Salicylic Acid and Jasmonic Acid Increase the Polysaccharide Production of Nostoc flagelliforme via the Regulation of the Intracellular NO Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Strains and Growth Conditions
2.3. Extraction and Purification of the CPS
2.4. Yield of Crude Polysaccharide
2.5. Chemical Analysis
2.6. Structural Characterization
2.6.1. Ultraviolet (UV) Spectra
2.6.2. Fourier-Transform Infrared (FT-IR) Spectra
2.7. Evaluation of Antioxidant Activity
2.8. Determination of Nitric Oxide Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Screening of Chemical Inducers for Increasing the Polysaccharide Accumulation
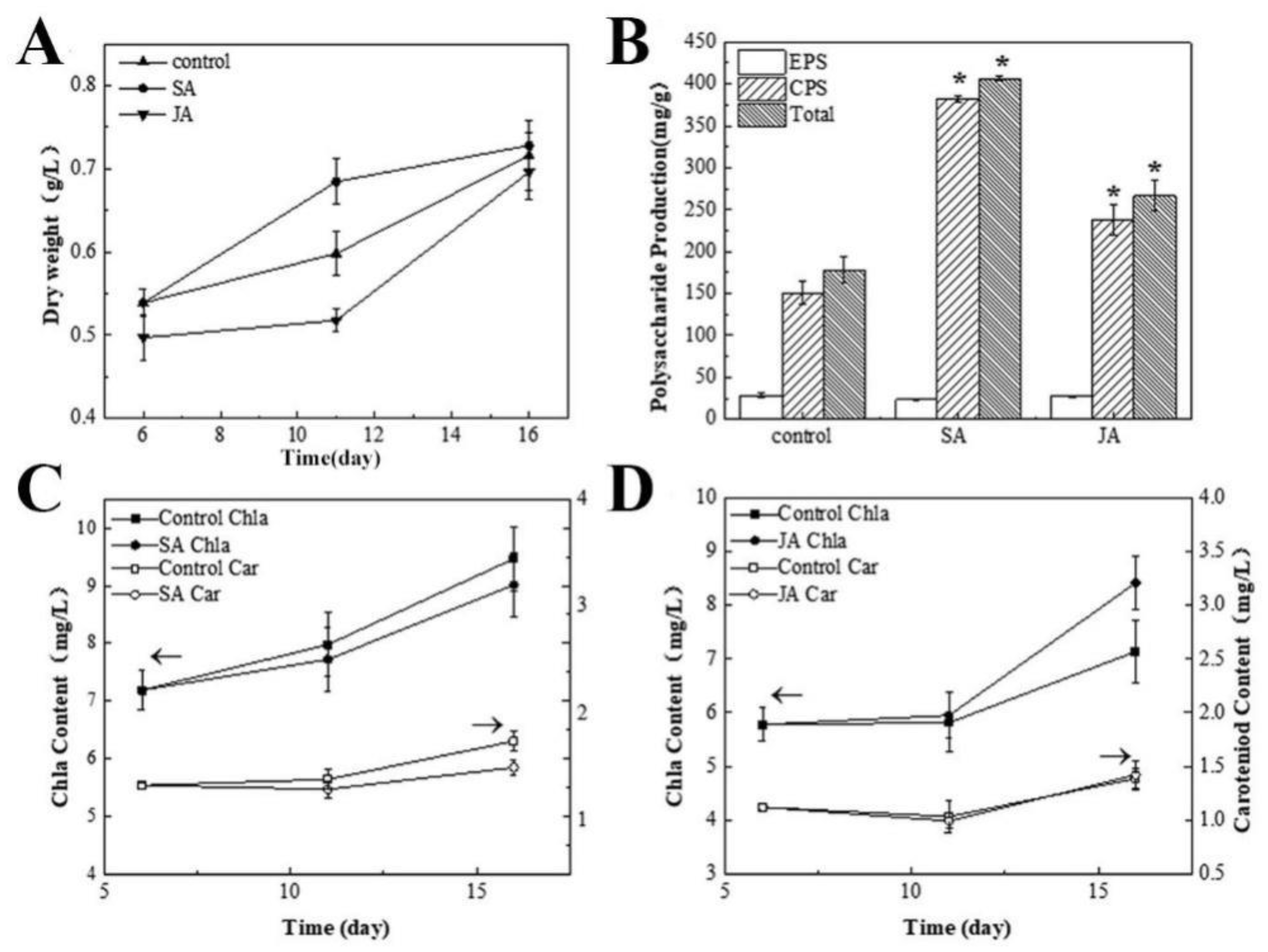
3.2. The Effects of SA and JA on the Culture of N. flagelliforme
3.3. The Effects of SA and JA on the Physicochemical Properties and Antioxidant Activity of Polysaccharides
