Effect of Sucrose on the Formation of Advanced Glycation End-Products of Ground Pork during Freeze–Thaw Cycles and Subsequent Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation
2.3. Heat Treatment
2.4. Determination of Trichloroacetic Acid (TCA)-Soluble Peptides
2.5. Detection of Schiff bases
2.6. Extraction and Detection of GO and MGO
2.7. Extraction and Detection of CML and CEL
2.8. Data Analysis
3. Results and Discussion
3.1. Changes of TCA-Soluble Peptides in Ground Pork during Freeze–Thaw Cycles and Subsequent Heating
3.2. Changes of Schiff Bases in Ground Pork during Freeze–Thaw Cycles and Subsequent Heating
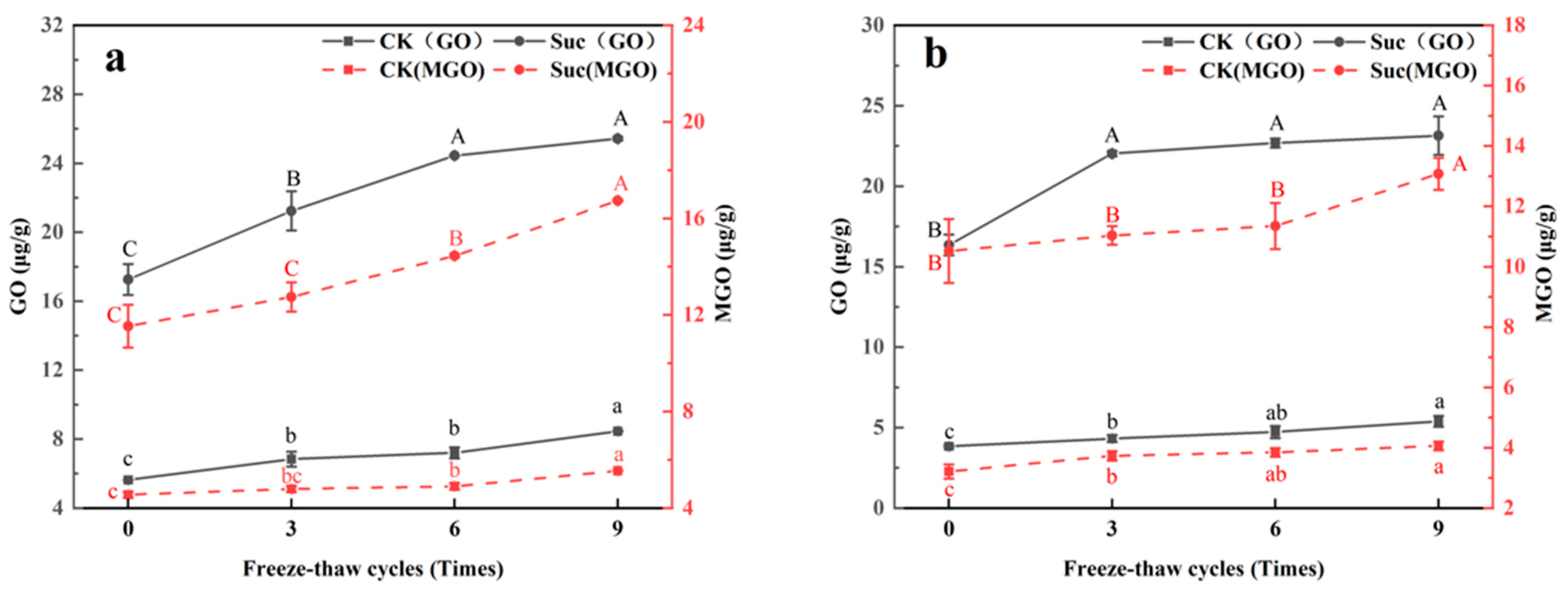
3.3. Changes of Dicarbonyl Compounds (GO, MGO) in Ground Pork during Freeze–Thaw Cycles and Subsequent Heating
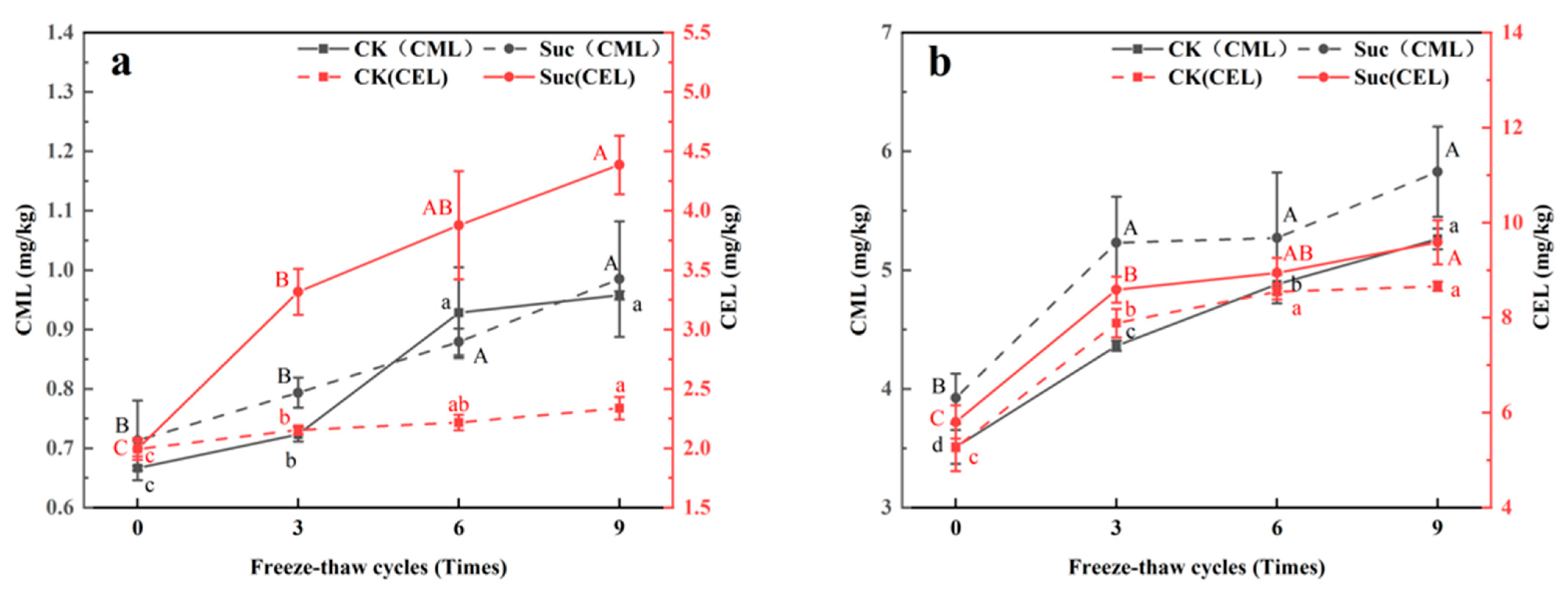
3.4. Changes of CML and CEL in Ground Pork during Freeze–Thaw Cycles and Subsequent Heating
3.5. Correlation Analysis between Freeze–Thaws (FT), TCA-Soluble Peptides, Schiff Bases, GO, MGO, CML and CEL Indicators
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, X.; Luo, X.; Zhu, L.; Mao, Y.; Lu, X.; Chen, X.; Hopkins, D.L.; Zhang, Y. Effect of medium voltage electrical stimulation and prior ageing on beef shear force during superchilled storage. Meat Sci. 2021, 172, 108320. [Google Scholar] [CrossRef]
- Du, X.; Chang, P.; Tian, J.; Kong, B.; Sun, F.; Xia, X. Effect of ice structuring protein on the quality, thermal stability and oxidation of mirror carp (Cyprinus carpio L.) induced by freeze-thaw cycles. LWT 2020, 124, 109140. [Google Scholar] [CrossRef]
- Li, T.; Niu, L.; Li, X.; Wang, F.; Huang, Y.; Liu, Y. Formation of advanced glycation end-products in silver carp (Hypophthalmichthys molitrix) surimi products during heat treatment as affected by freezing-thawing cycles. Food Chem. 2022, 395, 133612. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Wang, J.; Li, X.; Li, J.; Cui, F.; Yi, S.; Xu, Y.; Zhu, W.; Mi, H. Effects of ultrasound-assisted freezing on the quality of large yellow croaker (Pseudosciaena crocea) subjected to multiple freeze-thaw cycles. Food Chem. 2023, 404, 134530. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.K.; Rasco, B.A.; Tang, J.; Sablani, S.S. State/phase transitions, ice recrystallization, and quality changes in frozen foods subjected to temperature fluctuations. Food Eng. Rev. 2020, 12, 421–451. