Development of a DNA Metabarcoding Method for the Identification of Insects in Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction
2.3. Reference Sequences and Primer Design
2.4. Library Preparation and NGS
2.5. NGS Data Analysis Using Galaxy
3. Results and Discussion
3.1. Analysis of DNA Extracts from Reference Samples
3.2. Analysis of DNA Extracts from Model Foods
3.3. Analysis of Insect Samples Commercially Available
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Athanassiou, C.G. ‘Insects as Food and Feed: If You Can’t Beat Them, Eat Them!’—To the Magnificent Seven and Beyond. J. Insect Sci. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Li, Y.O. Edible insects as a means to address global malnutrition and food insecurity issues. Food Qual. Saf. 2018, 2, 17–26. [Google Scholar] [CrossRef]
- Halloran, A.; Muenke, C.; Vantomme, P.; van Huis, A. Insects in the human food chain: Global status and opportunities. Food Chain 2014, 4, 103–118. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods, amending Regulation (EU) No. 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No. 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No. 1852/2001. Off. J. Eur. Union 2011, L327, 1–22. [Google Scholar]
- European Parliament and the Council of the European Union. Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 authorising the placing on the market of frozen, dried and powder forms of yellow mealworm (Tenebrio molitor larva) as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2011, L28, 10–16. [Google Scholar]
- European Parliament and the Council of the European Union. Commission Implementing Regulation (EU) 2021/1975 of 12 November 2021 authorising the placing on the market of frozen, dried and powder forms of Locusta migratoria as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2011, L402, 10–16. [Google Scholar]
- European Parliament and the Council of the European Union. Commission Implementing Regulation (EU) 2022/188 of 10 February 2022 authorising the placing on the market of frozen, dried and powder forms of Acheta domesticus as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2011, L30, 108–114. [Google Scholar]
- European Parliament and the Council of the European Union. Commission Implementing Regulation (EU) 2023/58 of 5 January 2023 authorising the placing on the market of the frozen, paste, dried and powder forms of Alphitobius diaperinus larvae (lesser mealworm) as a novel food and amending Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2011, L5, 10–16. [Google Scholar]
- Reichmuth, C. Vorratsschädlinge und Vorratsschutz im Wandel der Zeit. In Beiträge zum Göttinger Umwelthistorischen Kolloquium 2008–2009; Herrmann, B., Ed.; Universitätsverlag Göttingen: Göttingen, Germany, 2009; pp. 41–42. [Google Scholar]