3.3.1. Analysis of the Basic Physicochemical Properties
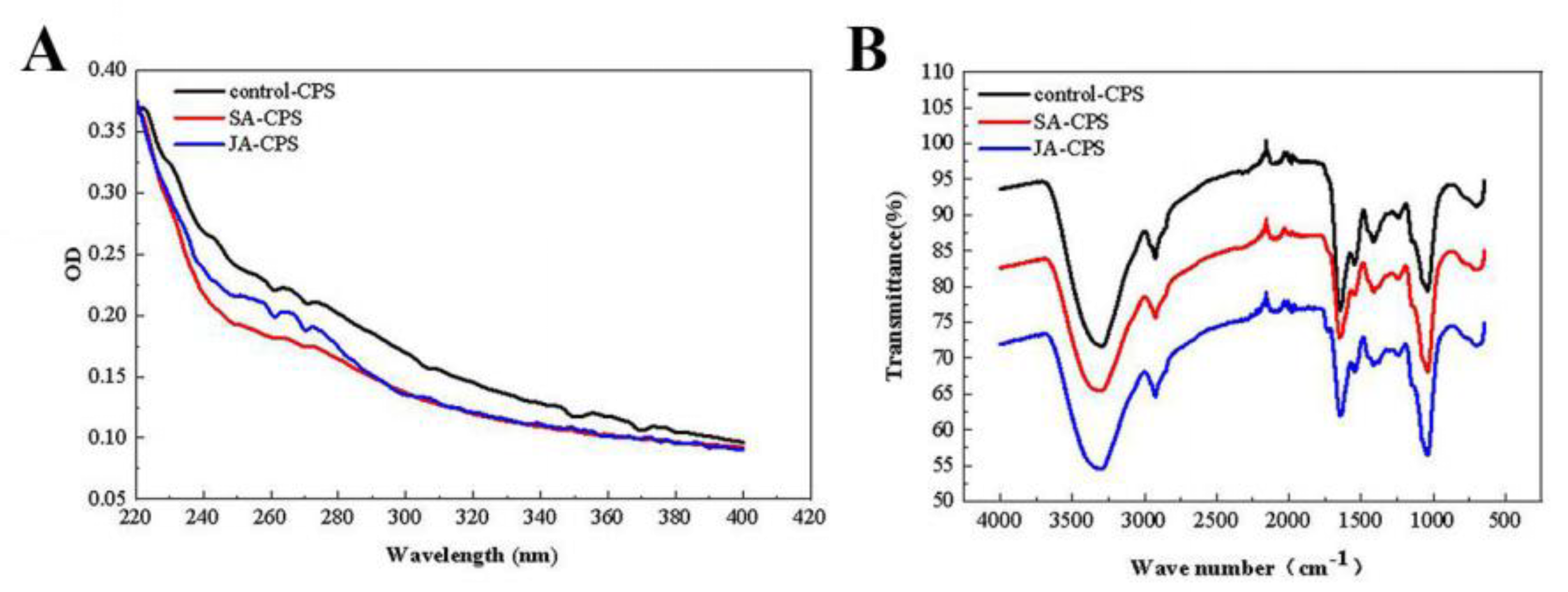
3.3.2. UV and FT-IR Analysis
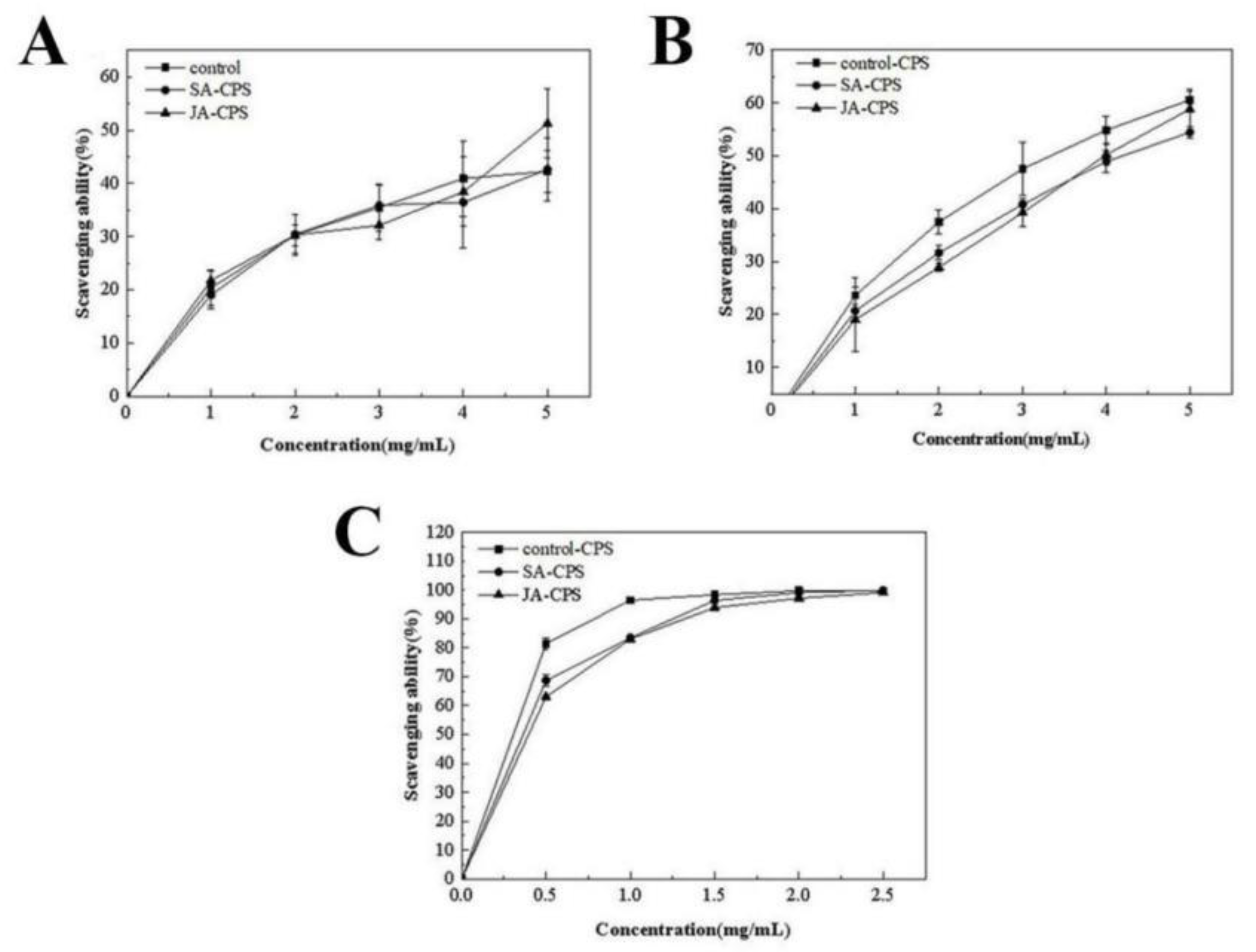
3.3.3. In Vitro Antioxidant Activity
3.4. The Possible Mechanisms through Which SA and JA Affect the Accumulation of Polysaccharides in N. flagelliforme
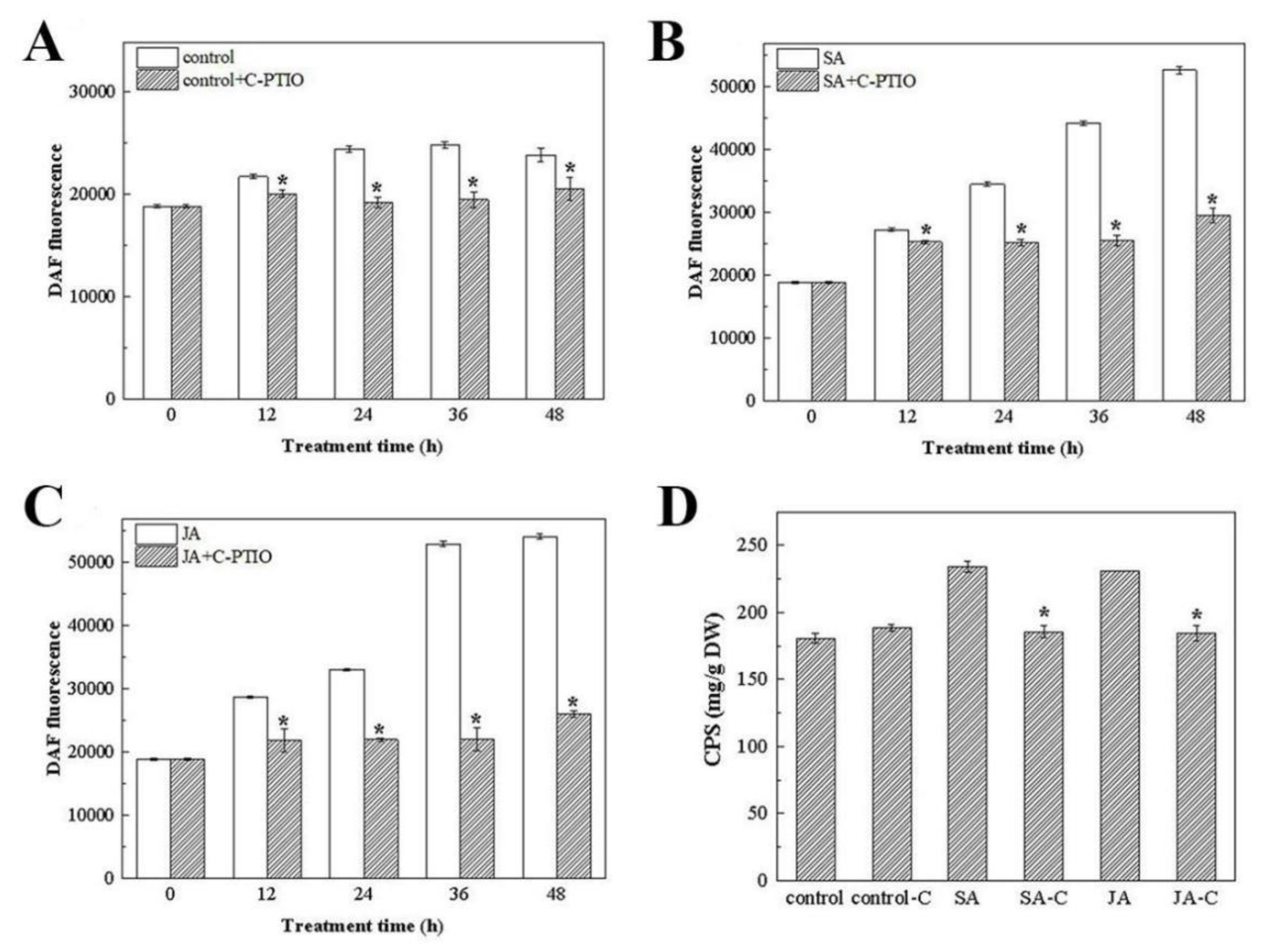
3.4.1. Changes in the NO Content under SA and JA Induction
3.4.2. The Effect of the Exogenous NO Scavenger C-PTIO on the NO Level and Polysaccharide Production
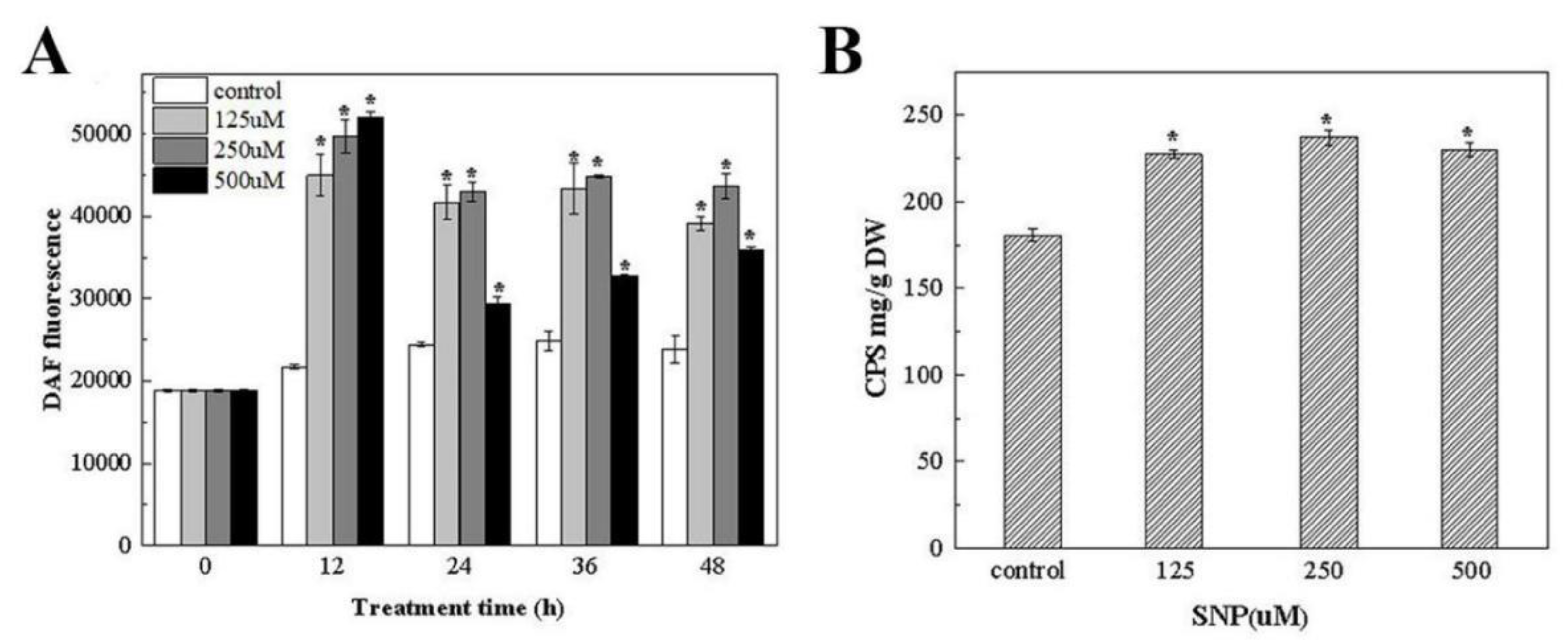
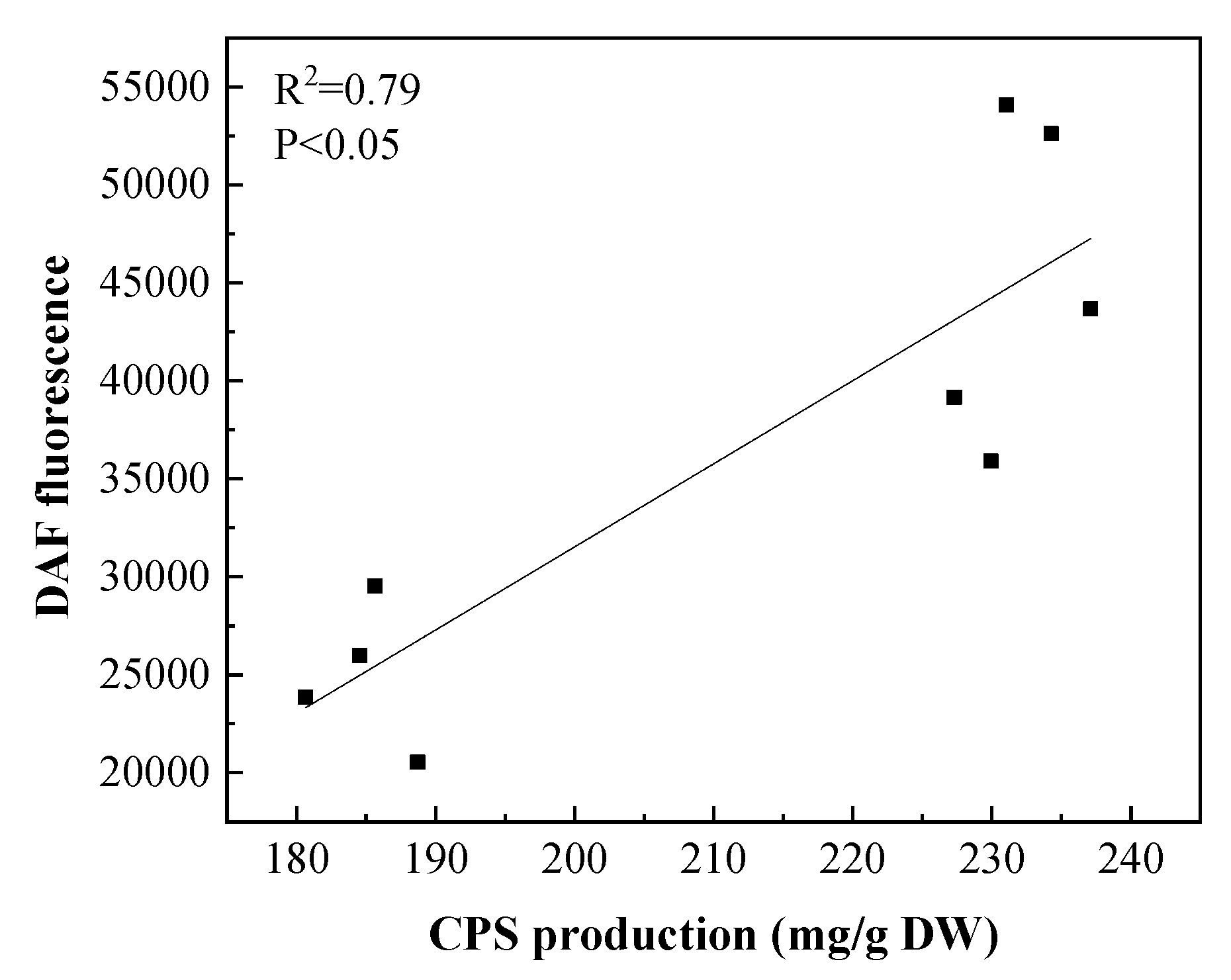
3.4.3. The Effect of the Exogenous NO Donor SNP on the NO Level and Polysaccharide Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, P.P.; Sun, Y.; Jia, S.R.; Zhong, C.; Tan, Z.L. Effects of light wavelengths on extracellular and capsular polysaccharide production by Nostoc flagelliforme. Carbohydr. Polym. 