Preparation and Characterization of Sodium Caseinate-Coated Papers Based on Glycerol and Sorbitol Contents for Packaging Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
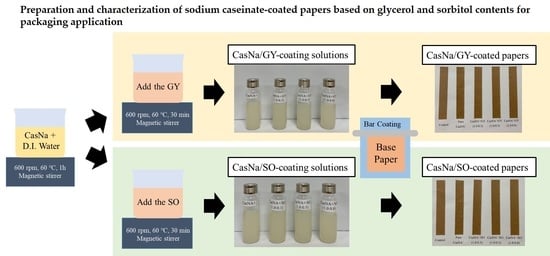
2.2. Preparation of Pristine CasNa, CasNa/GY, and CasNa/SO Coating Solution and Their Coated Papers
2.3. Chemical and Morphological Characterization
2.3.1. pH, Viscosity, and Grammage
2.3.2. Morphological Structure
2.3.3. Chemical Structure
2.4. Mechanical Properties
2.5. Air Permeability
2.6. Surface Properties
2.7. Thermal Properties
2.8. Statistical Analysis
3. Results and Discussion
3.1. CasNa Coating Solutions pH and Viscosity, and Coated Papers Grammage
3.2. Coated Papers Morphologies
3.3. Coated Papers Chemical Properties
3.4. Coated Papers Mechanical Properties
3.5. Coated Papers Air Permeability
3.6. Coated Papers Surface Properties
3.7. Coated Papers Thermal Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rate of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.-T.; Wang, B.-J.; Weng, Y.-M. Preparation and characterization of genipin crosslinked and lysozyme incorporated antimicrobial sodium caseinate edible films. Food Packag. Shelf Life 2020, 242, 100601. [Google Scholar] [CrossRef]
- Publications Office of the European Union. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment (Text with EEA Relevance); Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Valentino, M.; Volpe, S.; Giuseppe, F.A.D.; Cavella, S.; Torrieri, E. Active biopolymer coating based on sodium caseinate: Physical characterization and antioxidant activity. Coatings 2020, 10, 706. [Google Scholar] [CrossRef]
- Khaldia, K. Water vapor barrier and mechanical properties of paper-sodium caseinate and paper-sodium caseinate-paraffin wax films. J. Food Biochem. 2010, 34, 998–1013. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G.; Debeaufort, F. Mechanical and barrier properties of extruded film made from sodium and calcium caseinates. Food Packag. Shelf Life 2014, 2, 65–72. [Google Scholar] [CrossRef]
- Christophliemk, H.; Johansson, C.; Ullsten, H.; Järnström, L. Oxygen and water vapor transmission rates of starch-poly(vinyl alcohol) barrier coatings for flexible packaging paper. Prog. Org. Coat. 2017, 113, 218–224. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Li, Z.; Sirinakbumrung, N.; Rabnawaz, M. Zein and PVOH-based bilayer approach for plastic-free, repulpable and biodegradable oil- and water-resistant paper as a replacement for single-use plastics. Ind. Eng. Chem. Res. 2020, 59, 17856. [Google Scholar] [CrossRef]
- Sharma, M.; Aguado, R.; Murtinho, D.; Valente, A.J.M.; Sousa, A.P.M.D.; Ferreira, J.T. A review on cationic starch and nanocellulose as paper coating components. Int. J. Biol. Macromol. 2020, 162, 578–598. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, G.L.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Colak, B.Y.; Gouance, F.; Degraeve, P.; Espuche, E.; Prochazka, F. Study of the influences of film processing conditions and glycerol amount on the water sorption and gas barrier properties of novel sodium caseinate films. J. Membr. Sci. 2015, 478, 1–11. [Google Scholar] [CrossRef]
- Rezvani, E.; Schleining, G.; Sümen, G.; Taherian, A.R. Assessment of physical and mechanical properties of sodium caseinate and stearic acid based film-forming emulsions and edible films. J. Food Eng. 2013, 116, 598–605. [Google Scholar] [CrossRef]
- Siew, D.C.W.; Heilmann, C.; Easteal, A.J.; Cooney, R.P. Solution and film properties of sodium caseinate/glycerol and sodium caseinate/polyethylene glycol edible coating systems. J. Agr. Food Chem. 1999, 47, 3432–3440. [Google Scholar] [CrossRef]
- KS M ISO 2758:2014; Paper-Determination of Bursting Strength. Korea Agency for Technology and Standards: Maengdong-myeon, Republic of Korea, 2021.
