; Wang, Y.P.; Zhang, X.L.; Cui, Y.L.; Song, M.; Cao, Z.; Li, Y.F. PINK1/Parkin-mediated mitophagy as a protective mechanism against AFB1-induced liver injury in mice. Food Chem. Toxicol. 2022, 164, 113043. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.O. Mycotoxic Fungi. In Microbial Food Poisoning; Eley, A.R., Ed.; Springer: Boston, MA, USA, 1996; pp. 75–93. [Google Scholar]
- Iram, W.; Anjum, T.; Abbas, M.; Khan, A.M. Aflatoxins and ochratoxin A in maize of Punjab, Pakistan. Food Addit. Contam. Part B Surveill. 2014, 7, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Akinmusire, O.O.; El-Yuguda, A.D.; Musa, J.A.; Oyedele, O.A.; Sulyok, M.; Somorin, Y.M.; Ezekiel, C.N.; Krska, R. Mycotoxins in poultry feed and feed ingredients in Nigeria. Mycotoxin Res. 2019, 35, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahuku, G.; Nzioki, H.S.; Mutegi, C.; Kanampiu, F.; Narrod, C.; Makumbi, D. Pre-harvest management is a critical practice for minimizing aflatoxin contamination of maize. Food Control 2019, 96, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cao, Z.; Zhang, J.; Ji, Q.; Li, Y.F. Aflatoxin B1 promotes autophagy associated with oxidative stress-related PI3K/AKT/mTOR signaling pathway in mice testis. Environ. Pollut. 2019, 255, 113317. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Y.F.; Wang, Y.P.; Wang, Q.; Huo, S.M.; Zhang, X.L.; Cao, Z.; Song, M.; Li, Y.F. PINK1/Parkin-mediated mitophagy is activated to protect against AFB1-induced immunosuppression in mice spleen. Toxicol. Lett. 2022, 366, 33–44. [Google Scholar] [CrossRef]
- Wang, Y.P.; Song, M.; Wang, Q.; Guo, C.; Zhang, J.; Zhang, X.L.; Cui, Y.L.; Cao, Z.; Li, Y.F. PINK1/Parkin-mediated mitophagy is activated to protect against AFB1-induced kidney damage in mice. Chem. Biol. Interact. 2022, 358, 109884. [Google Scholar] [CrossRef]
- Karamkhani, M.; Asilian-Mahabadi, H.; Daraei, B.; Seidkhani-Nahal, A.; Noori-Zadeh, A. Liver and kidney serum profile abnormalities in workers exposed to aflatoxin B1 in urban solid waste management centers. Environ. Monit. Assess. 2020, 192, 472. [Google Scholar] [CrossRef]
- Da Silveira, A.R.; Rosa, É.V.F.; Sari, M.H.M.; Sampaio, T.B.; Dos Santos, J.T.; Jardim, N.S.; Müller, S.G.; Oliveira, M.S.; Nogueira, C.W.; Furian, A.F. Therapeutic potential of beta-caryophyllene against aflatoxin B1-Induced liver toxicity: Biochemical and molecular insights in rats. Chem. Biol. Interact. 2021, 348, 109635. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhan, D.L.; Chen, Y.Y.; Wang, W.H.; He, C.Y.; Lin, Y.; Lin, Y.C.; Lin, Z.N. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: An in vitro, ex vivo and in vivo study. Arch. Toxicol. 2019, 93, 3305–3320. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wen, L.; Shi, Q.F.; Gao, F.; Huang, B.; Meng, J.; Hu, C.P.; Wang, C.M. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; McGeough, M.D.; Inzaugarat, M.E.; Eguchi, A.; Schuster, S.; Johnson, C.D.; Peña, C.A.; Geisler, L.J.; Papouchado, B.G.; Hoffman, H.M.; et al. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology 2018, 67, 736–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Liu, P.L.; Cui, Y.L.; Song, M.; Zhang, X.L.; Zhang, C.; Jiang, Y.B.; Li, Y.F. T-2 toxin caused mice testicular inflammation injury via ROS-mediated NLRP3 inflammasome activation. J. Agric. Food Chem. 2022, 70, 14043–14051. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chen, S.J.; Zhou, S.C.; Wu, S.Z.; Wang, H. Inflammasomes and fibrosis. Front. Immunol. 2021, 12, 643149. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, R.L.; Huang, Z.Q.; Zhang, N.S.; Zhang, L.; Li, X.C.; Wang, P.M. Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis. Mediat. Inflamm. 2019, 2019, 2165918. [Google Scholar] [CrossRef]
- Gao, C.; Yan, Y.; Chen, G.; Wang, T.; Luo, C.L.; Zhang, M.Y.; Chen, X.P.; Tao, L.Y. Autophagy activation represses pyroptosis through the IL-13 and JAK1/STAT1 pathways in a mouse model of moderate traumatic brain injury. ACS Chem. Neurosci. 2020, 11, 4231–4239. [Google Scholar] [CrossRef]