- Haiminen, N.; Edlund, S.; Chambliss, D.; Kunitomi, M.; Weimer, B.C.; Ganesan, B.; Baker, R.; Markwell, P.; Davis, M.; Huang, B.C.; et al. Food authentication from shotgun sequencing reads with an application on high protein powders. NPJ Sci. Food 2019, 3, 24. [Google Scholar] [CrossRef]
- Busch, U. Molekulare Lebensmittelanalytik. In Molekularbiologische Methoden in der Lebensmittelanalytik; Busch, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–2. [Google Scholar]
- Debode, F.; Marien, A.; Gérard, A.; Francis, F.; Fumière, O.; Berben, G. Development of real-time PCR tests for the detection of Tenebrio molitor in food and feed. Food Addit. Contam. Part A 2017, 34, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Köppel, R.; Schum, R.; Habermacher, M.; Sester, C.; Piller, L.E.; Meissner, S.; Pietsch, K. Multiplex real-time PCR for the detection of insect DNA and determination of contents of Tenebrio molitor, Locusta migratoria and Achaeta domestica in food. Eur. Food Res. Technol. 2019, 245, 559–567. [Google Scholar] [CrossRef]
- Ali, M.A.; Gyulai, G.; Hidvégi, N.; Kerti, B.; Al Hemaid, F.M.; Pandey, A.K.; Lee, J. The changing epitome of species identification—DNA barcoding. Saudi J. Biol. Sci. 2014, 21, 204–231. [Google Scholar] [CrossRef]
- Marquina, D.; Andersson, A.F.; Ronquist, F. New mitochondrial primers for metabarcoding of insects, designed and evaluated using in silico methods. Mol. Ecol. Resour. 2018, 19, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Littlefair, J.E.; Clare, E.L. Barcoding the food chain: From Sanger to high-throughput sequencing. Genome 2016, 59, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shokralla, S.; Gibson, J.F.; Nikbakht, H.; Janzen, D.H.; Hallwachs, W.; Hajibabaei, M. Data from: Next-generation DNA barcoding: Using next-generation sequencing to enhance and accelerate DNA barcode capture from single specimens. Mol. Ecol. Resour. 2014, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Jinbo, U.; Kato, T.; Ito, M. Current progress in DNA barcoding and future implications for entomology. Èntomol. Sci. 2011, 14, 107–124. [Google Scholar] [CrossRef]
- Piper, A.M.; Batovska, J.; Cogan, N.O.I.; Weiss, J.; Cunningham, J.P.; Rodoni, B.C.; Blacket, M.J. Prospects and challenges of implementing DNA metabarcoding for high-throughput insect surveillance. GigaScience 2019, 8, giz092. [Google Scholar] [CrossRef]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef]