2014, 105, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Wang, H.; Guo, G.L.; Pu, Y.F.; Yan, B.L. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.X.; Zhu, H.R.; Chen, S.G. The ecological conditions for Nostoc flagelliforme and their analysis. Acta Phytoecol. Et Geobot. Sin. 1989, 13, 97–105. [Google Scholar]
- Takenaka, H.; Yamaguchi, Y.; Sakaki, S.; Watarai, K.; Tanaka, N.; Hori, M.; Seki, H.; Tsuchida, M.; Yamada, A.; Nishimori, T.; et al. Safety evaluation of Nostoc flagelliforme (nostocales, cyanophyceae) as a potential food. Food Chem. Toxicol. 1998, 12, 1073–1077. [Google Scholar] [CrossRef]
- Hayashi, K.K.K.; Ohta, Y.; Lee, J.B.; Hayashi, T. Anti-influenza a virus activity of an acidic polysaccharide from a blue-green alga Nostoc flagelliforme. J. Exerc. Physiol. Online Planta Med. 2008, 74, 946. [Google Scholar] [CrossRef]
- Kanekiyo, K.H.K.; Takenaka, H.; Lee, J.B.; Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef]
- Kanekiyo, J.B.L.; Hayashi, K.K.; Takenaka, H.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037. [Google Scholar] [CrossRef]
- Sara, P.; Andrea, Z.; Ernesto, M.; Pedro, M.F.; Roberto, D.P.; Paula, T. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2010, 33, 917–941. [Google Scholar]
- Rehm, H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578. [Google Scholar] [CrossRef]
- Han, P.P.; Guo, R.J.; Shen, S.G.; Yan, R.R.; Wu, Y.K.; Yao, S.Y.; Wang, H.Y.; Jia, S.R. Proteomic profiling of Nostoc flagelliforme reveals the common mechanism in promoting polysaccharide production by different light qualities. Biochem. Eng. J. 2018, 132, 68–78. [Google Scholar] [CrossRef]