- Coppock, R.W.; Christian, R.G.; Jacobsen, B.J. Aflatoxins. In Veterinary Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 983–994. [Google Scholar]
- Robinson, R.M.; Ray, A.C.; Reagor, J.C.; Holland, L.A. Waterfowl mortality caused by aflatoxicosis in Texas. J. Wildl. Dis. 1982, 18, 311–313. [Google Scholar] [CrossRef] [Green Version]
- Verheecke, C.; Liboz, T.; Mathieu, F. Microbial degradation of aflatoxin B1: Current status and future advances. Int. J. Food Microbiol. 2016, 237, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Gao, J.S.; Huang, W.Y.; Yan, J.L.; Shan, A.S.; Gao, X. Curcumin mitigates deoxynivalenol-induced intestinal epithelial barrier disruption by regulating Nrf2/p53 and NF-κB/MLCK signaling in mice. Food Chem. Toxicol. 2022, 167, 113281. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Shang, Z.P.; Lu, Y.Y.; Li, P.; Sun, L.; Guo, Q.L.; Bo, T.; Le, Z.Y.; Bai, Z.L.; Zhang, X.L.; et al. Analysis of curcuminoids and volatile components in 160 batches of turmeric samples in China by high-performance liquid chromatography and gas chromatography mass spectrometry. J. Pharm. Biomed. Anal. 2020, 188, 113465. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.J.; Yang, H.; Jiao, Y.H.; Pang, Q.; Wang, Y.J.; Wang, M.; Shan, A.S.; Feng, X.J. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Wang, W.C.; Lin, C.X.; Fouad, A.M.; Chen, W.; Xia, W.G.; Wang, S.; Luo, X.; Zhang, W.H.; Yan, S.J.; et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal 2019, 13, 42–52. [Google Scholar] [CrossRef]
- Jin, S.J.; Yang, H.; Wang, Y.J.; Pang, Q.; Jiao, Y.H.; Shan, A.S.; Feng, X.J. Dietary curcumin alleviated aflatoxin B1-induced acute liver damage in ducks by regulating NLRP3-Caspase-1 signaling pathways. Foods 2021, 10, 3086. [Google Scholar] [CrossRef]
- Liu, F.J.; Wang, Y.J.; Zhou, X.; Liu, M.R.; Jin, S.J.; Shan, A.S.; Feng, X.J. Resveratrol relieved acute liver damage in ducks (Anas platyrhynchos) induced by AFB1 via modulation of apoptosis and Nrf2 signaling pathways. Animals 2021, 11, 3516. [Google Scholar] [CrossRef]
- World Health Organization. Environmental Health Criteria 11: Mycotoxins; Published under the Joint Sponsorship of the United Nations Environment Programme and the World Health Organization; World Health Organization: Geneva, Switzerland, 1979; p. 127.
- Yang, H.; Yu, C.T.; Yin, Z.S.; Guan, P.Y.; Jin, S.J.; Wang, Y.J.; Feng, X.J. Curcumin: A potential exogenous additive for the prevention of LPS-induced duck ileitis by the alleviation of inflammation and oxidative stress. J. Sci. Food Agric. 2023, 103, 1550–1560. [Google Scholar] [CrossRef]
- Cui, Y.L.; Song, M.; Xiao, B.N.; Huang, W.Y.; Zhang, J.; Zhang, X.L.; Shao, B.; Han, Y.F.; Li, Y.F. PINK1/Parkin-mediated mitophagy plays a protective role in the bone impairment caused by aluminum exposure. J. Agric. Food Chem. 2021, 69, 6054–6063. [Google Scholar] [CrossRef]
- Akinrinmade, F.J.; Akinrinde, A.S.; Amid, A. Changes in serum cytokine levels, hepatic and intestinal morphology in aflatoxin B1-induced injury: Modulatory roles of melatonin and flavonoid-rich fractions from Chromolena odorata. Mycotoxin Res. 2016, 32, 53–60. [Google Scholar] [CrossRef]
- Meissonnier, G.M.; Laffitte, J.; Loiseau, N.; Benoit, E.; Raymond, I.; Pinton, P.; Cossalter, A.M.; Bertin, G.; Oswald, I.P.; Galtier, P. Selective impairment of drug-metabolizing enzymes in pig liver during subchronic dietary exposure to aflatoxin B1. Food Chem. Toxicol. 2007, 45, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.X.; Xu, J.N.; Jiang, L.Q.; Liu, W.; Hong, H.R.; Qian, Y.X.; Li, S.R.; Huang, W.L.; Zhao, H.G.; Yang, Z.T.; et al. Morin alleviates aflatoxin B1-induced liver and kidney injury by inhibiting heterophil extracellular traps release, oxidative stress and inflammatory responses in chicks. Poult. Sci. 2021, 100, 101513. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Schuppan, D. Targeting cancer associated fibroblasts in liver fibrosis and liver cancer using nanocarriers. Cells 2020, 9, 2027. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef]