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, T.; Sun, D.-W. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Lin, H.; Lai, K.; Zhang, J.; Wang, F.; Liu, Y.; Rasco, B.A.; Huang, Y. Heat-induced formation of advanced glycation end-products in ground pork as affected by the addition of acetic acid or citric acid and the storage duration prior to the heat treatments. Food Chem. X 2022, 15, 100387. [Google Scholar] [CrossRef]
- Bosch, L.; Alegria, A.; Farre, R.; Clemente, G. Fluorescence and color as markers for the Maillard reaction in milk–cereal based infant foods during storage. Food Chem. 2007, 105, 1135–1143. [Google Scholar] [CrossRef]
- Kong, S.; Chu, F.; Huang, Y.; Niu, L.; Lai, K. Effects of salt concentrations on the advanced glycation end-products in dried salted spanish mackerel fillets during storage. J. Food Meas. Charact. 2022, 16, 3469–3476. [Google Scholar] [CrossRef]
- Chen, H.; Kong, B.; Guo, Y.; Xia, X.; Diao, X.; Li, P. The effectiveness of cryoprotectants in inhibiting multiple freeze-thaw-induced functional andrheological changes in the myofibrillar proteins of common carp (Cyprinus carpio) surimi. Food Biophys. 2013, 8, 302–310. [Google Scholar] [CrossRef]
- Shi, H.; Qin, R.; Wu, R.; Rong, J.; Jia, C.; Liu, R. Effect of cryoprotectants on the formation of advanced glycation end products and acrylamide in fried fish cakes. Food Biosci. 2021, 44, 101433. [Google Scholar] [CrossRef]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Fan, Y.; Huang, Y. Combination effects of salts and cold storage on the formation of protein-bound N(varepsilon)-(carboxymethyl)lysine and N(varepsilon)-(carboxyethyl)lysine in raw and subsequently commercially sterilized ground pork. Food Chem. 2018, 264, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, J.; Huang, Y.; Wang, F.; Liu, Y.; Niu, L. Effects of acetic acid and citric acid on quality properties of ground pork during storage and subsequent commercial sterilization. J. Food Meas. Charact. 2022, 17, 155–166. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Renerre, M.; Dumont, F.; Gatellier, P. Antioxidant enzyme activities in beef in relation to oxidation of lipid and myoglobin. J. Meat Sci. 1996, 43, 111–121. [Google Scholar] [CrossRef]
