Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations
Abstract
:1. Introduction
2. Sustainable Food Packaging Design
- A focus on real demand and problems and try to find solutions with social, environmental, and economic benefits altogether.
- A shift in the design dynamics from the application requirements up, thinking in terms of functions and services rather than the product itself.
- Consideration of realistic and updated life-cycle and process thinking, having an integrated view of the supply chain, thus taking into account the product manufacturing, distribution, consumption and end-of-life.
- Inclusion of users, stakeholders, and different experts in the design process as much as possible.
- Research and innovation need to be grounded on justifiable priorities within the available time frame and scope of the project.
3. Strategies to Improve Barrier and Hydrophobicity Properties for Food Packaging
3.1. Enhanced Gas and Water Vapor Barrier Properties
3.2. Enhanced Hydrophobicity
3.3. Enhanced UV-Light Barrier Characteristics
4. Active and Intelligent Food Packaging
4.1. Active Materials and Packaging Systems
4.2. Intelligent and Smart Food Packaging
5. Biobased Packaging
5.1. Bioadhesives for Food Packaging
5.2. Biobased Inks and Dyes in the Food Industry
| Color | Classification | Source | Example | Reference |
|---|---|---|---|---|
| Yellow/ Orange/ Red | Curcumin | Plant/Vegetable | Turmeric (Curcuma longa L.) | [348] |
| Carotenoids | Plant/Vegetable | Carrot (Daucus carota L.), Annatto (Bixa orellana), Tomato (Solanum lycopersicum), Paprika (Capsicum annuum L.), petals of marigold (Tagetes erecta L.) | [349,350] | |
| Aryl carotenoids | Microorganisms | Brevibacterium linens, Streptomyces mediolani, Mycobacterium aurum | [351] | |
| Red/Pink | Betalains | Plant/Vegetable | Beetroot (Beta vulgaris L.), Opuntia lasiacantha | [339,349] |
| Carminic acid | Microorganisms | Cochineal (Dactylopius coccus) | [350] | |
| Anthocyanins | Plant/Vegetable | Hibiscus rosa sinensis flowers | [352] | |
| Blue/ Purple | Anthocyanins | Plant/Vegetable | Grapes (Vitus labruscana L.), red cabbage (Brassica oleracea var. capitata f. rubra), cherry (Prunus cerasus), blueberry (Vaccinium sect. Cyanococcus), red onion skin (Allium cepa L.), Beetroot (Beta vulgaris L.) | [323,326,350] |
| Tyrian purple (6,6′-dibromoíndigo) | Animal | Mollusks Bolinus brandaris | [349] | |
| Ultramarine Blue | Mineral | Lapis lazuli | [349] | |
| Green | Chlorophylls | Plant/Vegetable | Spinach (Spinacia oleracea), kiwi pomace (Actinidiaceae), green beans (Phaseolus vulgaris), grass, alfalfa (Medicago sativa) | [57,350] |
| Terre-Verte (Green Earth) | Mineral | Mixture of hydrosilicates of Fe, Mg, Al, and K (gluconite and celadenite) but other minerals are likely to be present | [349] | |
| Malachite | Copper carbonate hydroxide |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN. World Population Prospects 2022—Summary of Results; UN: New York, NY, USA, 2022. [Google Scholar]
- FAO. The Future of Food and Agriculture Trends and Challenges; FAO: Roma, Italy, 2017. [Google Scholar]
- Chakori, S.; Aziz, A.A.; Smith, C.; Dargusch, P. Untangling the Underlying Drivers of the Use of Single-Use Food Packaging. Ecol. Econ. 2021, 185, 107063. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased Materials for Active Food Packaging: A Review. Food Hydrocoll 2022, 125, 107419. [Google Scholar] [CrossRef]
- Wohner, B.; Pauer, E.; Heinrich, V.; Tacker, M. Packaging-Related Food Losses and Waste: An Overview of Drivers and Issues. Sustainability 2019, 11, 264. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Orikasa, T.; Nakamura, N.; Hayashi, K.; Yasaka, Y.; Makino, N.; Shobatake, K.; Koide, S.; Shiina, T. Determination of the Most Environmentally Friendly Packaging for Peach during Transportation by Modeling the Relationship between Food Loss Reduction and Environmental Impact. J. Food Eng. 2022, 331, 111120. [Google Scholar] [CrossRef]
- Blakeney, M. Chapter 1: Food Loss and Waste and Food Security. In Food Loss and Food Waste; Edward Elgar Publishing: Cheltenham, UK, 2019; pp. 1–26. ISBN 9781788975391. [Google Scholar]
- Bishop, G.; Styles, D.; Lens, P.N.L. Environmental Performance of Bioplastic Packaging on Fresh Food Produce: A Consequential Life Cycle Assessment. J. Clean. Prod. 2021, 317, 128377. [Google Scholar] [CrossRef]
- Kan, M.; Miller, S.A. Environmental Impacts of Plastic Packaging of Food Products. Resour. Conserv. Recycl. 2022, 180, 106156. [Google Scholar] [CrossRef]
- WEF. The New Plastics Economy–Rethinking the Future of Plastics. Industry Agenda; WEF: Geneva, Switzerland, 2016. [Google Scholar]
- Brivio, E.; Petsa, I.; McPhie, T. Single-Use Plastics: New EU Rules to Reduce Marine Litter. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_18_3927 (accessed on 23 November 2022).
- Plastics Europe. Plastics-the Facts 2022; Plastics Europe: Brussels, Belgium, 2022. [Google Scholar]
- Bandara, R.; Indunil, G.M. Food Packaging from Recycled Papers: Chemical, Physical, Optical Properties and Heavy Metal Migration. Heliyon 2022, 8, e10959. [Google Scholar] [CrossRef]
- Hosen, M.D.; Hossain, M.S.; Islam, M.A.; Haque, A.N.M.A.; Naebe, M. Utilisation of Natural Wastes: Water-Resistant Semi-Transparent Paper for Food Packaging. J. Clean. Prod. 2022, 364, 132665. [Google Scholar] [CrossRef]
- Markevičiūtė, Z.; Varžinskas, V. Plant-Origin Feedstock Applications in Fully Green Food Packaging: The Potential for Tree-Free Paper and Plant-Origin Bio-Plastics in the Baltic Sea Region. Sustainability 2022, 14, 7393. [Google Scholar] [CrossRef]
- Garrido-Romero, M.; Aguado, R.; Moral, A.; Brindley, C.; Ballesteros, M. From Traditional Paper to Nanocomposite Films: Analysis of Global Research into Cellulose for Food Packaging. Food Packag. Shelf Life 2022, 31, 100788. [Google Scholar] [CrossRef]
- Gonçalves de Moura, I.; Vasconcelos de Sá, A.; Lemos Machado Abreu, A.S.; Alves Machado, A.V. Bioplastics from Agro-Wastes for Food Packaging Applications. In Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 223–263. [Google Scholar]
- Mostafa, N.A.; Farag, A.A.; Abo-dief, H.M.; Tayeb, A.M. Production of Biodegradable Plastic from Agricultural Wastes. Arab. J. Chem. 2018, 11, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of Agricultural Wastes Liquefaction Process and Preparing Bio-Based Polyurethane Foams by the Obtained Polyols. Ind. Crops Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Yun, D.; Liu, J. Recent Advances on the Development of Food Packaging Films Based on Citrus Processing Wastes: A Review. J. Agric. Food Res. 2022, 9, 100316. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Otles, S.; Kartal, C. Food Waste Valorization. In Sustainable Food Systems from Agriculture to Industry; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 371–399. [Google Scholar]
- Six, L.; Velghe, F.; Verstichel, S.; de Meester, S. Sustainability Considerations on the Valorization of Organic Waste. In Biotransformation of Agricultural Waste and By-Products; Poltronieri, P., D’Urso, O.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 287–307. ISBN 9780128036488. [Google Scholar]
- Jõgi, K.; Bhat, R. Valorization of Food Processing Wastes and By-Products for Bioplastic Production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Vilaplana, F.; Strömberg, E.; Karlsson, S. Environmental and Resource Aspects of Sustainable Biocomposites. Polym. Degrad. Stab. 2010, 95, 2147–2161. [Google Scholar] [CrossRef]
- Springle, N.; Li, B.; Soma, T.; Shulman, T. The Complex Role of Single-Use Compostable Bioplastic Food Packaging and Foodservice Ware in a Circular Economy: Findings from a Social Innovation Lab. Sustain. Prod. Consum. 2022, 33, 664–673. [Google Scholar] [CrossRef]
- Gioia, C.; Giacobazzi, G.; Vannini, M.; Totaro, G.; Sisti, L.; Colonna, M.; Marchese, P.; Celli, A. End of Life of Biodegradable Plastics: Composting versus Re/Upcycling. ChemSusChem 2021, 14, 4167–4175. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of Biodegradable Plastics: New Problem or Solution to Solve the Global Plastic Pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Wang, G.X.; Huang, D.; Ji, J.H.; Völker, C.; Wurm, F.R. Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution. Adv. Sci. 2021, 8, 2001121. [Google Scholar] [CrossRef]
- Suwanmanee, U.; Varabuntoonvit, V.; Chaiwutthinan, P.; Tajan, M.; Mungcharoen, T.; Leejarkpai, T. Life Cycle Assessment of Single Use Thermoform Boxes Made from Polystyrene (PS), Polylactic Acid, (PLA), and PLA/Starch: Cradle to Consumer Gate. Int. J. Life Cycle Assess. 2013, 18, 401–417. [Google Scholar] [CrossRef]
- Saibuatrong, W.; Cheroennet, N.; Suwanmanee, U. Life Cycle Assessment Focusing on the Waste Management of Conventional and Bio-Based Garbage Bags. J. Clean. Prod. 2017, 158, 319–334. [Google Scholar] [CrossRef]
- Cheroennet, N.; Pongpinyopap, S.; Leejarkpai, T.; Suwanmanee, U. A Trade-off between Carbon and Water Impacts in Bio-Based Box Production Chains in Thailand: A Case Study of PS, PLAS, PLAS/Starch, and PBS. J. Clean. Prod. 2017, 167, 987–1001. [Google Scholar] [CrossRef]
- Banar, M.; Cokaygil, Z. A Comparative Life Cycle Analysis of Two Different Juice Packages. Environ. Eng. Sci. 2008, 25, 549–555. [Google Scholar] [CrossRef]
- Tsiropoulos, I.; Faaij, A.P.C.; Lundquist, L.; Schenker, U.; Briois, J.F.; Patel, M.K. Life Cycle Impact Assessment of Bio-Based Plastics from Sugarcane Ethanol. J. Clean. Prod. 2015, 90, 114–127. [Google Scholar] [CrossRef]
- Civancik-Uslu, D.; Puig, R.; Hauschild, M.; Fullana-i-Palmer, P. Life Cycle Assessment of Carrier Bags and Development of a Littering Indicator. Sci. Total Environ. 2019, 685, 621–630. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kassidi, E. Life Cycle Assessment Applied to Egg Packaging Made from Polystyrene and Recycled Paper. J. Clean. Prod. 2003, 11, 549–559. [Google Scholar] [CrossRef]
- Chen, L.; Pelton, R.E.O.; Smith, T.M. Comparative Life Cycle Assessment of Fossil and Bio-Based Polyethylene Terephthalate (PET) Bottles. J. Clean. Prod. 2016, 137, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Abejón, R.; Bala, A.; Vázquez-Rowe, I.; Aldaco, R.; Fullana-i-Palmer, P. When Plastic Packaging Should Be Preferred: Life Cycle Analysis of Packages for Fruit and Vegetable Distribution in the Spanish Peninsular Market. Resour. Conserv. Recycl. 2020, 155, 104666. [Google Scholar] [CrossRef]
- Ferrara, C.; Migliaro, V.; Ventura, F.; de Feo, G. An Economic and Environmental Analysis of Wine Packaging Systems in Italy: A Life Cycle (LC) Approach. Sci. Total Environ. 2023, 857, 159323. [Google Scholar] [CrossRef]
- Casson, A.; Giovenzana, V.; Frigerio, V.; Zambelli, M.; Beghi, R.; Pampuri, A.; Tugnolo, A.; Merlini, A.; Colombo, L.; Limbo, S.; et al. Beyond the Eco-Design of Case-Ready Beef Packaging: The Relationship between Food Waste and Shelf-Life as a Key Element in Life Cycle Assessment. Food Packag. Shelf Life 2022, 34, 100943. [Google Scholar] [CrossRef]
- Razza, F.; Innocenti, F.D.; Dobon, A.; Aliaga, C.; Sanchez, C.; Hortal, M. Environmental Profile of a Bio-Based and Biodegradable Foamed Packaging Prototype in Comparison with the Current Benchmark. J. Clean. Prod. 2015, 102, 493–500. [Google Scholar] [CrossRef]
- Bishop, G.; Styles, D.; Lens, P.N.L. Environmental Performance Comparison of Bioplastics and Petrochemical Plastics: A Review of Life Cycle Assessment (LCA) Methodological Decisions. Resour. Conserv. Recycl. 2021, 168, 105451. [Google Scholar] [CrossRef]
- Accorsi, R.; Battarra, I.; Guidani, B.; Manzini, R.; Ronzoni, M.; Volpe, L. Augmented Spatial LCA for Comparing Reusable and Recyclable Food Packaging Containers Networks. J. Clean. Prod. 2022, 375, 134027. [Google Scholar] [CrossRef]
- Briassoulis, D.; Pikasi, A.; Hiskakis, M. Recirculation Potential of Post-Consumer /Industrial Bio-Based Plastics through Mechanical Recycling—Techno-Economic Sustainability Criteria and Indicators. Polym. Degrad. Stab. 2021, 183, 109217. [Google Scholar] [CrossRef]
- Briassoulis, D.; Pikasi, A.; Hiskakis, M. End-of-Waste Life: Inventory of Alternative End-of-Use Recirculation Routes of Bio-Based Plastics in the European Union Context. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1835–1892. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Urbina, L.; Eceiza, A.; Gabilondo, N.; Corcuera, M.Á.; Retegi, A. Valorization of Apple Waste for Active Packaging: Multicomponent Polyhydroxyalkanoate Coated Nanopapers with Improved Hydrophobicity and Antioxidant Capacity. Food Packag. Shelf Life 2019, 21, 100356. [Google Scholar] [CrossRef]