- Han, P.P.; Yao, S.Y.; Guo, R.J.; Yan, R.R.; Wu, Y.K.; Shen, S.G.; Jia, S.R. Influence of culture conditions on extracellular polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Nostoc flagelliforme. RSC Adv. 2017, 7, 45075–45084. [Google Scholar] [CrossRef]
- Shen, S.G.; Jia, S.R.; Wu, Y.K.; Yan, R.R.; Lin, Y.H. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Zhao, J.; Li, J.H.; Li, S.S.; Zhang, L.H.; Wu, M. Effects of Calcium Levels on Colonial Aggregation and Buoyancy of Microcystis aeruginosa. Curr. Microbiol. 2011, 62, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Q.; Meng, C.X.; Zhang, X.W.; Xu, D.; Zhao, Y.F.; Wang, Y.T.; Lv, H.X.; Yang, L.M.; Chen, L.L.; Ye, N.H. Differential Expression of Carotenogenic Genes, Associated Changes on Astaxanthin Production and Photosynthesis Features Induced by JA in H. pluvialis. PLoS ONE 2012, 7, 42243. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, F.; Wang, C.; Wang, Z.Y.; Li, Y.Q. The boosted biomass and lipid accumulation in Chlorella vulgaris by supplementation of synthetic phytohormone analogs. Bioresour. Technol. 2017, 232, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Z.X.; Sun, H.; Guo, L.P. Smoke-Isolated Karrikins Stimulated Tanshinones Biosynthesis in Salvia miltiorrhiza through Endogenous Nitric Oxide and Jasmonic Acid. Molecules 2019, 24, 1229. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Li, Z.P. Effect of Nitric Oxide on Polysaccharide Production of Ganoderma lucidum. J. Anhui Sci. Technol. Univ. 2013, 27, 4–5. [Google Scholar]
- Zhang, K.; Gao, S.; Liu, S. Study on the Signaling Role of NO in Low Temperature Induced Polysaccharide Synthesis of Dendrobium candidum Protocorm. 2015 China Ornamental Horticulture Academic Seminar 2015.