- Li, L.; Kong, S.; Liu, Y.; Huang, Y.; Li, Y.; Lai, K. Effects of acetic acid, ethanol, and sodium chloride on the formation of Nε-carboxymethyllysine, Nε-carboxyethyllysine and their precursors in commercially sterilized pork. J. Food Meas. Charact. 2021, 15, 5337–5344. [Google Scholar] [CrossRef]
- Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Formation of advanced glycation endproducts in ground beef under pasteurisation conditions. Food Chem. 2015, 172, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Yu, H.; Zhang, L.; Zhao, Q.; Lai, K.; Liu, Y.; Huang, Y. Advanced glycation end-products in raw and commercially sterilized pork tenderloin and offal. J. Food Compos. Anal. 2022, 112, 104681. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, Y.H.B.; Puolanne, E.; Ertbjerg, P. Role of freezing-induced myofibrillar protein denaturation in the generation of thaw loss: A review. Meat Sci. 2022, 190, 108841. [Google Scholar] [CrossRef]
- Ni, D.; Chen, Z.; Tian, Y.; Xu, W.; Zhang, W.; Kim, B.G.; Mu, W. Comprehensive utilization of sucrose resources via chemical and biotechnological processes: A review. Biotechnol. Adv. 2022, 60, 107990. [Google Scholar] [CrossRef]
- Parvathy, U.; George, S. Influence of cryoprotectant levels on storage stability of surimi from Nemipterus japonicus and quality of surimi-based products. J. Food Sci. Technol. 2014, 51, 982–987. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.-M.; Li, D.-Y.; Liu, Z.-Q.; Liu, Y.-X.; Zhou, J.-Z.; Zhang, M.; Zhou, D.-Y.; Zhu, B.-W. Effects of heat treatments on texture of abalone muscles and its mechanism. Food Biosci. 2021, 44, 101402. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gursul Aktag, I.; Gokmen, V. Multiresponse kinetic modelling of alpha-dicarbonyl compounds formation in fruit juices during storage. Food Chem. 2020, 320, 126620. [Google Scholar] [CrossRef]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Fan, Y.; Huang, Y. Formation of advanced glycation end-products in fish muscle during heating: Relationship with fish freshness. J. Food Compos. Anal. 2017, 63, 133–138. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Sen, D.; Gokmen, V. Kinetic modeling of Maillard and caramelization reactions in sucrose-rich and low moisture foods applied for roasted nuts and seeds. Food Chem. 2022, 395, 133583. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Santos, S.A.O.; Silvestre, A.J.D.; Barros, A.S.; Ferreira, A.C.S.; Silva, A.M.S. Quantification of 3-deoxyglucosone (3DG) as an aging marker in natural and forced aged wines. J. Food Compos. Anal. 2016, 50, 70–76. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Chen, X.-M.; Kitts, D.D. Identification and quantification of α-dicarbonyl compounds produced in different sugar-amino acid Maillard reaction model systems. Food Res. Int. 2011, 44, 2775–2782. [Google Scholar] [CrossRef]
- Wu, R.; Jiang, Y.; Qin, R.; Shi, H.; Jia, C.; Rong, J.; Liu, R. Study of the formation of food hazard factors in fried fish nuggets. Food Chem. 2022, 373, 131562. [Google Scholar] [CrossRef] [PubMed]
- Luan, A.; Wang, C.; Kevin, L.; Yu, J.; Wang, F.; Liu, Y.; Li, X.; Li, Y. Effects of silver carp drying process on quality and content of advanced glycation end products. Food Mach. 2022, 38, 13–20. [Google Scholar] [CrossRef]
| Group | Freeze–Thaw Cycle/Times | TCA-Soluble Peptides (μmol/g) | |
|---|---|---|---|
| Raw | Cooked | ||
| CK | 0 | 1.73 ± 0.03 d | 1.92 ± 0.05 c |
| 3 | 1.93 ± 0.01 c | 2.11 ± 0.01 b | |
| 6 | 1.99 ± 0.01 b | 2.15 ± 0.03 ab | |
| 9 | 2.11 ± 0.01 a | 2.23 ± 0.02 a | |
| Suc | 0 | 1.93 ±0.02 C | 2.02 ± 0.02 C |
| 3 | 2.00 ± 0.02 BC | 2.05 ± 0.02 C | |
| 6 | 2.03 ± 0.02 B | 2.11 ± 0.01 B | |
| 9 | 2.13 ± 0.04 A | 2.25 ± 0.01 A | |
| Group | Freeze–Thaw Cycle/Times | Fluorescence (a.u.) | |
|---|---|---|---|
| Raw | Cooked | ||
| CK | 0 | 122.86 ± 11.06 a | 368.97 ± 21.92 c |
| 3 | 130.26 ± 3.16 a | 411.10 ± 20.42 bc | |
| 6 | 131.97 ± 3.80 b | 441.66 ± 18.69 b | |
| 9 | 131.68 ± 8.53 a | 453.41 ± 25.77 a | |
| Suc | 0 | 140.91 ± 9.07 A | 335.26 ± 20.50 B |
| 3 | 140.79 ± 13.55 A | 358.97 ± 17.79 AB | |
| 6 | 141.44 ± 0.70 A | 387.22 ± 18.86 A | |
| 9 | 141.03 ± 12.68 A | 385.53 ± 8.42 A | |
| Group | Index | FT | TCA- Soluble Peptides | Schiff Bases | GO | MGO | CML | CEL |
|---|---|---|---|---|---|---|---|---|
| CK | ||||||||
| FT | 1 | |||||||
| TCA-soluble peptides | 0.970 ** | 1 | ||||||
| Schiff bases | 0.491 | 0.484 | 1 | |||||