- Bibi, F.; Guillaume, C.; Gontard, N.; Sorli, B. A Review: RFID Technology Having Sensing Aptitudes for Food Industry and Their Contribution to Tracking and Monitoring of Food Products. Trends Food Sci. Technol. 2017, 62, 91–103. [Google Scholar] [CrossRef]
- Gustavo, J.U.; Pereira, G.M.; Bond, A.J.; Viegas, C.V.; Borchardt, M. Drivers, Opportunities and Barriers for a Retailer in the Pursuit of More Sustainable Packaging Redesign. J. Clean. Prod. 2018, 187, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of Poly(Lactic) Acid and Starch for Biodegradable Food Packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for Food Packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research Advances in Chemical Modifications of Starch for Hydrophobicity and Its Applications: A Review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef] [PubMed]
- Ashter, S.A. Additives and Modifiers for Biopolymers. In Introduction to Bioplastics Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–178. [Google Scholar]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in Research and Development of Bioplastic for Food Packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased Composites from Agro-Industrial Wastes and by-Products. Emergent Mater. 2022, 5, 873–921. [Google Scholar] [CrossRef]
- Chakrapani, G.; Zare, M.; Ramakrishna, S. Biomaterials from the Value-Added Food Wastes. Bioresour. Technol. Rep. 2022, 19, 101181. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Mazzocchi, C.; Esti, M. Natural Colorants from Vegetable Food Waste: Recovery, Regulatory Aspects, and Stability—A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2715–2737. [Google Scholar] [CrossRef]
- Ding, X.; Dai, R.; Chen, H.; Shan, Z. Gelatin as Green Adhesive for the Preparation of a Multifunctional Biobased Cryogel Derived from Bamboo Industrial Waste. Carbohydr. Polym 2021, 255, 117340. [Google Scholar] [CrossRef]
- Song, Y.; Chen, S.; Chen, Y.; Xu, Y.; Xu, F. Biodegradable and Transparent Films with Tunable UV-Blocking Property from Lignocellulosic Waste by a Top-down Approach. Cellulose 2021, 28, 8629–8640. [Google Scholar] [CrossRef]
- Espinosa, E.; Rincón, E.; Morcillo-Martín, R.; Rabasco-Vílchez, L.; Rodríguez, A. Orange Peel Waste Biorefinery in Multi-Component Cascade Approach: Polyphenolic Compounds and Nanocellulose for Food Packaging. Ind. Crops Prod. 2022, 187, 115413. [Google Scholar] [CrossRef]
- Castillo, L.A.; López, O.; Ninago, M.D.; Versino, F.; Barbosa, S.E.; García, M.A.; Villar, M.A. Composites and Nanocomposites Based on Starches. Effect of Mineral and Organic Fillers on Processing, Structure, and Final Properties of Starch. In Starch-Based Materials in Food Packaging; Villar, M.A., Barbosa, S.E., García, M.A., Castillo, L.A., López, O., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 125–151. ISBN 9780128122570. [Google Scholar]
- Versino, F.; López, O.V.; García, M.A. Green Biocomposites for Packaging Applications. In Biocomposite Materials Design and Mechanical Properties Characterization; Sultan, M.T.H., Majid, M.S.A., Azmi, M.R.M.J., Iskandar, A., Saba, N., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 1–30. ISBN 9789813340916. [Google Scholar]
- European Commission. Ecodesign Your Future: How Ecodesign Can Help the Environment by Making Products Smarter. Available online: https://op.europa.eu/en/publication-detail/-/publication/4d42d597-4f92-4498-8e1d-857cc157e6db (accessed on 13 December 2022).
- Verghese, K.; Lewis, H.; Lockrey, S.; Williams, H. Packaging’s Role in Minimizing Food Loss and Waste Across the Supply Chain. Packag. Technol. Sci. 2015, 28, 603–620. [Google Scholar] [CrossRef]
- Wikström, F.; Verghese, K.; Auras, R.; Olsson, A.; Williams, H.; Wever, R.; Grönman, K.; Kvalvåg Pettersen, M.; Møller, H.; Soukka, R. Packaging Strategies That Save Food: A Research Agenda for 2030. J. Ind. Ecol. 2019, 23, 532–540. [Google Scholar] [CrossRef]
- Heller, M.C.; Selke, S.E.M.; Keoleian, G.A. Mapping the Influence of Food Waste in Food Packaging Environmental Performance Assessments. J. Ind. Ecol. 2019, 23, 480–495. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhang, D.; Zhou, R.; Zhu, R.; Fei, P.; Zhu, Z.Z.; Cheng, S.Y.; Ding, W.P. Hydrophobic Interface Starch Nanofibrous Film for Food Packaging: From Bioinspired Design to Self-Cleaning Action. J. Agric. Food Chem. 2021, 69, 5067–5075. [Google Scholar] [CrossRef]
- Pardo-Figuerez, M.; López-Córdoba, A.; Torres-Giner, S.; Lagaron, J.M. Superhydrophobic Bio-Coating Made by Co-Continuous Electrospinning and Electrospraying Polyethylene Terephthalate Films Proposed as Easy Emptying Transparent Food Packaging. Coatings 2018, 8, 364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bi, J.; Wang, S.; Cao, Q.; Li, Y.; Zhou, J.; Zhu, B.W. Functional Food Packaging for Reducing Residual Liquid Food: Thermo-Resistant Edible Super-Hydrophobic Coating from Coffee and Beeswax. J. Colloid Interface Sci. 2019, 533, 742–749. [Google Scholar] [CrossRef]
- Lindh, H.; Williams, H.; Olsson, A.; Wikström, F. Elucidating the Indirect Contributions of Packaging to Sustainable Development: A Terminology of Packaging Functions and Features. Packag. Technol. Sci. 2016, 29, 225–246. [Google Scholar] [CrossRef]
- Wikström, F.; Williams, H.; Venkatesh, G. The Influence of Packaging Attributes on Recycling and Food Waste Behaviour—An Environmental Comparison of Two Packaging Alternatives. J. Clean. Prod. 2016, 137, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; de Meulenaer, B. Intelligent Food Packaging: The next Generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Mendy, T.K.; Misran, A.; Mahmud, T.M.M.; Ismail, S.I. Application of Aloe Vera Coating Delays Ripening and Extend the Shelf Life of Papaya Fruit. Sci. Hortic. 2019, 246, 769–776. [Google Scholar] [CrossRef]
- Agamuthu, P.; Visvanathan, C. Extended Producers’ Responsibility Schemes for Used Beverage Carton Recycling. Waste Manag. Res. 2014, 32, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geueke, B.; Groh, K.; Muncke, J. Food Packaging in the Circular Economy: Overview of Chemical Safety Aspects for Commonly Used Materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Palombini, F.L.; Cidade, M.K.; de Jacques, J.J. How Sustainable Is Organic Packaging? A Design Method for Recyclability Assessment via a Social Perspective: A Case Study of Porto Alegre City (Brazil). J. Clean. Prod. 2017, 142, 2593–2605. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Körner, I.; Redemann, K.; Stegmann, R. Behaviour of Biodegradable Plastics in Composting Facilities. Waste Manag. 2005, 25, 409–415. [Google Scholar] [CrossRef]
- Changwichan, K.; Silalertruksa, T.; Gheewala, S.H. Eco-Efficiency Assessment of Bioplastics Production Systems and End-of-Life Options. Sustainability 2018, 10, 952. [Google Scholar] [CrossRef] [Green Version]
- López, O.V.; Versino, F.; Villar, M.A.; García, M.A. Agro-Industrial Residue from Starch Extraction of Pachyrhizus Ahipa as Filler of Thermoplastic Corn Starch Films. Carbohydr. Polym. 2015, 134, 324–332. [Google Scholar] [CrossRef]
- Versino, F.; López, O.; García, M.A. Sunflower Oil Industry By-Product as Natural Filler of Biocomposite Foams for Packaging Applications. J. Polym. Environ. 2021, 29, 1869–1879. [Google Scholar] [CrossRef]
- Bascón-Villegas, I.; Pereira, M.; Espinosa, E.; Sánchez-Gutiérrez, M.; Rodríguez, A.; Pérez-Rodríguez, F. A New Eco-Friendly Packaging System Incorporating Lignocellulose Nanofibres from Agri-Food Residues Applied to Fresh-Cut Lettuce. J. Clean. Prod. 2022, 372, 133597. [Google Scholar] [CrossRef]
- dos Santos, J.W.S.; Garcia, V.A.d.S.; Venturini, A.C.; de Carvalho, R.A.; da Silva, C.F.; Yoshida, C.M.P. Sustainable Coating Paperboard Packaging Material Based on Chitosan, Palmitic Acid, and Activated Carbon: Water Vapor and Fat Barrier Performance. Foods 2022, 11, 4037. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, K.; Gundabala, V. Antimicrobial Bio-Inspired Active Packaging Materials for Shelf Life and Safety Development: A Review. Food Biosci. 2022, 48, 101730. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Riaz, S.; Khan, M.U.; Sultan, M.; Munekata, P.E.S.; Lorenzo, J.M. Biodegradable Active, Intelligent, and Smart Packaging Materials for Food Applications. Food Packag. Shelf Life 2022, 33, 100903. [Google Scholar] [CrossRef]
- Manfredi, M.; Fantin, V.; Vignali, G.; Gavara, R. Environmental Assessment of Antimicrobial Coatings for Packaged Fresh Milk. J. Clean. Prod. 2015, 95, 291–300. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable Green Packaging with Antimicrobial Functions Based on the Bioactive Compounds from Tropical Plants and Their By-Products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Zindani, D.; Kumar, K.; Davim, J.P. Sustainability Manufacturing Systems Design. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 512–518. [Google Scholar]
- Karwasz, A.; Dostatni, E.; Diakun, J.; Grajewski, D.; Wichniarek, R.; Stachura, M. Estimating the Cost of Product Recycling with the Use of Ecodesign Support System. Manag. Prod. Eng. Rev. 2016, 7, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Döhler, N.; Wellenreuther, C.; Wolf, A. Market Dynamics of Biodegradable Bio-Based Plastics: Projections and Linkages to European Policies. EFB Bioeconomy J. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Dang, S.; Chu, L. Evaluation Framework and Verification for Sustainable Container Management as Reusable Packaging. J. Bus Res. 2016, 69, 1949–1955. [Google Scholar] [CrossRef]
- Hellström, D.; Saghir, M. Packaging and Logistics Interactions in Retail Supply Chains. Packag. Technol. Sci. 2007, 20, 197–216. [Google Scholar] [CrossRef]
- Bernstad Saraiva, A.; Pacheco, E.B.A.V.; Gomes, G.M.; Visconte, L.L.Y.; Bernardo, C.A.; Simões, C.L.; Soares, A.G. Comparative Lifecycle Assessment of Mango Packaging Made from a Polyethylene/Natural Fiber-Composite and from Cardboard Material. J. Clean. Prod. 2016, 139, 1168–1180. [Google Scholar] [CrossRef]
- Meherishi, L.; Narayana, S.A.; Ranjani, K.S. Sustainable Packaging for Supply Chain Management in the Circular Economy: A Review. J. Clean. Prod. 2019, 237, 117582. [Google Scholar] [CrossRef]
- Padhi, S.S.; Pati, R.K.; Rajeev, A. Framework for Selecting Sustainable Supply Chain Processes and Industries Using an Integrated Approach. J. Clean. Prod. 2018, 184, 969–984. [Google Scholar] [CrossRef]
- Navaranjan, N.; Fletcher, G.C.; Summers, G.; Parr, R.; Anderson, R. Thermal Insulation Requirements and New Cardboard Packaging for Chilled Seafood Exports. J. Food Eng. 2013, 119, 395–403. [Google Scholar] [CrossRef]
- Yin, D.; Mi, J.; Zhou, H.; Wang, X.; Tian, H. Fabrication of Branching Poly (Butylene Succinate)/Cellulose Nanocrystal Foams with Exceptional Thermal Insulation. Carbohydr. Polym. 2020, 247, 116708. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in Barrier Coatings and Film Technologies for Achieving Sustainable Packaging of Food Products—A Review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, A.; Dar, M.; Nanda, V. Novel Food Packaging Technologies: Innovations and Future Prospective. J. Saudi Soc. Agric. Sci. 2018, 17, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Dekle, J.L.; Leads, R.R.; Hunter, R.A. Degradation of Bio-Based and Biodegradable Plastics in a Salt Marsh Habitat: Another Potential Source of Microplastics in Coastal Waters. Mar. Pollut. Bull. 2020, 160, 111518. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of Bioplastics in Natural Environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Magni, S.; Bonasoro, F.; della Torre, C.; Parenti, C.C.; Maggioni, D.; Binelli, A. Plastics and Biodegradable Plastics: Ecotoxicity Comparison between Polyvinylchloride and Mater-Bi® Micro-Debris in a Freshwater Biological Model. Sci. Total Environ. 2020, 720, 137602. [Google Scholar] [CrossRef]
- Ortega, F.; Sobral, P.; Jios, J.L.; Arce, V.B.; García, M.A. Starch Nanocomposite Films: Migration Studies of Nanoparticles to Food Simulants and Bio-Disintegration in Soil. Polymers 2022, 14, 1636. [Google Scholar] [CrossRef]
- Abe, M.M.; Branciforti, M.C.; Nallin Montagnolli, R.; Marin Morales, M.A.; Jacobus, A.P.; Brienzo, M. Production and Assessment of the Biodegradation and Ecotoxicity of Xylan- and Starch-Based Bioplastics. Chemosphere 2022, 287, 132290. [Google Scholar] [CrossRef]
- Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Rodriguez, A.; Sain, M.M. Biosynthesis, Microstructural Characterisations and Investigation of In-Vitro Mutagenic and Eco-Toxicological Response of a Novel Microbial Exopolysaccharide Based Biopolymer. J. Polym. Environ. 2018, 26, 365–374. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Bioplastics: Missing Link in the Era of Microplastics. Sci. Total Environ. 2019, 697, 134139. [Google Scholar] [CrossRef]
- Konstantoglou, A.; Folinas, D.; Fotiadis, T. Investigating Food Packaging Elements from a Consumer’s Perspective. Foods 2020, 9, 1097. [Google Scholar] [CrossRef]
- van Herpen, E.; van den Broek, E.; van Trijp, H.C.M.; Yu, T. Can a Virtual Supermarket Bring Realism into the Lab? Comparing Shopping Behavior Using Virtual and Pictorial Store Representations to Behavior in a Physical Store. Appetite 2016, 107, 196–207. [Google Scholar] [CrossRef]
- Herbes, C.; Beuthner, C.; Ramme, I. Consumer Attitudes towards Biobased Packaging—A Cross-Cultural Comparative Study. J. Clean. Prod. 2018, 194, 203–218. [Google Scholar] [CrossRef]
- Tischner, U.; Hora, M. Sustainable Electronic Product Design. In Waste Electrical and Electronic Equipment (WEEE) Handbook; Elsevier: Amsterdam, The Netherlands, 2019; pp. 443–482. ISBN 9780081021583. [Google Scholar]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [Green Version]
- Lamba, A.; Garg, V. Recent Innovations in Food Packaging: A Review. Int. J. Food Sci. Nutr. Int. 2019, 4, 123–129. [Google Scholar]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and Challenges in the Development of Bio-Based Barrier Coating Materials for Paper/Cardboard Food Packaging; a Review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Zabihzadeh Khajavi, M.; Ebrahimi, A.; Yousefi, M.; Ahmadi, S.; Farhoodi, M.; Mirza Alizadeh, A.; Taslikh, M. Strategies for Producing Improved Oxygen Barrier Materials Appropriate for the Food Packaging Sector. Food Eng. Rev. 2020, 12, 346–363. [Google Scholar] [CrossRef]
- Blanke, M.M. Reducing Ethylene Levels along the Food Supply Chain: A Key to Reducing Food Waste? J. Sci. Food Agric. 2014, 94, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Giacinti Baschetti, M.; Minelli, M. Test Methods for the Characterization of Gas and Vapor Permeability in Polymers for Food Packaging Application: A Review. Polym. Test 2020, 89, 106606. [Google Scholar] [CrossRef]
- Ameer, A.; Seleshe, S.; Kang, S.N. Effect of Modified Atmosphere Packaging Varying in CO2 and N2 Composition on Quality Characteristics of Dry Fermented Sausage during Refrigeration Storage. Food Sci. Anim. Resour. 2022, 42, 639–654. [Google Scholar] [CrossRef]
- Turan, D. Water Vapor Transport Properties of Polyurethane Films for Packaging of Respiring Foods. Food Eng. Rev. 2021, 13, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Orsuwan, A.; Bumbudsanpharoke, N.; Yoon, C.; Choi, J.; Ko, S. A Short Review of Light Barrier Materials for Food and Beverage Packaging. Korean J. Packag. Sci. Technol. 2018, 24, 141–148. [Google Scholar] [CrossRef]
- Csapó, J.; Prokisch, J.; Albert, C.; Sipos, P. Effect of UV Light on Food Quality and Safety. Acta Univ. Sapientiae Aliment. 2019, 12, 21–41. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.E.; Hannah, S. Light-Protective Packaging Materials for Foods and Beverages. In Emerging Food Packaging Technologies; Elsevier: Amsterdam, The Netherlands, 2012; pp. 303–322. [Google Scholar]
- Cassar, J.R.; Ouyang, B.; Krishnamurthy, K.; Demirci, A. Microbial Decontamination of Food by Light-Based Technologies: Ultraviolet (UV) Light, Pulsed UV Light (PUV), and UV Light-Emitting Diodes (UV-LED). In Food Safety Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–521. [Google Scholar]
- Yadav, M.; Liu, Y.-K.; Chiu, F.-C. Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application. Nanomaterials 2019, 9, 1523. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.L.; Tran, T.H.; Hao, L.T.; Jeon, H.; Koo, J.M.; Shin, G.; Hwang, D.S.; Hwang, S.Y.; Park, J.; Oh, D.X. Biorenewable, Transparent, and Oxygen/Moisture Barrier Nanocellulose/Nanochitin-Based Coating on Polypropylene for Food Packaging Applications. Carbohydr. Polym. 2021, 271, 118421. [Google Scholar] [CrossRef]
- Kim, T.; Tran, T.H.; Hwang, S.Y.; Park, J.; Oh, D.X.; Kim, B.S. Crab-on-a-Tree: All Biorenewable, Optical and Radio Frequency Transparent Barrier Nanocoating for Food Packaging. ACS Nano 2019, 13, 3796–3805. [Google Scholar] [CrossRef]
- Kiryukhin, M.; Lau, H.H.; Goh, S.H.; Teh, C.; Korzh, V.; Sadovoy, A. A Membrane Film Sensor with Encapsulated Fluorescent Dyes towards Express Freshness Monitoring of Packaged Food. Talanta 2018, 182, 187–192. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A Review of Multilayer and Composite Films and Coatings for Active Biodegradable Packaging. NPJ Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; López, D.; López, J.; Kenny, J.M. An Overview of Nanoparticles Role in the Improvement of Barrier Properties of Bioplastics for Food Packaging Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128043028. [Google Scholar]
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to Improve the Barrier and Mechanical Properties of Pectin Films for Food Packaging: Comparing Nanocomposites with Bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Bizymis, A.P.; Giannou, V.; Tzia, C. Improved Properties of Composite Edible Films Based on Chitosan by Using Cellulose Nanocrystals and Beta-Cyclodextrin. Appl. Sci. 2022, 12, 8729. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Yang, B.; Fan, B.; Zhang, H.; Weng, Y. Enhancement of Mechanical and Barrier Property of Hemicellulose Film via Crosslinking with Sodium Trimetaphosphate. Polymers 2021, 13, 927. [Google Scholar] [CrossRef]
- Petronilho, S.; Oliveira, A.; Domingues, M.R.; Nunes, F.M.; Coimbra, M.A.; Gonçalves, I. Hydrophobic Starch-Based Films Using Potato Washing Slurries and Spent Frying Oil. Foods 2021, 10, 2897. [Google Scholar] [CrossRef]
- Alias, A.R.; Wan, M.K.; Sarbon, N.M. Emerging Materials and Technologies of Multi-Layer Film for Food Packaging Application: A Review. Food Control 2022, 136, 108875. [Google Scholar] [CrossRef]
- Messin, T.; Marais, S.; Follain, N.; Guinault, A.; Gaucher, V.; Delpouve, N.; Sollogoub, C. Biodegradable PLA/PBS Multinanolayer Membrane with Enhanced Barrier Performances. J. Membr. Sci. 2020, 598, 117777. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Parsons, A.J.; Rudd, C.D.; Ahmed, I.; Thielemans, W. Mechanical, Crystallisation and Moisture Absorption Properties of Melt Drawn Polylactic Acid Fibres. Eur. Polym. J. 2014, 53, 270–281. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Wang, W.; Qin, C.; Wu, M. Facile Preparation of Reactive Hydrophobic Cellulose Nanofibril Film for Reducing Water Vapor Permeability (WVP) in Packaging Applications. Cellulose 2019, 26, 3271–3284. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Dielectric Barrier Discharge Atmospheric Air Plasma Treatment of High Amylose Corn Starch Films. LWT 2015, 63, 1076–1082. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of Plasma Treatment on the Surface Properties of Polylactic Acid Films. Polym. Test 2021, 96, 107097. [Google Scholar] [CrossRef]
- Goiana, M.L.; de Brito, E.S.; Alves Filho, E.G.; Miguel, E.d.C.; Fernandes, F.A.N.; de Azeredo, H.M.C.; Rosa, M.d.F. Corn Starch Based Films Treated by Dielectric Barrier Discharge Plasma. Int. J. Biol. Macromol. 2021, 183, 2009–2016. [Google Scholar] [CrossRef]
- Hoque, M.; McDonagh, C.; Tiwari, B.K.; Kerry, J.P.; Pathania, S. Effect of Cold Plasma Treatment on the Packaging Properties of Biopolymer-Based Films: A Review. Appl. Sci. 2022, 12, 1346. [Google Scholar] [CrossRef]
- Bahrami, R.; Zibaei, R.; Hashami, Z.; Hasanvand, S.; Garavand, F.; Rouhi, M.; Jafari, S.M.; Mohammadi, R. Modification and Improvement of Biodegradable Packaging Films by Cold Plasma; a Critical Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1936–1950. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning Polysaccharides into Hydrophobic Materials: A Critical Review. Part 1. Cellulose. Cellulose 2010, 17, 875–889. [Google Scholar] [CrossRef]
- Wang, C.; Shao, R.; Wang, G.; Sun, S. Hierarchical Hydrophobic Surfaces with Controlled Dual Transition between Rose Petal Effect and Lotus Effect via Structure Tailoring or Chemical Modification. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126661. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Guo, Z. Tomato-Lotus Inspired Edible Superhydrophobic Artificial Lotus Leaf. Chem. Eng. J. 2020, 400, 125883. [Google Scholar] [CrossRef]
- Allred, T.P.; Weibel, J.A.; Garimella, S. The Petal Effect of Parahydrophobic Surfaces Offers Low Receding Contact Angles That Promote Effective Boiling. Int. J. Heat Mass Transf. 2019, 135, 403–412. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Ramos, A. Production of Hydrophobic Zein-Based Films Bioinspired by the Lotus Leaf Surface: Characterization and Bioactive Properties. Microorganisms 2019, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Gama, R.; Bretas, R.E.S.; Oréfice, R.L. Control of the Hydrophilic/Hydrophobic Behavior of Biodegradable Natural Polymers by Decorating Surfaces with Nano- and Micro-Components. Adv. Polym. Technol. 2018, 37, 654–661. [Google Scholar] [CrossRef]
- Gonçalves, I.; Lopes, J.; Barra, A.; Hernández, D.; Nunes, C.; Kapusniak, K.; Kapusniak, J.; Evtyugin, D.; Lopes da Silva, J.A.; Ferreira, P.; et al. Tailoring the Surface Properties and Flexibility of Starch-Based Films Using Oil and Waxes Recovered from Potato Chips Byproducts. Int. J. Biol. Macromol. 2020, 163, 251–259. [Google Scholar] [CrossRef]
- Ruzi, M.; Celik, N.; Onses, M.S. Superhydrophobic Coatings for Food Packaging Applications: A Review. Food Packag. Shelf Life 2022, 32, 100823. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Tedeschi, G.; Goldoni, L.; Benítez, J.J.; Ceseracciu, L.; Koschella, A.; Heinze, T.; Athanassiou, A.; Heredia-Guerrero, J.A. Greaseproof, Hydrophobic, and Biodegradable Food Packaging Bioplastics from C6-Fluorinated Cellulose Esters. Food Hydrocoll. 2022, 128, 107562. [Google Scholar] [CrossRef]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart Monitoring of Gas/Temperature Changes within Food Packaging Based on Natural Colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef]
- Nikvarz, N.; Khayati, G.R.; Sharafi, S. Preparation of UV Absorbent Films Using Polylactic Acid and Grape Syrup for Food Packaging Application. Mater. Lett. 2020, 276, 128187. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-Blocking, and High Barrier Cellulose-Based Bioplastics with Naringin as Active Food Packaging Materials. Int. J. Biol. Macromol. 2022, 209, 1985–1994. [Google Scholar] [CrossRef]
- Gonçalves, I.; Hernández, D.; Cruz, C.; Lopes, J.; Barra, A.; Nunes, C.; da Silva, J.A.L.; Ferreira, P.; Coimbra, M.A. Relevance of Genipin Networking on Rheological, Physical, and Mechanical Properties of Starch-Based Formulations. Carbohydr. Polym. 2021, 254, 117236. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Gonçalves, I.; Barra, A.; Nunes, C.; Ferreira, P.; Coimbra, M.A. Coffee Silverskin and Starch-Rich Potato Washing Slurries as Raw Materials for Elastic, Antioxidant, and UV-Protective Biobased Films. Food Res. Int. 2020, 138, 109733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of PH Indicator and Antimicrobial Cellulose Nanofibre Packaging Film Based on Purple Sweet Potato Anthocyanin and Oregano Essential Oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and Active Furcellaran-Gelatin Films Containing Green or Pu-Erh Tea Extracts: Characterization, Antioxidant and Antimicrobial Potential. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef]
- Tracey, C.T.; Predeina, A.L.; Krivoshapkina, E.F.; Kumacheva, E. A 3D Printing Approach to Intelligent Food Packaging. Trends Food Sci. Technol. 2022, 127, 87–98. [Google Scholar] [CrossRef]
- Digvijay, P.; Onkar, S. Smart Packaging Market by Type: Global Opportunity Analysis and Industry Forecast, 2021–2030; Allied Market Research: Pune, India, 2022. [Google Scholar]
- European Commission. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Available online: https://eur-lex.europa.eu/eli/reg/2004/1935/oj (accessed on 13 December 2022).