- Batovska, J.; Piper, A.M.; Valenzuela, I.; Cunningham, J.P.; Blacket, M.J. Developing a non-destructive metabarcoding protocol for detection of pest insects in bulk trap catches. Sci. Rep. 2021, 11, 7946. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Weinbrecht, K. Next-Generation Sequencing: Methodology and Application. J. Investig. Dermatol. 2013, 133, e11. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-Y.; Chen, X.; Murphy, R.W. Assessing DNA Barcoding as a Tool for Species Identification and Data Quality Control. PLoS ONE 2013, 8, e57125. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolny, S.; Blaschitz, M.; Weinmaier, T.; Pechatschek, J.; Cichna-Markl, M.; Indra, A.; Hufnagl, P.; Hochegger, R. Development of a DNA metabarcoding method for the identification of fifteen mammalian and six poultry species in food. Food Chem. 2018, 272, 354–361. [Google Scholar] [CrossRef]
- Gense, K.; Peterseil, V.; Licina, A.; Wagner, M.; Cichna-Markl, M.; Dobrovolny, S.; Hochegger, R. Development of a DNA Metabarcoding Method for the Identification of Bivalve Species in Seafood Products. Foods 2021, 10, 2618. [Google Scholar] [CrossRef] [PubMed]
- Preckel, L.; Brünen-Nieweler, C.; Denay, G.; Petersen, H.; Cichna-Markl, M.; Dobrovolny, S.; Hochegger, R. Identification of Mammalian and Poultry Species in Food and Pet Food Samples Using 16S rDNA Metabarcoding. Foods 2021, 10, 2875. [Google Scholar] [CrossRef]
- Dobrovolny, S.; Uhlig, S.; Frost, K.; Schlierf, A.; Nichani, K.; Simon, K.; Cichna-Markl, M.; Hochegger, R. Interlaboratory Validation of a DNA Metabarcoding Assay for Mammalian and Poultry Species to Detect Food Adulteration. Foods 2022, 11, 1108. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
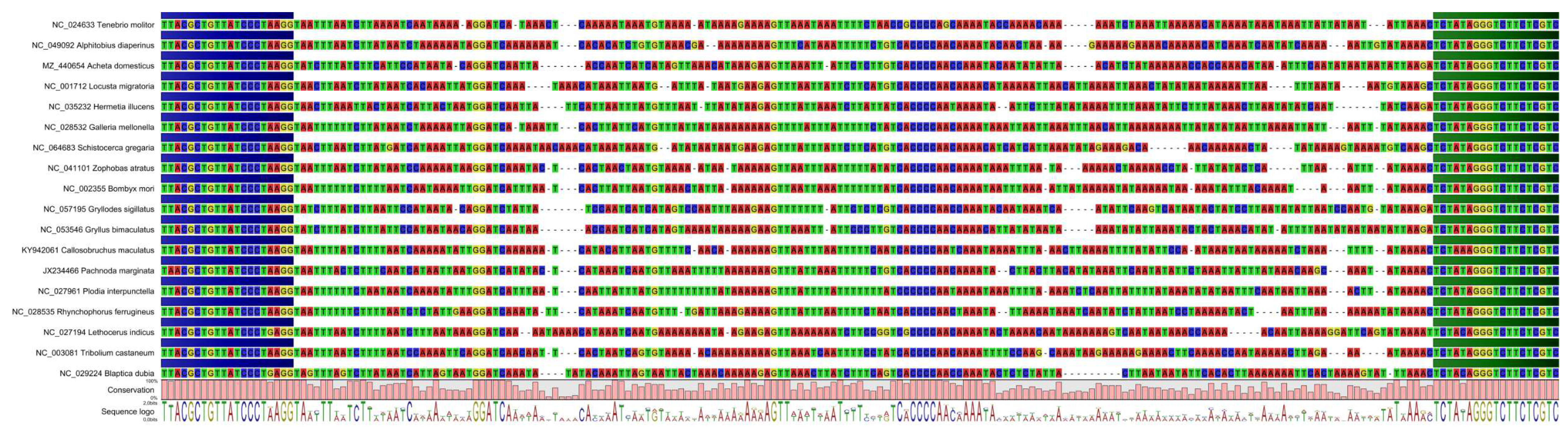
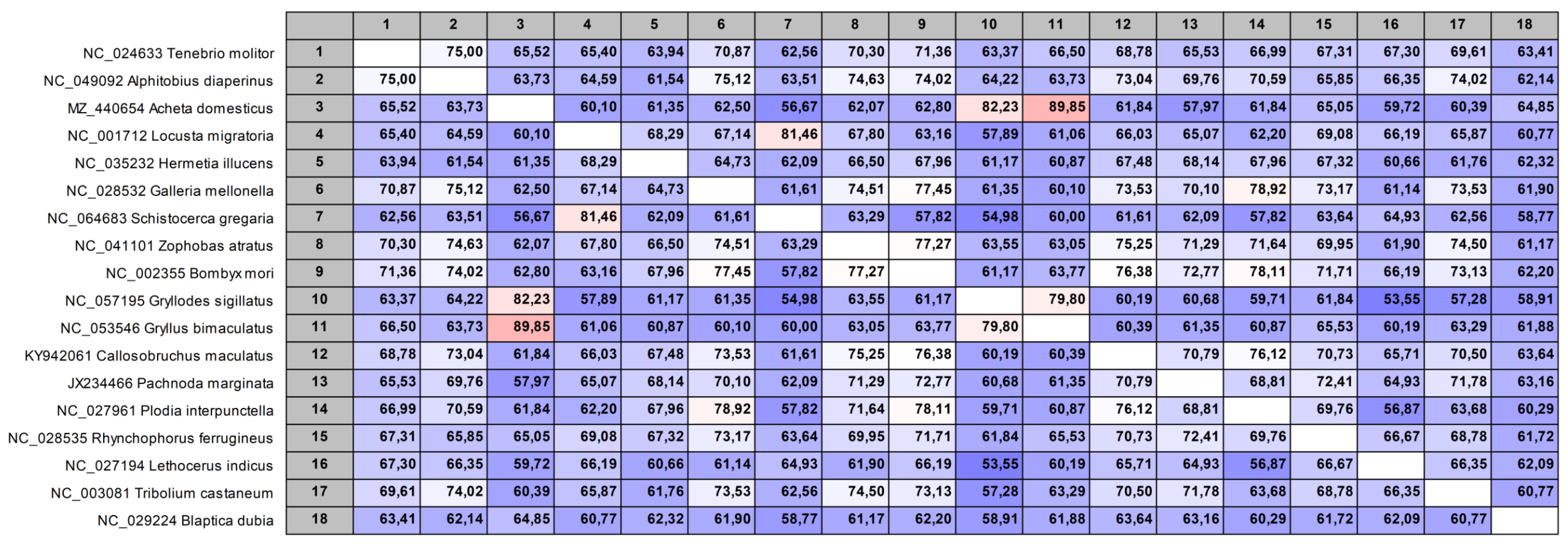
| Scientific Name | Commercial Name (English) |
|---|---|
| Tenebrio molitor | Mealworm |
| Alphitobius diaperinus | Buffalo worm |
| Acheta domesticus | House cricket |
| Locusta migratoria | Migratory locust |
| Hermetia illucens | Black soldier fly |
| Galleria mellonella | Greater wax moth |
| Schistocerca gregaria | Desert locust |
| Zophobas atratus | Superworm |
| Bombyx mori | Silkworm moth |
| Gryllodes sigillatus | Tropical house cricket |
| Gryllus bimaculatus | Mediterranean field cricket |
| Callosobruchus maculatus | Cowpea weevil |
| Pachnoda marginata | Sun beetle |
| Plodia interpunctella | Indian meal moth |
| Rhynchophorus ferrugineus | Red palm weevil |
| Lethocerus indicus | Water bug |
| Tribolium castaneum | Red flour beetle |
| Blaptica dubia | Cockroach |
| Name | Sequence 5′ → 3′ |
|---|---|
| Fwd-I-1 | TTACGCTGTTATCCCTAAGG |
| Fwd-I-2 | TAACGCTGTTATCCCTAAGG |
| Fwd-I-3 | TWACGCTGTTATCCCTAAGG |
| Fwd-I-4 | ACGCTGTTATCCCTAAGG |
| Rev-I-1 | GACGAGAAGACCCTATAGA |
| Rev-I-2 | GACGATAAGACCCTATAGA |
| Rev-I-3 | GACGAKAAGACCCTATAGA |
| Illumina Overhang Adapter Sequences | |
| Forward | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG |
| Reverse | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG |
| Scientific Name | Identified Species | Total Raw Reads | Total Reads Passing the Pipeline | Reads Assigned Correctly |