- KS M ISO 1924-2:2008; Paper and Board—Determination of Tensile Properties-Part 2: Constant Rate of Elongation Method (20 mm/min). Korea Agency for Technology and Standards: Maengdong-myeon, Republic of Korea, 2020.
- KS M ISO 5636-5:2013; Paper and Board-Determination of Air Permeance (Medium Range)—Part 5: Gurley Method. Korea Agency for Technology and Standards: Maengdong-myeon, Republic of Korea, 2022.
- Kim, D.; Lim, M.; Seo, J. Preparation of polypropylene/octadecane composite films and their use in the packaging of cherry tomatoes. J. Appl. Polym. Sci. 2016, 133, 44087. [Google Scholar] [CrossRef]
- Kobori, T.; Matsumoto, A.; Sugiyama, S. pH-Dependent interaction between sodium caseinate and xanthan gum. Carbohydr. Polym. 2009, 75, 719–723. [Google Scholar] [CrossRef]
- Barreto, P.L.M.; Roeder, J.; Crespo, J.S.; Maciel, G.R.; Terenzi, H.; Pires, A.T.N.; Soldi, V. Effect of concentration, temperature and plasticizer content on rheological properties of sodium caseinate and sodium caseinate/sorbitol solutions and glass transition of their films. Food Chem. 2003, 82, 425–431. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Microstructure and optical properties of sodium caseinate films containing oleic acid-beewax mixtures. Food Hydrocoll. 2009, 23, 676–683. [Google Scholar] [CrossRef]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Faust, S.; Foerster, J.; Lindner, M.; Schimid, M. Effect of glycerol and sorbitol on the mechanical and barrier properties of films based on pea protein isolate produced by high-moisture extrusion processing. Polym. Eng. Sci. 2022, 62, 95–102. [Google Scholar] [CrossRef]
- Chevalier, E.; Assezat, G.; Prochazka, F.; Oulahal, N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocoll. 2018, 75, 182–191. [Google Scholar] [CrossRef]
- Danish, M.; Mumtaz, M.W.; Fakhar, M.; Rashid, U. Response surface methodology based optimized purification of the residual glycerol from biodiesel production process. Chiang Mai J. Sci. 2017, 44, 1570–1582. [Google Scholar]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.D.C.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Castro, E.D.S.G.D.; Cassella, R.J. Direct determination of sorbitol and sodium glutamate by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) in the thermostabilizer employed in the production of yellow-fever vaccine. Talanta 2016, 152, 33–38. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Ahmadian, Z.; Habibi-Najafi, H. Interactions between polyols and wheat biopolymers in a bread model system fortified with inulin: A Fourier transform infrared study. Heliyon 2018, 4, e01017. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-C.; Wang, B.-J.; Weng, Y.-M. Development and characterization of sodium caseinate edible films cross-linked with genipin. LWT—Food Sci. Technol. 2020, 118, 108813. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and characterization of sodium caseinate edible films made by brown-film extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Gustaw, W.; Świeca, M.; Baraniak, B. A study on the mechanical properties of pea protein isolate films. J. Food Process. Preserv. 2014, 38, 1726–1736. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.; Seo, J.; Han, H.; Khan, S.B. Preparation and properties of poly(urethane acrylate) (PUA) and tetrapod ZnO whisker (TZnO-W) composite films. Polym. Int. 2012, 62, 257–265. [Google Scholar] [CrossRef]
- Gómez, N.; Quintana, E.; Villar, J.C. Effect of paper surface properties on coated paper wettability with different fountain solutions. Bioresources 2014, 9, 4226–4241. [Google Scholar] [CrossRef] [Green Version]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]






| Sample Code | Compositions (g) | pH | Viscosity (mPa·s) | Grammage (g/m2) | |||
|---|---|---|---|---|---|---|---|
| CasNa | D.I. Water | GY | SO | ||||
| Base Paper | - | - | - | - | - | - | 80.0 |
| Pristine CasNa | 10.0 | 90.0 | 0.0 | - | 6.6 | 58.1 a,A | 90.1 a,A |
| CasNa/GY (1.0:0.3) | 10.0 | 90.0 | 3.0 | - | 6.7 | 50.8 b | 90.9 ab |
| CasNa/GY (1.0:0.5) | 10.0 | 90.0 | 5.0 | - | 6.7 | 45.7 c | 91.3 ab |
| CasNa/GY (1.0:0.8) | 10.0 | 90.0 | 8.0 | - | 6.9 | 45.7 c | 93.1 b |
| CasNa/SO (1.0:0.3) | 10.0 | 90.0 | - | 3.0 | 6.8 | 57.5 B | 91.1 AB |
| CasNa/SO (1.0:0.5) | 10.0 | 90.0 | - | 5.0 | 6.9 | 56.1 C | 92.2 B |
| CasNa/SO (1.0:0.8) | 10.0 | 90.0 | - | 8.0 | 6.9 | 51.7 D | 94.3 C |
| Sample Code | Static Contact Angle (°) | Surface Energy (mN/m2) | |||
|---|---|---|---|---|---|
| Water | Diiodomethane | Polar | Dispersive | Total | |
| Base Paper | 73.9 ± 0.62 aA | 31.0 ± 0.35 a | 5.1 | 41.3 | 46.4 |
| Pristine CasNa | 61.2 ± 0.37 bB | 30.0 ± 0.87 bBC | 11.2 | 40.5 | 51.7 |
| CasNa/GY (1.0:0.3) | 61.2 ± 0.47 b | 23.5 ± 0.59 b | 10.4 | 42.9 | 53.3 |
| CasNa/GY (1.0:0.5) | 60.5 ± 0.30 b | 23.5 ± 0.47 b | 10.7 | 42.9 | 53.6 |
| CasNa/GY (1.0:0.8) | 57.3 ± 0.04 c | 22.9 ± 0.36 b | 12.5 | 42.8 | 55.3 |
| CasNa/SO (1.0:0.3) | 61.9 ± 0.22 BC | 25.6 ± 0.35 C | 10.3 | 42.3 | 52.5 |
| CasNa/SO (1.0:0.5) | 62.5 ± 0.09 C | 22.9 ± 0.54 B | 9.6 | 43.3 | 52.9 |
| CasNa/SO (1.0:0.8) | 61.5 ± 0.22 B | 20.9 ± 0.16 D | 9.9 | 43.8 | 53.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Hwang, J. Preparation and Characterization of Sodium Caseinate-Coated Papers Based on Glycerol and Sorbitol Contents for Packaging Application. Foods 2023, 12, 940. https://doi.org/10.3390/foods12050940
Kim D, Hwang J. Preparation and Characterization of Sodium Caseinate-Coated Papers Based on Glycerol and Sorbitol Contents for Packaging Application. Foods. 2023; 12(5):940. https://doi.org/10.3390/foods12050940
Chicago/Turabian StyleKim, Dowan, and Jihyeon Hwang. 2023. "Preparation and Characterization of Sodium Caseinate-Coated Papers Based on Glycerol and Sorbitol Contents for Packaging Application" Foods 12, no. 5: 940. https://doi.org/10.3390/foods12050940