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Silva, A.S.; Pastrana, L.; Carneiro, O.S.; et al. Active Flexible Films for Food Packaging: A Review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef]
- Rivero, S.; Giannuzzi, L.; García, M.A.; Pinotti, A. Controlled Delivery of Propionic Acid from Chitosan Films for Pastry Dough Conservation. J. Food Eng. 2013, 116, 524–531. [Google Scholar] [CrossRef]
- López, O.; Giannuzzi, L.; Zaritzky, N.E.; García, M.A. Potassium Sorbate Controlled Release from Corn Starch Films. Mater. Sci. Eng. C 2013, 33, 1583–1591. [Google Scholar] [CrossRef]
- Villarruel, S.; Giannuzzi, L.; Rivero, S.; Pinotti, A. Changes Induced by UV Radiation in the Presence of Sodium Benzoate in Films Formulated with Polyvinyl Alcohol and Carboxymethyl Cellulose. Mater. Sci. Eng. C 2015, 56, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Neogi, S. Oxygen Scavengers for Food Packaging Applications: A Review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Li, S.; Xu, L.; Wang, R.; Chen, R.; Sun, Y. The Size-Controllable Preparation of Chitosan/Silver Nanoparticle Composite Microsphere and Its Antimicrobial Performance. Carbohydr. Polym. 2019, 220, 22–29. [Google Scholar] [CrossRef]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active Composite Starch Films Containing Green Synthesized Silver Nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Passaretti, M.G.; Ninago, M.D.; Paulo, C.I.; Petit, A.; Irassar, E.F.; Vega, D.A.; Armando, V.M.; Lopez, O.V. Biocomposites Based on Thermoplastic Starch and Granite Sand Quarry Waste. J. Renew. Mater. 2019, 7, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites Materials for Food Packaging Applications: Concepts and Future Outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O. Active Films Based on Thermoplastic Corn Starch and Chitosan Oligomer for Food Packaging Applications. Food Packag. Shelf Life 2017, 14, 128–136. [Google Scholar] [CrossRef]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N. Chitosan from Marine Crustaceans: Production, Characterization and Applications. In Biological Activities and Application of Marine Polysaccharides; InTech: London, UK, 2017; pp. 39–54. [Google Scholar]
- Varghese, S.A.; Siengchin, S.; Parameswaranpillai, J. Essential Oils as Antimicrobial Agents in Biopolymer-Based Food Packaging—A Comprehensive Review. Food Biosci. 2020, 38, 100785. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Bof, M.J.; Jiménez, A.; Locaso, D.E.; García, M.A.; Chiralt, A. Grapefruit Seed Extract and Lemon Essential Oil as Active Agents in Corn Starch–Chitosan Blend Films. Food Bioproc. Tech. 2016, 9, 2033–2045. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Development and Characterization of Carrageenan/Grapefruit Seed Extract Composite Films for Active Packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Bof, M.J.; Laurent, F.E.; Massolo, F.; Locaso, D.E.; Versino, F.; García, M.A. Bio-Packaging Material Impact on Blueberries Quality Attributes under Transport and Marketing Conditions. Polymers 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, W. Antioxidant and Antibacterial Chitosan Film with Tea Polyphenols-Mediated Green Synthesis Silver Nanoparticle via a Novel One-Pot Method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A Novel Active Bionanocomposite Film Incorporating Rosemary Essential Oil and Nanoclay into Chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Romani, V.P.; Prentice-Hernández, C.; Martins, V.G. Active and Sustainable Materials from Rice Starch, Fish Protein and Oregano Essential Oil for Food Packaging. Ind. Crops Prod. 2017, 97, 268–274. [Google Scholar] [CrossRef]
- Reyes, L.M.; Landgraf, M.; Sobral, P.J.A. Gelatin-Based Films Activated with Red Propolis Ethanolic Extract and Essential Oils. Food Packag. Shelf Life 2021, 27, 100607. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of Eugenol Loaded Gelatin Nanofibers by Electrospinning Technique as Active Packaging Material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Stoleru, E.; Pamfil, D.; Zaharescu, T.; Tarţău, L.; Tudorachi, N.; Brebu, M.A.; Pricope, G.M.; Dumitriu, R.P.; et al. Evaluation of the Rosemary Extract Effect on the Properties of Polylactic Acid-Based Materials. Materials 2018, 11, 1825. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ge, X.; Zhou, L.; Wang, Y. Eugenol Embedded Zein and Poly(Lactic Acid) Film as Active Food Packaging: Formation, Characterization, and Antimicrobial Effects. Food Chem. 2022, 384, 132482. [Google Scholar] [CrossRef]
- Ren, J.; Li, Y.; Lin, Q.; Li, Z.; Zhang, G. Development of Biomaterials Based on Plasticized Polylactic Acid and Tea Polyphenols for Active-Packaging Application. Int. J. Biol. Macromol. 2022, 217, 814–823. [Google Scholar] [CrossRef]
- Lamarra, J.; Giannuzzi, L.; Rivero, S.; Pinotti, A. Assembly of Chitosan Support Matrix with Gallic Acid-Functionalized Nanoparticles. Mater. Sci. Eng. C 2017, 79, 848–859. [Google Scholar] [CrossRef]
- Mariño-Cortegoso, S.; Stanzione, M.; Andrade, M.A.; Restuccia, C.; Rodríguez-Bernaldo de Quirós, A.; Buonocore, G.G.; Barbosa, C.H.; Vilarinho, F.; Silva, A.S.; Ramos, F.; et al. Development of Active Films Utilizing Antioxidant Compounds Obtained from Tomato and Lemon By-Products for Use in Food Packaging. Food Control 2022, 140, 109128. [Google Scholar] [CrossRef]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial Tragacanth Gum-Based Nanocomposite Films Carrying Ascorbic Acid Antioxidant for Bioactive Food Packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Peng, L.; Wang, J.; Lei, Y.; Li, S.; Li, Q.; Yuan, X.; Zhou, M.; Zhang, Z. Development and Characterization of Antioxidant Composite Films Based on Starch and Gelatin Incorporating Resveratrol Fabricated by Extrusion Compression Moulding. Food Hydrocoll. 2023, 139, 108509. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant Edible Films Based on Chitosan and Starch Containing Polyphenols from Thyme Extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Lombo Vidal, O.; Barros Santos, M.C.; Batista, A.P.; Andrigo, F.F.; Baréa, B.; Lecomte, J.; Figueroa-Espinoza, M.C.; Gontard, N.; Guillard, V.; Rezende, C.M.; et al. Active packaging films containing antioxidant extracts from green coffee oil by-products to prevent lipid oxidation. J. Food Eng. 2021, 136, 110744. [Google Scholar]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Oxygen Scavenging Films in Food Packaging. Environ. Chem. Lett. 2018, 16, 523–538. [Google Scholar] [CrossRef]
- Uruchaya, S.; Promsorn, J.; Bumbudsanpharoke, N.; Chonhenchob, V.; Harnkarnsujarit, N. Polyesters Incorporating Gallic Acid as Oxygen Scavenger in Biodegradable Packaging. Encycl. Renew. Sustain. Mater. 2022, 14, 5296. [Google Scholar] [CrossRef]
- di Giuseppe, F.; Coffigniez, F.; Aouf, C.; Guillard, V.; Torrieri, E. Activated Gallic Acid as Radical and Oxygen Scavenger in Biodegradable Packaging Film. Food Packag. Shelf Life 2022, 31, 100811. [Google Scholar] [CrossRef]
- Juan-Polo, A.; Maestre Pérez, S.E.; Monedero Prieto, M.; Sánchez Reig, C.; Tone, A.M.; Herranz Solana, N.; Beltrán Sanahuja, A. Oxygen Scavenger and Antioxidant LDPE/EVOH/PET-Based Films Containing β-Carotene Intended for Fried Peanuts (Arachis hypogaea L.) Packaging: Pilot Scale Processing and Validation Studies. Polymers 2022, 14, 3550. [Google Scholar] [CrossRef]
- Lamarra, J.; Calienni, M.N.; Rivero, S.; Pinotti, A. Electrospun Nanofibers of Poly(Vinyl Alcohol) and Chitosan-Based Emulsions Functionalized with Cabreuva Essential Oil. Int. J. Biol. Macromol. 2020, 160, 307–318. [Google Scholar] [CrossRef]
- Castro Coelho, S.; Nogueiro Estevinho, B.; Rocha, F. Encapsulation in Food Industry with Emerging Electrohydrodynamic Techniques: Electrospinning and Electrospraying—A Review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; García, M.A.; Arce, V.B. Nanocomposite Films with Silver Nanoparticles Synthesized in Situ: Effect of Corn Starch Content. Food Hydrocoll. 2019, 97, 105200. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Nassiri, R.; Sheibani, S.; Ariffin, F.; Karim, A.A. Preparation and Characterization of Bionanocomposite Films Filled with Nanorod-Rich Zinc Oxide. Carbohydr. Polym. 2013, 96, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Humayoun, U.; Kumar, M.; Zaidi, S.F.A.; Yoo, J.H.; Ali, N.; Jeong, D.I.; Lee, J.H.; Yoon, D.H. Citric Acid Mediated Green Synthesis of Copper Nanoparticles Using Cinnamon Bark Extract and Its Multifaceted Applications. J. Clean. Prod. 2021, 292, 125974. [Google Scholar] [CrossRef]
- Ortega, F.; Arce, V.B.; Garcia, M.A. Nanocomposite Starch-Based Films Containing Silver Nanoparticles Synthesized with Lemon Juice as Reducing and Stabilizing Agent. Carbohydr. Polym. 2021, 252, 117208. [Google Scholar] [CrossRef]
- Hu, Q.; Fang, Y.; Yang, Y.; Ma, N.; Zhao, L. Effect of Nanocomposite-Based Packaging on Postharvest Quality of Ethylene-Treated Kiwifruit (Actinidia Deliciosa) during Cold Storage. Food Res. Int. 2011, 44, 1589–1596. [Google Scholar] [CrossRef]
- Lee, D.S. Carbon Dioxide Absorbers for Food Packaging Applications. Trends Food Sci. Technol. 2016, 57, 146–155. [Google Scholar] [CrossRef]
- Abreu, A.S.; Oliveira, M.; de Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial Nanostructured Starch Based Films for Packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Almasi, H.; Forghani, S.; Moradi, M. Recent Advances on Intelligent Food Freshness Indicators; an Update on Natural Colorants and Methods of Preparation. Food Packag. Shelf Life 2022, 32, 100839. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A Critical Review on Intelligent and Active Packaging in the Food Industry: Research and Development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A Novel PH-Sensing Indicator Based on Bacterial Cellulose Nanofibers and Black Carrot Anthocyanins for Monitoring Fish Freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent PH-Sensitive Indicator Based on Starch-Cellulose and Alizarin Dye to Track Freshness of Rainbow Trout Fillet. Int. J. Biol. Macromol. 2019, 132, 157–165. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, Y.; Zou, Y.; Dong, Q.; Ding, F.; Liu, X.; Li, H. A Novel Colorimetric Indicator Based on Agar Incorporated with Arnebia Euchroma Root Extracts for Monitoring Fish Freshness. Food Hydrocoll. 2019, 90, 198–205. [Google Scholar] [CrossRef]
- Wang, H.; Li, B.; Ding, F.; Ma, T. Improvement of Properties of Smart Ink via Chitin Nanofiber and Application as Freshness Indicator. Prog. Org. Coat. 2020, 149, 105921. [Google Scholar] [CrossRef]
- Materon, E.M.; Wong, A.; Gomes, L.M.; Ibáñez-Redín, G.; Joshi, N.; Oliveira, O.N.; Faria, R.C. Combining 3D Printing and Screen-Printing in Miniaturized, Disposable Sensors with Carbon Paste Electrodes. J. Mater. Chem. C Mater. 2021, 9, 5633–5642. [Google Scholar] [CrossRef]
- Bautista, L.; Molina, L.; Niembro, S.; García, J.M.; López, J.; Vílchez, A. Emerging Nanotechnologies in Food Science. In Emerging Nanotechnologies in Food Science; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 149–173. [Google Scholar]
- Negi, Y.S.; Ruchir, P.; Kulshreshta, A. Intelligent Packaging Systems for Food Applications. Available online: https://packaging360.in/insights/intelligent-packaging-systems-for-food/ (accessed on 25 November 2022).