- Dolivo, D.; Weathers, P.; Dominko, T. Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics. Acta Pharm. Sin. B 2021, 11, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Arana, S.; Alves, V.A.; Sabino, M.; Tabata, Y.A.; Nonogaki, S.; Zaidan-Dagli, M.L.; Hernandez-Blazquez, F.J. Immunohistochemical evidence for myofibroblast-like cells associated with liver injury induced by aflatoxin B1 in rainbow trout (Oncorhynchus mykiss). J. Comp. Pathol. 2014, 150, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.A.Z.; Abdel-Maksoud, F.M.; Abd Elaziz, H.O.; Al-Brakati, A.; Elmahallawy, E.K. Descriptive histopathological and ultrastructural study of hepatocellular alterations induced by aflatoxin B1 in rats. Animals 2021, 11, 509. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho Ribeiro, M.; Szabo, G. Role of the inflammasome in liver disease. Annu. Rev. Pathol. 2022, 17, 345–365. [Google Scholar] [CrossRef]
- Zhao, J.; Qi, Y.F.; Yu, Y.R. STAT3: A key regulator in liver fibrosis. Ann. Hepatol. 2021, 21, 100224. [Google Scholar] [CrossRef]
- Inzaugarat, M.E.; Johnson, C.D.; Holtmann, T.M.; McGeough, M.D.; Trautwein, C.; Papouchado, B.G.; Schwabe, R.; Hoffman, H.M.; Wree, A.; Feldstein, A.E. NLR family pyrin domain-containing 3 inflammasome activation in hepatic stellate cells induces liver fibrosis in mice. Hepatology 2019, 69, 845–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.L.; Xu, D.; She, L.L.; Wang, Z.X.; Yang, N.; Sun, R.B.; Zhang, Y.R.; Yan, C.X.; Wei, Q.L.; Aa, J.Y.; et al. Silybin inhibits NLRP3 inflammasome assembly through the NAD+/SIRT2 pathway in mice with nonalcoholic fatty liver disease. FASEB J. 2018, 32, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Qin, X.; Zhang, Y.C.; Qiu, P.; Wang, L.G.; Zha, W.L.; Ren, J. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc. Diagn. Ther. 2020, 10, 752–769. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.; Liu, H.Y.; Wu, S.F.; Huang, R.M.; Tang, Z.X.; Zhang, N.; Hu, L.M. Curcumin alleviates arsenic trioxide-induced inflammation and pyroptosis via the NF-κB/NLRP3 signaling pathway in the hypothalamus of ducks. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wu, J.; Ye, B.; Wang, Q.; Xie, X.D.; Shen, H. Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement. Altern. Med. 2016, 16, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.M.; Xu, R.; Huang, X.Y.; Cheng, S.M.; Huang, M.F.; Yue, H.Y.; Wang, X.; Zou, Y.; Lu, A.P.; Liu, D.Y. Curcumin suppressed activation of dendritic cells via JAK/STAT/SOCS signal in mice with experimental colitis. Front. Pharmacol. 2016, 7, 455. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.J.; Yang, H.; Liu, F.J.; Pang, Q.; Shan, A.S.; Feng, X.J. Effect of dietary curcumin supplementation on duck growth performance, antioxidant capacity and breast meat quality. Foods 2021, 10, 2981. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Wang, Q.; Zhang, X.; Yang, X.; Shi, Y.; Li, Y.; Song, M. Curcumin Alleviates Aflatoxin B1-Induced Liver Pyroptosis and Fibrosis by Regulating the JAK2/NLRP3 Signaling Pathway in Ducks. Foods 2023, 12, 1006. https://doi.org/10.3390/foods12051006
Cui Y, Wang Q, Zhang X, Yang X, Shi Y, Li Y, Song M. Curcumin Alleviates Aflatoxin B1-Induced Liver Pyroptosis and Fibrosis by Regulating the JAK2/NLRP3 Signaling Pathway in Ducks. Foods. 2023; 12(5):1006. https://doi.org/10.3390/foods12051006
Chicago/Turabian StyleCui, Yilong, Qi Wang, Xuliang Zhang, Xu Yang, Yun Shi, Yanfei Li, and Miao Song. 2023. "Curcumin Alleviates Aflatoxin B1-Induced Liver Pyroptosis and Fibrosis by Regulating the JAK2/NLRP3 Signaling Pathway in Ducks" Foods 12, no. 5: 1006. https://doi.org/10.3390/foods12051006