- Dong, J.F.; Xu, M.J.; Zhu, M.Y. Effect of nitric oxide on catharanthine production and growth of Catharanthus roseus suspension cells. Biotechnol. Biotechnol. Bioeng 2005, 89, 367–372. [Google Scholar]
- Xu, M.J.; Dong, J.F.; Zhu, M.Y. Nitric Oxide Mediates the Fungal Elicitor-Induced Hypericin Production of Hypericum perforatum Cell Suspension Cultures through a Jasmonic-Acid-Dependent Signal Pathway. Plant Physiol. 2005, 139, 991–998. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.D.; Ansary, M.M.U.; Fujita, M.; Tran, L.S.P. Interactive Effects of Salicylic Acid and Nitric Oxide in Enhancing Rice Tolerance to Cadmium Stress. Int. J. Mol. Sci. 2019, 20, 5798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhang, Y.; Nie, S.; Xu, F.; He, S.; Gong, D.; Wu, G.; Tan, L. Physicochemical properties and in vitro antioxidant activities of polysaccharide from Artocarpus heterophyllus Lam. Carbohydr. Polym. 2017, 155, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.P.; Ding, S.D.; Ai, L.Z.; Deng, K. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohydr. Polym. 2012, 90, 870–874. [Google Scholar] [CrossRef]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Yao, S.Y.; Guo, R.J.; Shen, S.G.; Yan, R.R.; Tan, Z.L.; Jia, S.R. The relationship between monosaccharide composition of extracellular polysaccharide and activities of related enzymes in Nostoc flagelliforme under different culture conditions. Carbohydr. Polym. 2017, 147, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shi, L.; Zhu, T.; Yang, T.; Ren, A.; Zhu, J.; Zhao, M.W. Cross Talk between Nitric Oxide and Calcium-Calmodulin Regulates Ganoderic Acid Biosynthesis in Ganoderma lucidum under Heat Stress. Appl Environ Microbiol 2018, 84, e00043-18. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Fanning, K.; Netzel, M.; Schenk, P.M. Induced carotenoid accumulation in Dunaliella salina and Tetraselmis suecica by plant hormones and UV-C radiation. Appl. Microbiol. Biotechnol. 2015, 99, 9407–9416. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; Cha, T.S. Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris (Trebouxiophyceae) during early stationary growth phase. Algal Res. 2015, 9, 14–20. [Google Scholar] [CrossRef]
- Zhang, H.X.; Xiang, R.J.; Zeng, Q.J.; Li, X.F.; Zhang, X.Z.; Gu, X.P. Effects of exogenous methyl jasmonate induction on polysaccharide metabolism and key enzyme gene expression in Lentinula edodes. J. Fungi 2020, 39, 2338–2345. [Google Scholar]
- Wu, W.L.; Zhu, Y.T.; Zhang, L.; Yang, R.W.; Zhou, Y.H. Extraction, preliminary structural characterization, and antioxidant activities of polysaccharides from Salvia miltiorrhiza Bunge. Carbohydr. Polym. 2012, 87, 1348–1353. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.S.; Zhao, M.M.; Liu, Y.; Wang, W.; Jiang, Y.M. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr. Res. 2006, 341, 634–638. [Google Scholar] [CrossRef]
- Xie, X.; Zou, G.; Li, C. Purification, characterization and in vitro antioxidant activities of polysaccharide from Chaenomeles speciosa. Int. J. Biol. Macromol. 2016, 92, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, B.; Ibrahim, S.A.; Gao, S.S.; Yang, H.; Huang, W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016, 145, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, B.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH. Free Radical Method. LWT—Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Khaskheli, S.G.; Zheng, W.; Sheikh, S.A.; Khaskheli, A.A.; Liu, Y.; Soomro, A.H.; Feng, X.; Sauer, M.B.; Wang, Y.F.; Huang, W. Characterization of Auricularia auricula polysaccharides and its antioxidant properties in fresh and pickled product. Int. J. Biol. Macromol. 2015, 81, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Galley, H.F.; Webster, N.R. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003, 90, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, S.S.; Ibrahim, S.A.; Li, E.H.; Yang, H.; Huang, W. Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chem. 2015, 185, 159–164. [Google Scholar] [CrossRef]