- Azeredo, H.M.C.; Correa, D.S. Smart Choices: Mechanisms of Intelligent Food Packaging. Curr. Res. Food Sci. 2021, 4, 932–936. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An Overview of the Intelligent Packaging Technologies in the Food Sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Guo, H.; Sun, X. Recent Progress on Cell-Based Biosensors for Analysis of Food Safety and Quality Control. Biosens. Bioelectron. 2019, 126, 389–404. [Google Scholar] [CrossRef]
- Fronczek, C.F.; Yoon, J.Y. Detection of Foodborne Pathogens Using Biosensors. In Antimicrobial Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 153–166. ISBN 9780128007235. [Google Scholar]
- Papkovsky, D.B.; Dmitriev, R.I. Biological Detection by Optical Oxygen Sensing. Chem. Soc. Rev. 2013, 42, 8700. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Bhandari, B.; Yang, C. Novel PH-Sensitive Films Containing Curcumin and Anthocyanins to Monitor Fish Freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Chen, H.; Bhandari, B. Freshness Monitoring Technology of Fish Products in Intelligent Packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with PH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Khairunnisa, A.; Suyatma, N.E.; Adawiyah, D.R. Development of Smart TTI Label Based on Kinetics Diffusion of Vegetable Oils Blends for Cold Supply Chain Monitoring. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019; Volume 335. [Google Scholar]
- Rahman, A.T.M.M.; Kim, D.H.; Jang, H.D.; Yang, J.H.; Lee, S.J. Preliminary Study on Biosensor-Type Time-Temperature Integrator for Intelligent Food Packaging. Sensors 2018, 18, 1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.A.; Jung, S.W.; Park, H.R.; Chung, K.Y.; Lee, S.J. Application of a Prototype of Microbial Time Temperature Indicator (TTI) to the Prediction of Ground Beef Qualities during Storage. Korean J. Food Sci. Anim. Resour. 2012, 32, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.Y.; Jung, S.W.; Kim, T.J.; Lee, S.J. A Prototype of Time Temperature Integrator (TTI) with Microbeads-Entrapped Microorganisms Maintained at a Constant Concentration. J. Food Eng. 2014, 120, 118–123. [Google Scholar] [CrossRef]
- Mataragas, M.; Bikouli, V.C.; Korre, M.; Sterioti, A.; Skandamis, P.N. Development of a Microbial Time Temperature Indicator for Monitoring the Shelf Life of Meat. Innov. Food Sci. Emerg. Technol. 2019, 52, 89–99. [Google Scholar] [CrossRef]
- Gao, T.; Tian, Y.; Zhu, Z.; Sun, D.W. Modelling, Responses and Applications of Time-Temperature Indicators (TTIs) in Monitoring Fresh Food Quality. Trends Food Sci. Technol. 2020, 99, 311–322. [Google Scholar] [CrossRef]
- Kim, K.; Kim, E.; Lee, S.J. New Enzymatic Time-Temperature Integrator (TTI) That Uses Laccase. J. Food Eng. 2012, 113, 118–123. [Google Scholar] [CrossRef]
- Jhuang, J.R.; Lin, S.; Chen, L.C.; Lou, S.N.; Chen, S.H.; Chen, H.H. Development of Immobilized Laccase-Based Time Temperature Indicator by Electrospinning Zein Fiber. Food Packag. Shelf Life 2020, 23, 100436. [Google Scholar] [CrossRef]
- Jhuang, J.R.; Lou, S.N.; Lin, S.; Chen, S.H.; Chen, L.C.; Chen, H.H. Immobilizing Laccase on Electrospun Chitosan Fiber to Prepare Time-Temperature Indicator for Food Quality Monitoring. Innov. Food Sci. Emerg. Technol. 2020, 63, 102370. [Google Scholar] [CrossRef]
- Lin, C.X.; Hsu, H.H.; Chang, Y.H.; Chen, S.H.; Lin, S.; Lou, S.N.; Chen, H.H. Expanding the Applicability of an Innovative Laccase TTI in Intelligent Packaging by Adding an Enzyme Inhibitor to Change Its Coloration Kinetics. Polymers 2021, 13, 3646. [Google Scholar] [CrossRef]
- Wu, D.; Hou, S.; Chen, J.; Sun, Y.; Ye, X.; Liu, D.; Meng, R.; Wang, Y. Development and Characterization of an Enzymatic Time-Temperature Indicator (TTI) Based on Aspergillus Niger Lipase. LWT 2015, 60, 1100–1104. [Google Scholar] [CrossRef]
- Chuang, W.-P.; Hsieh, B.-C. Development of a Gallic Acid Based Time Temperature Indicator with Adjustable Activation Energy. Food Control 2023, 144, 109396. [Google Scholar] [CrossRef]
- Ye, B.; Chen, J.; Ye, H.; Zhang, Y.; Yang, Q.; Yu, H.; Fu, L.; Wang, Y. Development of a Time–Temperature Indicator Based on Maillard Reaction for Visually Monitoring the Freshness of Mackerel. Food Chem. 2022, 373, 131448. [Google Scholar] [CrossRef]
- Jelinek, R.; Ritenberg, M. Polydiacetylenes-Recent Molecular Advances and Applications. RSC Adv. 2013, 3, 21192–21201. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.Y.; Chen, X.; Yoon, J. Recent Progress in Stimuli-Induced Polydiacetylenes for Sensing Temperature, Chemical and Biological Targets. Chem. Commun. 2016, 52, 9178–9196. [Google Scholar] [CrossRef]
- Dory, Y.L.; Caron, M.; Duguay, V.O.; Chicoine-Ouellet, L.; Fortin, D.; Baillargeon, P. Preparation and Single Crystal Structure Determination of the First Biobased Furan-Polydiacetylene Using Topochemical Polymerization. Crystals 2019, 9, 448. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Städler, B. Recent Developments in Polydiacetylene-Based Sensors. Chem. Mater. 2019, 31, 1196–1222. [Google Scholar] [CrossRef]
- Suppakul, P.; Kim, D.Y.; Yang, J.H.; Lee, S.B.; Lee, S.J. Practical Design of a Diffusion-Type Time-Temperature Indicator with Intrinsic Low Temperature Dependency. J. Food Eng. 2018, 223, 22–31. [Google Scholar] [CrossRef]
- Vivaldi, F.; Melai, B.; Bonini, A.; Poma, N.; Salvo, P.; Kirchhain, A.; Tintori, S.; Bigongiari, A.; Bertuccelli, F.; Isola, G.; et al. A Temperature-Sensitive RFID Tag for the Identification of Cold Chain Failures. Sens. Actuators A Phys. 2020, 313, 112182. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Sharma, H.; Pandey, S.; Mishra, P.K.; Wimmer, R. Bionanocomposites: Smart Biodegradable Packaging Material for Food Preservation. In Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–110. [Google Scholar]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU Regulation Aspects and Global Market of Active and Intelligent Packaging for Food Industry Applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Zuo, J.; Feng, J.; Gameiro, M.G.; Tian, Y.; Liang, J.; Wang, Y.; Ding, J.; He, Q. RFID-Based Sensing in Smart Packaging for Food Applications: A Review. Future Foods 2022, 6, 100198. [Google Scholar] [CrossRef] [PubMed]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart Gelatin Films Prepared Using Red Cabbage (Brassica oleracea L.) Extracts as Solvent. Food Hydrocoll. 2019, 89, 674–681. [Google Scholar] [CrossRef]
- Salgado, P.R.; di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent Developments in Smart Food Packaging Focused on Biobased and Biodegradable Polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Active Nanocomposite Films Based on Soy Proteins-Montmorillonite-Clove Essential Oil for the Preservation of Refrigerated Bluefin Tuna (Thunnus Thynnus) Fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca Starch Active Nanocomposite Films and Their Antimicrobial Effectiveness on Ready-to-Eat Chicken Meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of Antimicrobial Packaging Film Made from Poly(Lactic Acid) Incorporating Titanium Dioxide and Silver Nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.; Ashokkumar, V. Preparation and Characterization of Curdlan/Polyvinyl Alcohol/ Thyme Essential Oil Blending Film and Its Application to Chilled Meat Preservation. Carbohydr. Polym. 2020, 247, 116670. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of Whey Protein Edible Films Containing Plant Essential Oils on Microbial Inactivation of Sliced Kasar Cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Vargas, M.; Atarés, L.; Chiralt, A. Thermoplastic Cassava Starch-Chitosan Bilayer Films Containing Essential Oils. Food Hydrocoll. 2018, 75, 107–115. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M.; et al. Natural Biomaterial-Based Edible and PH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Ekiz, H.I.; López-Rubio, A. Phytochemical-Loaded Electrospun Nanofibres as Novel Active Edible Films: Characterization and Antibacterial Efficiency in Cheese Slices. Food Control 2020, 112, 107133. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lu, L.; Gunasekaran, S. Biopolymer/Gold Nanoparticles Composite Plasmonic Thermal History Indicator to Monitor Quality and Safety of Perishable Bioproducts. Biosens. Bioelectron. 2017, 92, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, B.C.N.; de Mello Aquilino, M.; Prata, A.S. Biodegradable Thermoactive Packaging Using Phase Change Material Particles on Cellulosic Materials. Cellulose 2021, 28, 6427–6437. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-Based PH-Sensitive Smart Packaging Films for Monitoring Food Freshness. J. Agric. Food Res. 2022, 9, 100340. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and Characterization of Antioxidant and Antimicrobial Packaging Films Based on Chitosan and Proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Lee, K.; Park, H.; Baek, S.; Han, S.; Kim, D.; Chung, S.; Yoon, J.Y.; Seo, J. Colorimetric Array Freshness Indicator and Digital Color Processing for Monitoring the Freshness of Packaged Chicken Breast. Food Packag. Shelf Life 2019, 22, 100408. [Google Scholar] [CrossRef]
- Hashim, S.B.H.; Tahir, H.E.; Lui, L.; Zhang, J.; Zhai, X.; Mahdi, A.A.; Ibrahim, N.A.; Mahunu, G.K.; Hassan, M.M.; Xiaobo, Z.; et al. Smart Films of Carbohydrate-Based/Sunflower Wax/Purple Chinese Cabbage Anthocyanins: A Biomarker of Chicken Freshness. Food Chem. 2023, 399, 133824. [Google Scholar] [CrossRef]
- Pirsa, S.; Karimi Sani, I.; Pirouzifard, M.K.; Erfani, A. Smart Film Based on Chitosan/Melissa Officinalis Essences/ Pomegranate Peel Extract to Detect Cream Cheeses Spoilage. Food Addit. Contam. Part A 2020, 37, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.-W. PH-Responsive Chitosan-Based Film Incorporated with Alizarin for Intelligent Packaging Applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Lyu, J.S.; Choi, I.; Hwang, K.S.; Lee, J.Y.; Seo, J.; Kim, S.Y.; Han, J. Development of a BTB−/TBA+ Ion-Paired Dye-Based CO2 Indicator and Its Application in a Multilayered Intelligent Packaging System. Sens. Actuators B Chem. 2019, 282, 359–365. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Badola, R.; Singh, A.K.; Minz, P.S.; Ganguly, S.; Alam, T. Characterization of Biopolymer-Based UV-Activated Intelligent Oxygen Indicator for Food-Packaging Applications. J. Packag. Technol. Res. 2018, 2, 29–43. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A Colorimetric Hydrogen Sulfide Sensor Based on Gellan Gum-Silver Nanoparticles Bionanocomposite for Monitoring of Meat Spoilage in Intelligent Packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Kwon, S.; Lee, W.; Ko, S. Optical Response of Photonic Cellulose Nanocrystal Film for a Novel Humidity Indicator. Int. J. Biol. Macromol. 2019, 140, 91–97. [Google Scholar] [CrossRef]