|---|---|---|---|---|
| Tenebrio molitor | Tenebrio molitor | 99,977 | 85,871 | 85,681 |
| Alphitobius diaperinus | Alphitobius diaperinus | 141,850 | 124,433 | 124,190 |
| Acheta domesticus | Acheta domesticus | 131,959 | 114,260 | 114,137 |
| Locusta migratoria | Locusta migratoria | 98,907 | 88,917 | 87,041 |
| Hermetia illucens | Hermetia illucens | 57,123 | 53,160 | 53,003 |
| Galleria mellonella | Galleria mellonella | 55,899 | 36,539 | 36,361 |
| Schistocerca gregaria | Schistocerca gregaria | 126,910 | 109,280 | 109,168 |
| Zophobas atratus | Zophobas atratus | 106,749 | 102,126 | 102,051 |
| Bombyx mori | Bombyx mori | 57,587 | 56,222 | 56,158 |
| Gryllodes sigillatus | Gryllodes sigillatus | 145,814 | 115,026 | 114,938 |
| Gryllus bimaculatus | Gryllus bimaculatus | 79,639 | 68,255 | 68,196 |
| Callosobruchus maculatus | Callosobruchus maculatus | 51,577 | 50,403 | 50,352 |
| Pachnoda marginata | Pachnoda marginata | 17,830 | 17,427 | 17,414 |
| Plodia interpunctella | Plodia interpunctella | 70,974 | 64,860 | 64,728 |
| Rhynchophorus ferrugineus | Rhynchophorus ferrugineus | 98,335 | 93,398 | 93,289 |
| Lethocerus indicus | Lethocerus indicus | 97,715 | 89,487 | 89,301 |
| Tribolium castaneum | Tribolium castaneum | 160,925 | 148,201 | 148,040 |
| Blaptica dubia | Blaptica dubia | 151,001 | 115,351 | 115,293 |
| Model Foods | Total Number of Raw Reads | Total Number of Reads Passing the Pipeline | Reads Assigned Correctly | ||||
|---|---|---|---|---|---|---|---|
| Cookies (Main component: wheat flour) | Species 1 | Species 2 | Species 3 | ||||
| Insect cookie 1 (proportion insects: 10% (w/w)) | 110,506 | 105,462 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 11,521 | 79,493 | 14,419 | ||
| Insect cookie 2 (proportion insects: 3.2% (w/w)) | 113,085 | 108,271 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 16,578 | 82,318 | 9259 | ||
| Insect cookie 3 (proportion insects: 1% (w/w)) | 103,454 | 98,752 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 22,975 | 65,476 | 10,247 | ||
| Insect cookie 4 (proportion insects: 0.32% (w/w)) | 76,268 | 72,307 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 17,810 | 45,327 | 9112 | ||
| Insect cookie 5 (proportion insects: 0.10% (w/w)) | 72,699 | 68,225 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 13,204 | 43,988 | 10,993 | ||
| Burgers (Main component: ground meat, asynchronous preparation) | |||||||
| Insect burger 1 (proportion insects: 3.2% (w/w)) | 126,563 | 110,249 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 1104 | 97,062 | 12,030 | ||
| Insect burger 2 (proportion insects: 1% (w/w)) | 98,628 | 60,013 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 45,442 | 12,137 | 2387 | ||