| GO | 0.968 ** | 0.947 ** | 0.480 | 1 | ||||
| MGO | 0.736 * | 0.670 | 0.610 | 0.767 * | 1 | |||
| CML | 0.934 ** | 0.869 ** | 0.403 | 0.882 ** | 0.534 | 1 | ||
| CEL | 0.776 * | 0.803 * | 0.424 | 0.824 * | 0.407 | 0.762 * | 1 | |
| Suc | ||||||||
| FT | 1 | |||||||
| TCA-soluble peptides | 0.952 ** | 1 | ||||||
| Schiff bases | 0.015 | −0.124 | 1 | |||||
| GO | 0.959 ** | 0.893 ** | −0.113 | 1 | ||||
| MGO | 0.972 ** | 0.929 ** | −0.123 | 0.929 ** | 1 | |||
| CML | 0.916 ** | 0.799 * | 0.054 | 0.892 ** | 0.944 ** | 1 | ||
| CEL | 0.947 ** | 0.926 ** | 0.022 | 0.954 ** | 0.876 ** | 0.802 * | 1 |
| Group | Index | FT | TCA- Soluble Peptides | Schiff Bases | GO | MGO | CML | CEL |
|---|---|---|---|---|---|---|---|---|
| CK | ||||||||
| FT | 1 | |||||||
| TCA-soluble peptides | 0.942 ** | 1 | ||||||
| Schiff bases | 0.879 ** | 0.867 ** | 1 | |||||
| GO | 0.851 ** | 0.774 * | 0.837 ** | 1 | ||||
| MGO | 0.751 * | 0.788 * | 0.651 | 0.828 * | 1 | |||
| CML | 0.882 ** | 0.941 ** | 0.846 ** | 0.746 * | 0.751 * | 1 | ||
| CEL | 0.869 ** | 0.924 ** | 0.887 ** | 0.698 | 0.727 * | 0.968 ** | 1 | |
| Suc | ||||||||
| FT | 1 | |||||||
| TCA-soluble peptides | 0.942 ** | 1 | ||||||
| Schiff bases | 0.816 * | 0.693 | 1 | |||||
| GO | 0.843 ** | 0.685 | 0.837 ** | 1 | ||||
| MGO | 0.821 * | 0.922 ** | 0.737 * | 0.654 | 1 | |||
| CML | 0.830 * | 0.729 * | 0.844 ** | 0.860 ** | 0.726 * | 1 | ||
| CEL | 0.783 * | 0.718 * | 0.622 | 0.859 ** | 0.691 | 0.702 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, F.; Lin, Y.; Huang, Y.; Niu, L.; Lai, K. Effect of Sucrose on the Formation of Advanced Glycation End-Products of Ground Pork during Freeze–Thaw Cycles and Subsequent Heat Treatment. Foods 2023, 12, 1024. https://doi.org/10.3390/foods12051024
Chu F, Lin Y, Huang Y, Niu L, Lai K. Effect of Sucrose on the Formation of Advanced Glycation End-Products of Ground Pork during Freeze–Thaw Cycles and Subsequent Heat Treatment. Foods. 2023; 12(5):1024. https://doi.org/10.3390/foods12051024
Chicago/Turabian StyleChu, Fuyu, Yi Lin, Yiqun Huang, Lihong Niu, and Keqiang Lai. 2023. "Effect of Sucrose on the Formation of Advanced Glycation End-Products of Ground Pork during Freeze–Thaw Cycles and Subsequent Heat Treatment" Foods 12, no. 5: 1024. https://doi.org/10.3390/foods12051024