- Choudhary, A.; Prasad, E. Bioplastic Market Outlook 2020–2027. In Bioplastic Market by Biodegradable Type and Application: Opportunity Analysis and Industry Forecast, 2020–2027; Allied Market Research: Pune, India, 2020. [Google Scholar]
- Jabeen, N.; Majid, I.; Nayik, G.A.; Yildiz, F. Bioplastics and Food Packaging: A Review. Cogent. Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Mohammed, A.; Gaduan, A.; Chaitram, P.; Pooran, A.; Lee, K.Y.; Ward, K. Sargassum Inspired, Optimized Calcium Alginate Bioplastic Composites for Food Packaging. Food Hydrocoll. 2023, 135, 108192. [Google Scholar] [CrossRef]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased Materials for Food Packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Leceta, I.; Cabezudo, S.; de La Caba, K.; Guerrero, P. Valorization of Soya By-Products for Sustainable Packaging. J. Clean. Prod. 2014, 64, 228–233. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent Advances and Future Prospects of Cellulose, Starch, Chitosan, Polylactic Acid and Polyhydroxyalkanoates for Sustainable Food Packaging Applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef]
- Nielsen, C.; Rahman, A.; Rehman, A.U.; Walsh, M.K.; Miller, C.D. Food Waste Conversion to Microbial Polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338–1352. [Google Scholar] [CrossRef]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the Polyhydroxyalkanoate Biopolymer by Cupriavidusnecator Using Beer Brewery Wastewater Containing Maltose as a Primary Carbon Source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Alsafadi, D.; Al-Mashaqbeh, O. A One-Stage Cultivation Process for the Production of Poly-3-(Hydroxybutyrate-Co-Hydroxyvalerate) from Olive Mill Wastewater by Haloferax Mediterranei. N. Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef]
- Lammi, S.; le Moigne, N.; Djenane, D.; Gontard, N.; Angellier-Coussy, H. Dry Fractionation of Olive Pomace for the Development of Food Packaging Biocomposites. Ind. Crops Prod. 2018, 120, 250–261. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery Approach for Cassava-Based Industrial Wastes: Current Status and Opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Domingos, I.; Ferreira, J.; Cruz-Lopes, L.; Esteves, B. Polyurethane Foams from Liquefied Orange Peel Wastes. Food Bioprod. Process. 2019, 115, 223–229. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.H.B. Bacterial Nanocellulose from Agro-Industrial Wastes: Low-Cost and Enhanced Production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef] [Green Version]
- Valencia, G.A.; de Andrade, C.J.; Ienczak, J.L.; Monteiro, A.R.; Gutiérrez, T.J. Bio-Valorization of Waste; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2021; ISBN 978-981-15-9695-7. [Google Scholar]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.H.; Kwon, E.E.; Jeon, Y.J. Production of Bioplastic through Food Waste Valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- Liu, M.; Arshadi, M.; Javi, F.; Lawrence, P.; Davachi, S.M.; Abbaspourrad, A. Green and Facile Preparation of Hydrophobic Bioplastics from Tea Waste. J. Clean. Prod. 2020, 276, 123353. [Google Scholar] [CrossRef]
- Chiarathanakrit, C.; Riyajan, S.A.; Kaewtatip, K. Transforming Fish Scale Waste into an Efficient Filler for Starch Foam. Carbohydr. Polym. 2018, 188, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, R. Degradación Ambiental y En Condiciones Adversas de Adhesivos Estructurales: Análisis y Consideraciones Técnicas Para Su Aplicación Industrial. Doctoral Thesis, E.T.S.I. Diseño Industrial (UPM), Madrid, Spain, 2017. [Google Scholar]
- Toenniessen, M. Packaging Materials: Adhesives for Food Packaging Applications. Available online: https://ilsi.eu/publication/packaging-materials-10-adhesives-for-food-packaging-applications/ (accessed on 20 December 2022).
- Ortiz-Fernández, A.; Ríos-Soberanis, C.R.; Chim-Chi, Y.A.; Moo-Huchin, V.M.; Estrada-León, R.J.; Pérez-Pacheco, E. Optimization of Biodegradable Starch Adhesives Using Response Surface Methodology. Polym. Bull. 2021, 78, 3729–3749. [Google Scholar] [CrossRef]
- Dohr, C.A.; Hirn, U. Influence of Paper Properties on Adhesive Strength of Starch Gluing. Nord. Pulp Paper Res. J. 2022, 37, 120–129. [Google Scholar] [CrossRef]
- Norström, E.; Demircan, D.; Fogelström, L.; Khabbaz, F.; Malmström, E. Green Binders for Wood Adhesives. In Applied Adhesive Bonding in Science and Technology; InTech: London, UK, 2018. [Google Scholar]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-Based Adhesives and Evaluation for Wood Composites Application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Solt, P.; Konnerth, J.; Gindl-Altmutter, W.; Kantner, W.; Moser, J.; Mitter, R.; van Herwijnen, H.W.G. Technological Performance of Formaldehyde-Free Adhesive Alternatives for Particleboard Industry. Int. J. Adhes. Adhes. 2019, 94, 99–131. [Google Scholar] [CrossRef]
- Bacigalupe, A.; Escobar, M.M. Soy Protein Adhesives for Particleboard Production—A Review. J. Polym. Environ. 2021, 29, 2033–2045. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wu, L.; Zhu, Z.; Xu, Z. Improving the Adhesion-to-Fibers and Film Properties of Corn Starch by Starch Sulfo-Itaconation for a Better Application in Warp Sizing. Polym. Test 2021, 98, 107194. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future Opportunities for Bio-Based Adhesives-Advantages beyond Renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef] [Green Version]
- Ebnesajjad, S.; Landrock, A.H. Material Surface Preparation Techniques. In Adhesives Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2015; pp. 35–66. [Google Scholar]
- Watcharakitti, J.; Win, E.E.; Nimnuan, J.; Smith, S.M. Modified Starch-Based Adhesives: A Review. Polymers 2022, 14, 2023. [Google Scholar] [CrossRef]
- Gadhave, R.V.I.; Gadhave, C.R. Adhesives for the Paper Packaging Industry: An Overview. Open J. Polym. Chem. 2022, 12, 55–79. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.; Du, G.; Zhou, X.; Wang, H.; Lei, H. Development and Characterization of a Bayberry Tannin-Based Adhesive for Particleboard. Bioresources 2017, 12, 6082–6093. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Guo, M. Preparation and Properties of a Chitosan-Lignin Wood Adhesive. Int. J. Adhes. Adhes. 2018, 82, 8–13. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood. Polymers 2017, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Monroy, Y.; Rivero, S.; García, M.A. Sustainable Panels Design Based on Modified Cassava Starch Bioadhesives and Wood Processing Byproducts. Ind. Crops Prod. 2019, 137, 171–179. [Google Scholar] [CrossRef]
- Monroy, Y.; Seré, P.; Rivero, S.; García, M.A. Sustainable Panels Based on Starch Bioadhesives: An Insight into Structural and Tribological Performance. Int. J. Biol. Macromol. 2020, 148, 898–907. [Google Scholar] [CrossRef]
- Schwarzenbrunner, R.; Barbu, M.C.; Petutschnigg, A.; Tudor, E.M. Water-Resistant Casein-Based Adhesives for Veneer Bonding in Biodegradable Ski Cores. Polymers 2020, 12, 1745. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Luo, J.; Li, K.; Gao, Q.; Li, J. A Novel Eco-Friendly Blood Meal-Based Bio-Adhesive: Preparation and Performance. J. Polym. Environ. 2018, 26, 607–615. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, P.; Yang, W.; Chu, H.; Wang, W.; Dong, W.; Chen, M.; Bai, H.; Ma, P. Soy Protein-Based Adhesive with Superior Bonding Strength and Water Resistance by Designing Densely Crosslinking Networks. Eur. Polym. J. 2021, 142, 110128. [Google Scholar] [CrossRef]
- Maaßen, W.; Oelmann, S.; Peter, D.; Oswald, W.; Willenbacher, N.; Meier, M.A.R. Novel Insights into Pressure-Sensitive Adhesives Based on Plant Oils. Macromol. Chem. Phys. 2015, 216, 1609–1618. [Google Scholar] [CrossRef]
- Mahieu, A.; Vivet, A.; Poilane, C.; Leblanc, N. Performance of Particleboards Based on Annual Plant Byproducts Bound with Bio-Adhesives. Int. J. Adhes. Adhes. 2021, 107, 102847. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging Films Formulated with Gelatin and Anthocyanins Nanocomplexes: Physical Properties, Antioxidant Activity and Its Application for Olive Oil Protection. Food Hydrocoll. 2019, 96, 617–624. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Biodegradable Composite Films/Coatings of Modified Corn Starch/Gelatin for Shelf Life Improvement of Cucumber. J. Food Sci. Technol. 2021, 58, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Olomo, V.D. Influence of Processing Variables on Some Physico-Chemical Properties and Quality of Manioc Starch-Based Adhesives. Open J. Polym. Chem. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and Techno-Functional Properties of Cassava Starch Modified by Ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef]
- Toenniessen, M. ILSI Europe Report Series: Adhesives for Food Packaging; International Life Sciences Institute Europe (ILSI): Bruxelles, Belgium, 2018. [Google Scholar]
- Altan, A.; Akıncı, E.; Arca, M. Comparing the Performances of Aqueous Solution of Boric Acid / Borax and Powder Boric Acid/Borax in Corrugated Board Production Process. Biol. Chem. Res. 2019, 6, 150–154. [Google Scholar]
- Romero Zaliz, L.; Labombarda, J.; Wildner Fox, C. Etiquetado de Botellas Retornables de Vidrio. Available online: https://www.tecnicomadhesivos.com.ar/wp-content/uploads/2020/04/Tecnicom_Etiquetado-de-Botellas-Retornables-de-Vidrio.pdf (accessed on 20 December 2022).
- Luque, G.C.; Stürtz, R.; Passeggi, M.C.G.; Gugliotta, L.M.; Gonzalez, V.D.G.; Minari, R.J. New Hybrid Acrylic/Collagen Nanocomposites and Their Potential Use as Bio-Adhesives. Int. J. Adhes. Adhes. 2020, 100, 102624. [Google Scholar] [CrossRef]
- Phan, K.; Raes, K.; van Speybroeck, V.; Roosen, M.; de Clerck, K.; de Meester, S. Non-Food Applications of Natural Dyes Extracted from Agro-Food Residues: A Critical Review. J. Clean. Prod. 2021, 301, 126920. [Google Scholar] [CrossRef]
- Iqbal, S.; Ansari, T.N. Extraction and Application of Natural Dyes. In Sustainable Practices in the Textile Industry; Rather, L.J., Shabbir, M., Haji, A., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; Volume 4, pp. 218–233. ISBN 978-1-119-81891-5. [Google Scholar]
- The Business Research Company. Synthetic Dye and Pigment Global Market Report. 2022. Available online: https://www.thebusinessresearchcompany.com/report/synthetic-dye-and-pigment-global-market-report (accessed on 27 December 2022).