| Insect burger 3 (proportion insects: 0.32% (w/w)) | 97,647 | 82,260 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 63,118 | 18,658 | 387 | ||
| Insect burger 4 (proportion insects: 0.1% (w/w)) | 111,195 | 100,029 | |||||
| Locusta migratoria | Acheta domesticus | Tenebrio molitor | 5080 | 94,180 | 744 | ||
| Sample ID | Food Product | Insect Species Labeled (Amount in %, if Available) | Identified Species | Total Number of Raw Reads | Total Number of Reads Passing the Pipeline | Reads Assigned Correctly |
|---|---|---|---|---|---|---|
| 1 | Insect mushroom soup | Buffalo worm meal (26%) | Alphitobius diaperinus | 116,024 | 112,783 | 112,695 |
| 2 | Insect pancake ready mix | Buffalo worm meal (7.1%) | Alphitobius diaperinus | 139,113 | 134,493 | 134,361 |
| 3 | Insect beetroot risotto mix | Alphitobius diaperinus | Alphitobius diaperinus | 132,667 | 128,963 | 128,839 |
| 4 | Insect brownie mix | Buffalo worm meal (5.5%) | Alphitobius diaperinus | 151,134 | 146,191 | 145,884 |
| 5 | Insect oatmeal patty | Alphitobius diaperinus | Alphitobius diaperinus | 100,524 | 97,449 | 97,409 |
| 6 | Insect bread | Buffalo worm meal (4.8%) | Alphitobius diaperinus | 106,194 | 102,849 | 102,635 |
| 7 | Chocolate with worm | Alphitobius diaperinus (0.1%) | Alphitobius diaperinus | 127,168 | 123,370 | 123,302 |
| 8 | Insect meal (buffalo worms) | Alphitobius diaperinus (100%) | Alphitobius diaperinus | 120,078 | 116,090 | 116,007 |
| 9 | Insect bar sour cherry | Milled buffalo worms (12%) | Alphitobius diaperinus | 155,677 | 150,368 | 150,267 |
| 10 | Insect bar apple strudel | Milled buffalo worms (12%) | Alphitobius diaperinus | 132,100 | 127,302 | 127,221 |
| 11 | Insect bar apricot | Milled buffalo worms (13%) | Alphitobius diaperinus | 175,897 | 170,155 | 170,112 |
| 12 | Protein drink with cocoa taste | Buffalo worm protein (50%) | Alphitobius diaperinus | 137,082 | 132,930 | 132,887 |
| 13 | Protein drink with strawberry taste | Buffalo worm protein (50%) | Alphitobius diaperinus | 117,959 | 114,836 | 114,761 |
| 14 | Protein drink with vanilla taste | Buffalo worm protein (50%) | Alphitobius diaperinus | 134,025 | 129,917 | 129,846 |
| 15 | Protein drink with banana taste | Buffalo worm protein (50%) | Alphitobius diaperinus | 112,744 | 109,573 | 109,482 |
| 16 | Mealworm flour | Mealworm | Alphitobius diaperinus | 115,509 | 111,621 | 111,530 |
| 17 | Porridge with chocolate-hazelnut taste | Buffalo worms (20%) | Alphitobius diaperinus | 129,371 | 125,431 | 125,395 |
| 18 | Porridge with raspberry-coco taste | Buffalo worms (20%) | Alphitobius diaperinus | 118,337 | 114,805 | 114,699 |
| 19 | Insect peanut creme | Buffalo worm protein (17%) | Alphitobius diaperinus | 126,108 | 122,465 | 122,420 |
| 20 | Insect meal (buffalo worms) | Buffalo worm | Alphitobius diaperinus | 128,800 | 125,408 | 125,348 |