- Osman, W.K.; Fernyhough, A.M.A. ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts. Biochem. Biophys. Res. Commun. 2006, 346, 321–329. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.P.; Wang, J.W. Nitric oxide elicitation for secondary metabolite production in cultured plant cells. Appl. Microbiol. Biotechnol. 2012, 93, 455–466. [Google Scholar] [CrossRef]
- Dong, J.F.; Xu, M.J. Synergistic Action between Jasmonic Acid and Nitric Oxide in Inducing Matrine Accumulation of Sophora flavescens Suspension Cells. J. Integr. Plant Biol. 2008, 50, 92–101. [Google Scholar]
- Klepper, L. No, evolution by soybean leaves treated with salicylic acid and selected derivatives. Pestic. Biochem. Physiol. 1991, 39, 43–48. [Google Scholar] [CrossRef]
- Ni, N.T.; Livaja, M.J.; Kieber, J.; Scherer, G. Zeatin-induced nitric oxide (NO) biosynthesis in Arabidopsis thaliana mutants of NO biosynthesis and of two-component signaling genes. New Phytol. 2010, 178, 515–531. [Google Scholar]
- Kolbert, Z.; Bartha, B.; Erdei, L. Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J. Plant Physiol. 2008, 165, 975. [Google Scholar] [CrossRef] [PubMed]
- Zottini, M.; Costa, A.; De Michele, R.; Ruzzene, M.; Carimi, F.; Lo Schiavo, F. Salicylic acid activates nitric oxide synthesis in Arabidopsis. J. Exp. Bot. 2007, 58, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Luis, S.; Pablo, A.; Isabel, M.; Inmaculada, S.V.; Tamara, L.; María, F.M.; Oscar, L. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015, 66, 2857–2868. [Google Scholar]

| Compound Classification | Compound | Optimum Concentration | Increase in Polysaccharide Production (%) |
|---|---|---|---|
| Phytohormones | 2,4-D | 0.25 mg/L | 109.7 |
| GA | 10 mg/L | 30.19 | |
| Metal ions | Ca2+ | 10 mg/L | 27.23 |
| Mg2+ | - | - | |
| Vitamins | VB12 | - | - |
| Oxidizing agents | AAPH | - | - |
| MB | 3 mg/L | 15.54 | |
| H2O2 | 60 mg/L | 18.92 | |
| Antioxidants | PG | 200 mg/L | 86.15 |
| Signaling reagents | JA | 1 mg/L | 58.57 |
| SA | 1 mg/L | 35.33 | |
| AHLs | 0.5 mg/L | 74.17 |
| Basic Physicochemical Properties | Sample Name | |||
|---|---|---|---|---|
| Control-CPS | SA-CPS | JA-CPS | ||
| Chemical composition (%) | Total sugar | 84.47 ± 2.17 | 87.67 ± 3.23 | 89.59 ± 2.75 |
| Protein | 0.49 ± 0.04 | 0.54 ± 0.02 | 0.44 ± 0.03 | |
| Uronic acid | 15.03 ± 1.32 | 11.79 ± 1.25 | 10.17 ± 1.46 | |
| Total polyphenols | ND | ND | ND | |
| Average molecular weights (kDa) | 2.06 × 103 | 2.16 × 103 | 2.04 × 103 | |
| Monosaccharide composition (percentage %) | Xylose | 0.85 ± 0.01 | 0.82 ± 0.11 | 0.68 ± 0.01 |
| Arabinose | 1.81 ± 0.01 | 2.08 ± 0.02 | 1.63 ± 0.04 | |
| Ribose | 2.98 ± 0.01 | 3.59 ± 0.02 | 3.47 ± 0.16 | |
| Rhamnose | 5.60 ± 0.12 | 6.67 ± 0.01 | 4.75 ± 0.11 | |
| Fucose | 1.98 ± 1.04 | 1.86 ± 0.03 | 1.28 ± 0.04 | |
| Fructose | 0.33 ± 0.05 | 0.44 ± 0.05 | 0.57 ± 0.02 | |
| Mannose | 16.87 ± 0.15 | 18.45 ± 0.26 | 16.41 ± 0.16 | |
| Galactose | 17.84 ± 0.18 | 19.74 ± 0.33 | 17.28 ± 0.12 | |
| Glucose | 46.39 ± 0.62 | 40.44 ± 0.44 | 48.80 ± 0.19 | |
| Glucuronic acid | 4.97 ± 0.16 | 5.89 ± 0.99 | 5.11 ± 0.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.-F.; Liu, S.-T.; Yan, R.-R.; Li, J.; Chen, N.; Zhang, L.-L.; Jia, S.-R.; Han, P.-P. Salicylic Acid and Jasmonic Acid Increase the Polysaccharide Production of Nostoc flagelliforme via the Regulation of the Intracellular NO Level. Foods 2023, 12, 915. https://doi.org/10.3390/foods12050915
Han C-F, Liu S-T, Yan R-R, Li J, Chen N, Zhang L-L, Jia S-R, Han P-P. Salicylic Acid and Jasmonic Acid Increase the Polysaccharide Production of Nostoc flagelliforme via the Regulation of the Intracellular NO Level. Foods. 2023; 12(5):915. https://doi.org/10.3390/foods12050915
Chicago/Turabian StyleHan, Cheng-Feng, Shu-Ting Liu, Rong-Rong Yan, Jian Li, Ni Chen, Le-Le Zhang, Shi-Ru Jia, and Pei-Pei Han. 2023. "Salicylic Acid and Jasmonic Acid Increase the Polysaccharide Production of Nostoc flagelliforme via the Regulation of the Intracellular NO Level" Foods 12, no. 5: 915. https://doi.org/10.3390/foods12050915
APA StyleHan, C.-F., Liu, S.-T., Yan, R.-R., Li, J., Chen, N., Zhang, L.-L., Jia, S.-R., & Han, P.-P. (2023). Salicylic Acid and Jasmonic Acid Increase the Polysaccharide Production of Nostoc flagelliforme via the Regulation of the Intracellular NO Level. Foods, 12(5), 915. https://doi.org/10.3390/foods12050915