- Echegaray, N.; Guzel, N.; Kumar, M.; Guzel, M.; Hassoun, A.; Lorenzo, J.M. Recent Advancements in Natural Colorants and Their Application as Coloring in Food and in Intelligent Food Packaging. Food Chem. 2023, 404, 134453. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. Starch and Agar-Based Color-Indicator Films Integrated with Shikonin for Smart Packaging Application of Shrimp. ACS Food Sci. Technol. 2021, 1, 1963–1969. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M.; Barros, L.; Ferreira, I.C.F.R. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.S.; He, P.H.; Chau, C.F.; Liou, B.K.; Li, S.; Pan, M.H. The Feasibility Study of Natural Pigments as Food Colorants and Seasonings Pigments Safety on Dried Tofu Coloring. Food Sci. Hum. Wellness 2018, 7, 220–228. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, Stability, Encapsulation and Application of Natural Pigments in Foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar] [CrossRef]
- Teixeira, V.M.C.; da Silva, R.F.G.; Gonçalves, O.H.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R.; Bona, E.; Leimann, F.V. Chemometric Approaches to Evaluate the Substitution of Synthetic Food Dyes by Natural Compounds: The Case of Nanoencapsulated Curcumin, Spirulina, and Hibiscus Extracts. LWT 2022, 154, 112786. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Trujillo-de Santiago, G.; Álvarez, M.M.; Chuck-Hernández, C. Advances and Prospective Applications of 3D Food Printing for Health Improvement and Personalized Nutrition. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5722–5741. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, D.J.; Kim, H.W.; Park, H.J. Effect of Particle Size on 3D Printing Performance of the Food-Ink System with Cellular Food Materials. J. Food Eng. 2019, 256, 1–8. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Bhandari, B.; Chitrakar, B.; Chang, L. Investigation of 3D Printing of Apple and Edible Rose Blends as a Dysphagia Food. Food Hydrocoll. 2023, 135, 108184. [Google Scholar] [CrossRef]
- Derossi, A.; Caporizzi, R.; Azzollini, D.; Severini, C. Application of 3D Printing for Customized Food. A Case on the Development of a Fruit-Based Snack for Children. J. Food Eng. 2018, 220, 65–75. [Google Scholar] [CrossRef]
- He, C.; Zhang, M.; Guo, C. 4D Printing of Mashed Potato/Purple Sweet Potato Puree with Spontaneous Color Change. Innov. Food Sci. Emerg. Technol. 2020, 59, 102250. [Google Scholar] [CrossRef]
- Ghazal, A.F.; Zhang, M.; Bhandari, B.; Chen, H. Investigation on Spontaneous 4D Changes in Color and Flavor of Healthy 3D Printed Food Materials over Time in Response to External or Internal PH Stimulus. Food Res. Int. 2021, 142, 110215. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, M.; Guo, C.; Chen, H. 4D Printing of Lotus Root Powder Gel: Color Change Induced by Microwave. Innov. Food Sci. Emerg. Technol. 2021, 68, 102605. [Google Scholar] [CrossRef]
- Ozcan, A.; Arman Kandirmaz, E. Natural Ink Production and Printability Studies for Smart Food Packaging. Color Res. Appl. 2020, 45, 495–502. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The Application of Natural Food Colorants as Indicator Substances in Intelligent Biodegradable Packaging Materials. Food Chem. Toxicol. 2020, 135, 110975. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Alomgir Hossen, M.; Dai, J.; Qin, W.; Liu, Y. Facile Fabrication of Sandwich-like Anthocyanin/Chitosan/Lemongrass Essential Oil Films via 3D Printing for Intelligent Evaluation of Pork Freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Moghaddas Kia, E.; Ghasempour, Z.; Ehsani, A. Preparation of Active Nanocomposite Film Consisting of Sodium Caseinate, ZnO Nanoparticles and Rosemary Essential Oil for Food Packaging Applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Rachmelia, D.; Imawan, C. Time Temperature Indicator Label Using Black Corn Extract and Chitosan Matrix. J. Phys. Conf. Ser. 2018, 1120, 012041. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Erna, K.H.; Felicia, W.X.L.; Rovina, K.; Vonnie, J.M.; Huda, N. Development of Curcumin/Rice Starch Films for Sensitive Detection of Hypoxanthine in Chicken and Fish Meat. Carbohydr. Polym. Technol. Appl. 2022, 3, 100189. [Google Scholar] [CrossRef]
- Boccalon, E.; Viscusi, G.; Lamberti, E.; Fancello, F.; Zara, S.; Sassi, P.; Marinozzi, M.; Nocchetti, M.; Gorrasi, G. Composite Films Containing Red Onion Skin Extract as Intelligent PH Indicators for Food Packaging. Appl. Surf. Sci. 2022, 593, 153319. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Z.; Xie, F.; Tang, S.; Fang, J.; Wang, X. 3D Printed Nanocellulose-Based Label for Fruit Freshness Keeping and Visual Monitoring. Carbohydr. Polym. 2021, 273, 118545. [Google Scholar] [CrossRef]
- Cerreti, M.; Liburdi, K.; del Franco, F.; Esti, M. Heat and Light Stability of Natural Yellow Colourants in Model Beverage Systems. Food Addit. Contam. Part A 2020, 37, 905–915. [Google Scholar] [CrossRef]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef] [Green Version]