| 21 | Energy bar with roasted worms | Dried buffalo worms (10%) | Alphitobius diaperinus | 135,324 | 130,434 | 130,348 |
| 22 | Insect candy lollipop | Dried buffalo worms | Alphitobius diaperinus | 215,121 | 209,388 | 209,339 |
| 23 | Insect burger patty | Alphitobius diaperinus (38%) | Alphitobius diaperinus | 104,859 | 101,862 | 101,861 |
| 24 | Insect meal (mealworms) | Tenebrio molitor (100%) | Tenebrio molitor | 87,354 | 73,377 | 73,242 |
| 25 | Dark chocolate with mealworms | Roasted freeze-dried mealworms (2%) | Tenebrio molitor | 104,484 | 89,683 | 89,535 |
| 26 | Milk chocolate with mealworms | Roasted freeze-dried mealworms (2%) | Tenebrio molitor | 92,543 | 76,521 | 76,371 |
| 27 | Insect candy lollipop | Roasted freeze-dried mealworms | Tenebrio molitor | 115,989 | 100,325 | 100,213 |
| 28 | Crickets | Cricket | Acheta domesticus | 105,620 | 102,960 | 102,931 |
| 29 | Cricket flour | Cricket | Acheta domesticus | 121,121 | 115,782 | 115,608 |
| 30 | Cricket pasta | Cricket | Acheta domesticus | 241,867 | 232,106 | 232,037 |
| 31 | Fried crickets | Cricket | Acheta domesticus | 99,982 | 97,911 | 97,854 |
| 32 | Spicy crickets | Acheta domesticus (90%) | Acheta domesticus | 103,818 | 98,562 | 98,519 |
| 33 | Smoked crickets | Cricket | Acheta domesticus | 110,766 | 107,170 | 107,050 |
| 34 | Insect candy lollipop | Roasted freeze-dried crickets | Acheta domesticus | 173,377 | 164,565 | 164,495 |
| 35 | Cricket cracker | Acheta domesticus (15%) | Acheta domesticus | 150,410 | 143,659 | 143,574 |
| 36 | Cricket cracker | Acheta domesticus (15%) | Acheta domesticus | 130,263 | 124,414 | 124,295 |
| 37 | Cricket cracker | Acheta domesticus (15%) | Acheta domesticus | 134,728 | 129,629 | 129,506 |
| 38 | Insect bar | Cricket meal (15%) | Acheta domesticus | 133,735 | 127,033 | 126,929 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillinger, S.; Saeckler, J.; Domig, K.J.; Dobrovolny, S.; Hochegger, R. Development of a DNA Metabarcoding Method for the Identification of Insects in Food. Foods 2023, 12, 1086. https://doi.org/10.3390/foods12051086
Hillinger S, Saeckler J, Domig KJ, Dobrovolny S, Hochegger R. Development of a DNA Metabarcoding Method for the Identification of Insects in Food. Foods. 2023; 12(5):1086. https://doi.org/10.3390/foods12051086
Chicago/Turabian StyleHillinger, Sophie, Julia Saeckler, Konrad J. Domig, Stefanie Dobrovolny, and Rupert Hochegger. 2023. "Development of a DNA Metabarcoding Method for the Identification of Insects in Food" Foods 12, no. 5: 1086. https://doi.org/10.3390/foods12051086
APA StyleHillinger, S., Saeckler, J., Domig, K. J., Dobrovolny, S., & Hochegger, R. (2023). Development of a DNA Metabarcoding Method for the Identification of Insects in Food. Foods, 12(5), 1086. https://doi.org/10.3390/foods12051086