- Coultate, T.; Blackburn, R.S. Food Colorants: Their Past, Present and Future. Color. Technol. 2018, 134, 165–186. [Google Scholar] [CrossRef]
- Dufossé, L. Current and Potential Natural Pigments From Microorganisms (Bacteria, Yeasts, Fungi, Microalgae). In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 337–354. ISBN 9780081003923. [Google Scholar]
- Vankar, P.S.; Shukla, D. Natural Dyeing with Anthocyanins from Hibiscus Rosa Sinensis Flowers. J. Appl. Polym. Sci. 2011, 122, 3361–3368. [Google Scholar] [CrossRef]
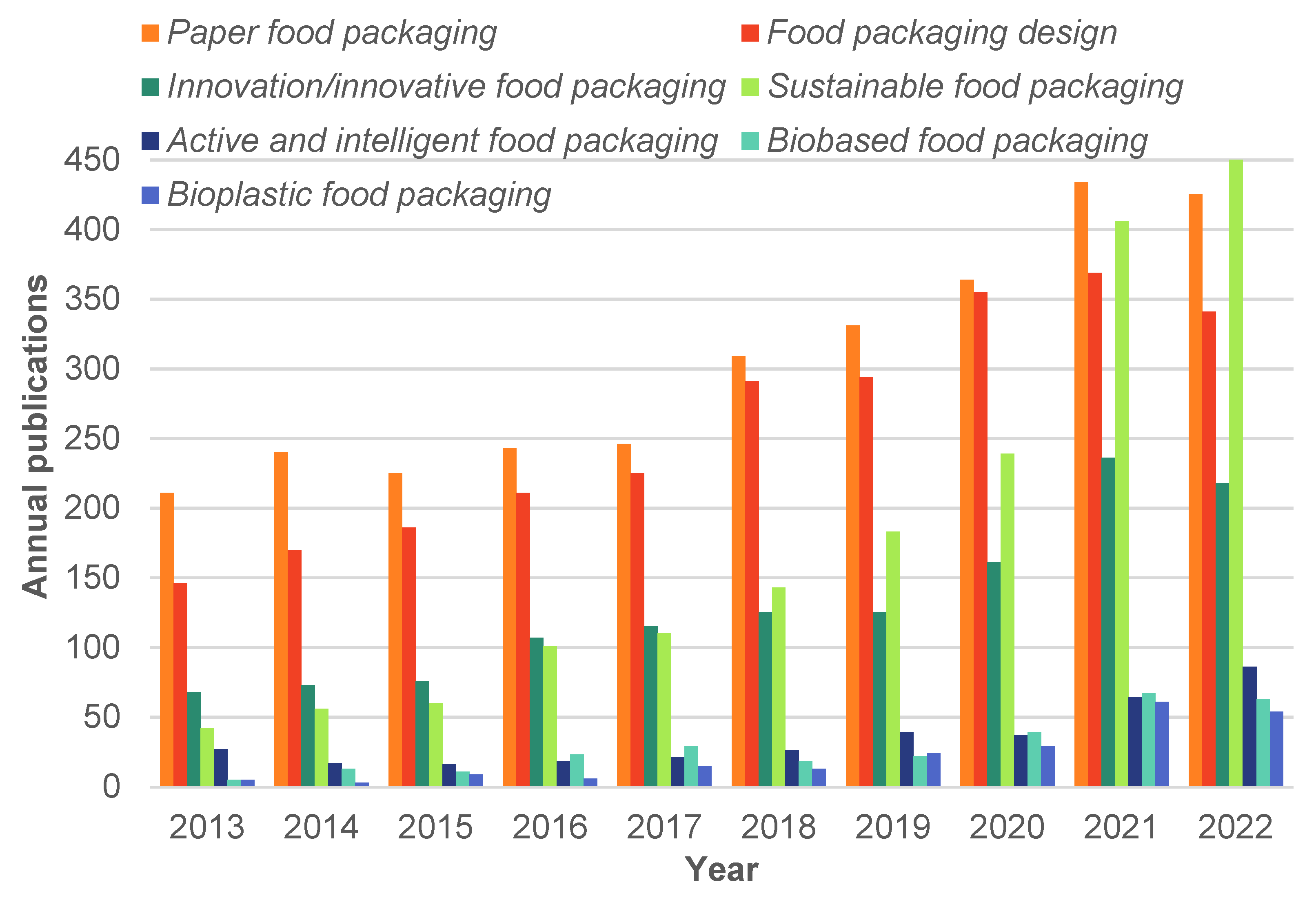
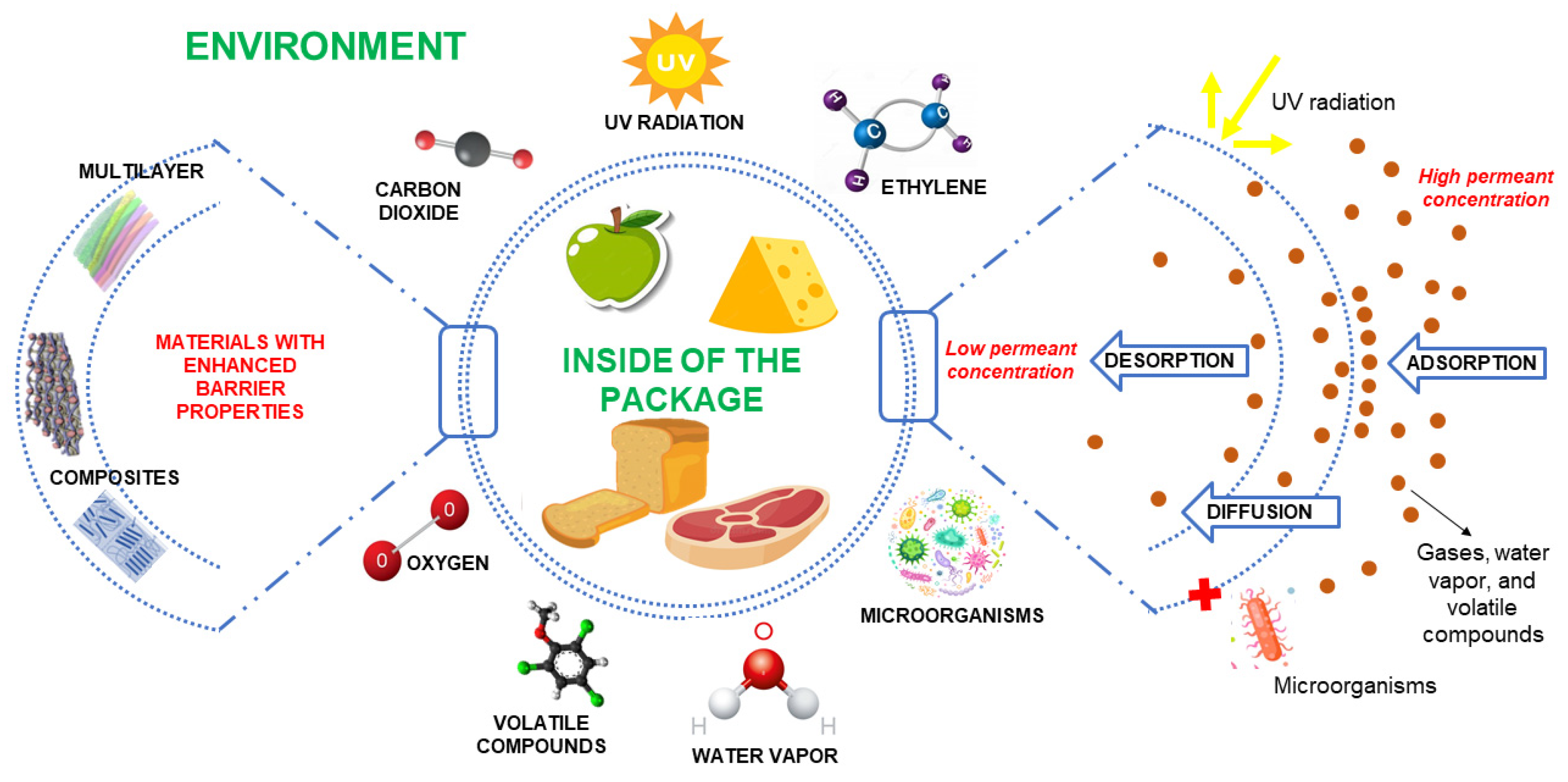
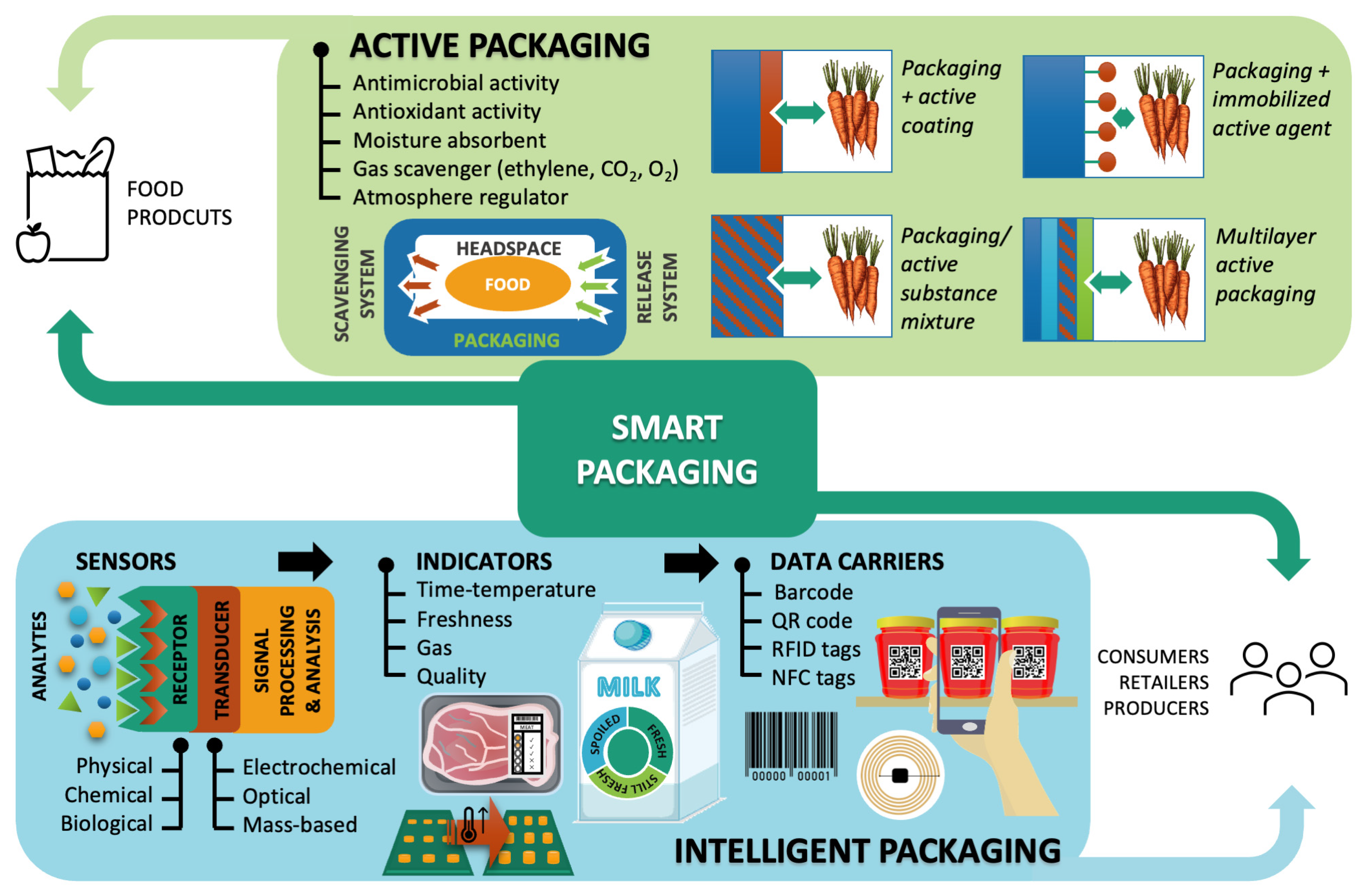

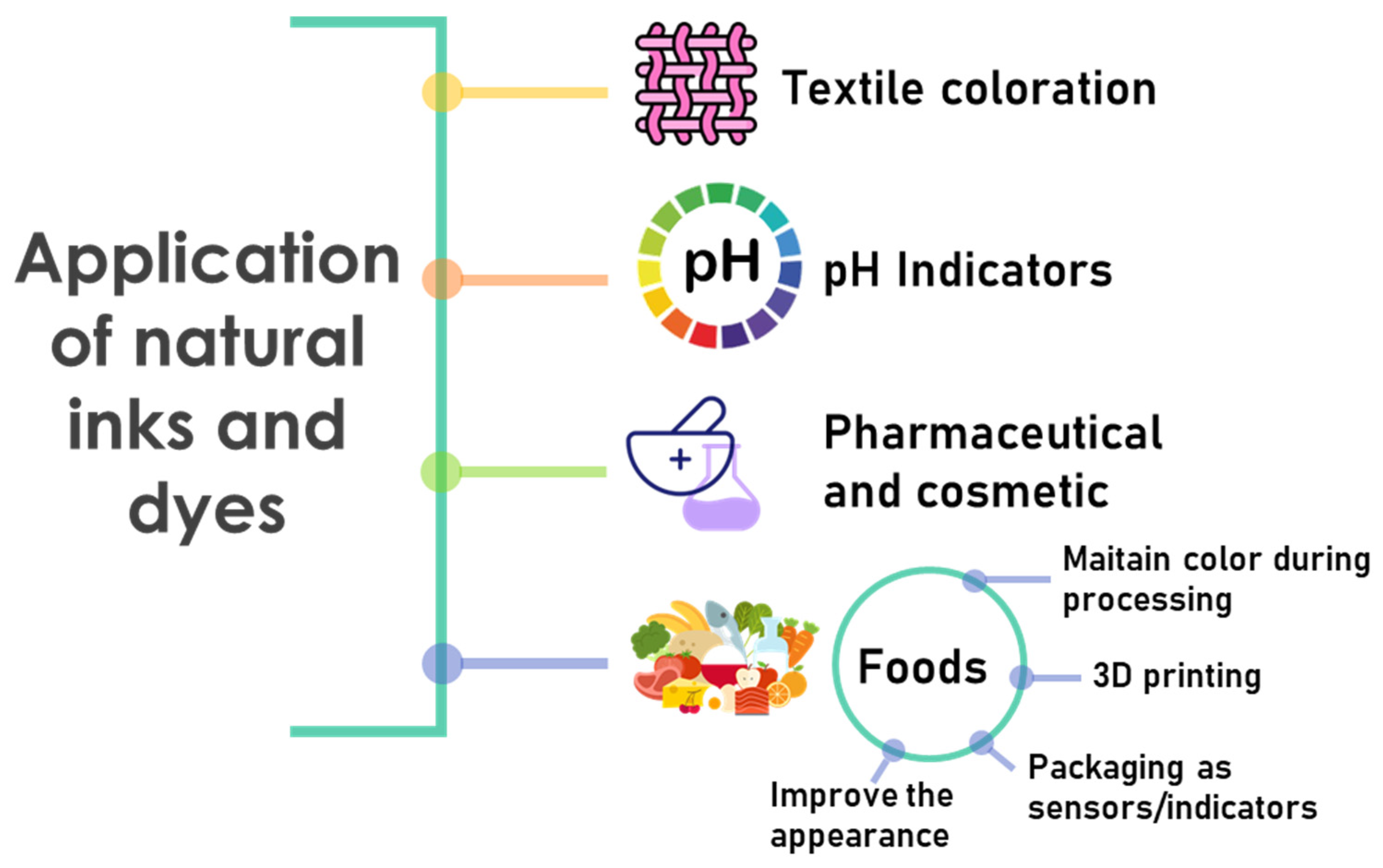
| Norm | Method | Permeant |
|---|---|---|
| ASTM D 1434 | Manometric/volumetric | All gasses |
| ASTM D 7709 | Gravimetric | Water vapor |
| ASTM E 3985 | Dynamic with electrochemical sensor | O2 |
| ASTM E 96 | Gravimetric | Water vapor |
| ASTM E 2945 | Static cells with analytic technique | All gasses |
| ASTM F 3136 | Accumulation method with optical sensor | O2 |
| ASTM F 1249 | IR sensor | Water vapor |
| ASTM F 2622 | Dynamic with sensor | O2 |
| ASTM F 3299 | Coulometric P2O5 sensor | Water vapor |
| ASTM F 2476 | Dynamic with IR sensor | CO2 |
| Active Biodegradable Films | |||
|---|---|---|---|
| Biopolymer | Active Compound | Food Application | References |
| Soy protein isolate | Montmorillonite (0.5%wt) + clove essential oil (0.5%v) | Bluefin tuna filets | [253] |
| Chitosan and Corn starch | Lemon essential oil (1–3%wt) and grapefruit seed extract (1–3%wt) | Blueberries conservation simulating transport and commercial conditions | [182] |
| Corn starch | Green synthesized AgNPs in situ (143 ppm) | Cheese preservation | [173] |
| Tapioca starch | Grape pomace extracts (8% v/v) and cellulose nanocrystals (10% v/v) | Ready to eat chicken meats | [254] |
| PLA | Commercial nanoparticles: TiO2 (3%wt); (2%wt) nano-TiO2 + (1%wt) nano-Ag | Cottage cheese preservation | [255] |
| Curdlan + PVA | Thyme essential oil (1–2%wt) | Chilled pork meat preservation | [256] |
| Whey protein | Oregano and garlic essential oils (2%wt) | Kasar cheese | [257] |
| Chitosan-Cassava TPS bilayer films | Oregano and or cinnamon leaf essential oils (0.25%wt) | Sliced pork meat | [258] |
| Gelatin/Gellan gum | Red radish anthocyanins 5, 10, 15, and 20 mg/100 mL | Milk and fish quality | [259] |
| Zein | Laurel or rosemary leaves extracts (1–10%) | Cheese slices | [260] |
| Chitosan | Propionic acid | Pastry dough | [168] |
| Intelligent Biodegradable Films | |||
| Function | Intelligent System and Innovative Characteristic | Food Application | References |
| Temperature sensor | A passive RFID tag modified with a copper-doped ionic liquid | Fresh products, for the identification of cold chain failures | [245] |
| Temperature abuse indicator | Au nanoparticles included in alginate hydrogel. Biobased, food safe, cost-effective, time sensible | Fresh products | [261] |
| Thermal insulation | Commercial pale-yellow carnauba wax. Biobased and biodegradable insulator | Beverages | [262] |
| pH-based freshness indicator | Biodegradable films containing anthocyanins from different sources. Real-time monitoring of food freshness. | Fresh products: cheese, yogurt, fish, pork, shrimp, and beef | [250,263,264,265,266,267] |
| pH sensitive | Natural compounds showing color changes with pH in biodegradable films | Fish and seafood products | [85,212,268] |
| CO2 detector | Labels containing natural (anthocyanins) or commercial (bromothymol blue and tetrabutyl-ammonium) dyes. | Fermented products such as kimchi | [269] |
| Oxygen indicator | UV-light activated oxygen sensitive biobased film with methyl blue indicator | Suggested for food products packed in modified atmosphere | [270] |
| Hydrogen sulfide indicator | Biobased films containing silver nanoparticles (detect up to 0.81 μmole H2S) or ferrous sulfate (detect 100 ppm H2S) and had a fast response (3 min). | Meat and meat products. Chicken breast and silver carp | [85,271] |
| Humidity indicator | Colorimetric-based sensor on photonic cellulose nanocrystals | Suggested for pharmaceutical products, cereals, and grain seeds storage | [272] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. https://doi.org/10.3390/foods12051057
Versino F, Ortega F, Monroy Y, Rivero S, López OV, García MA. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods. 2023; 12(5):1057. https://doi.org/10.3390/foods12051057
Chicago/Turabian StyleVersino, Florencia, Florencia Ortega, Yuliana Monroy, Sandra Rivero, Olivia Valeria López, and María Alejandra García. 2023. "Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations" Foods 12, no. 5: 1057. https://doi.org/10.3390/foods12051057
APA StyleVersino, F., Ortega, F., Monroy, Y., Rivero, S., López, O. V., & García, M. A. (2023). Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods, 12(5), 1057. https://doi.org/10.3390/foods12051057








