Impact of Air-Drying Temperature on Antioxidant Properties and ACE-Inhibiting Activity of Fungal Fermented Lentil Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fungal Solid-State Fermentation
2.2.1. Starter Culture Preparation
2.2.2. Fermentation Process
2.3. Drying and Milling of Fermented Grains/Seeds
2.4. Drying Kinetics and Modelling
2.5. Analytical Determinations
2.5.1. Proximal Substrate Composition
2.5.2. Reducing Sugars
2.5.3. Fungus Biomass
2.5.4. pH and Water Activity (aw)
2.5.5. Colour
2.5.6. Particle Size
2.5.7. Phytic Acid Content
2.5.8. Phenolic Compounds by HPLC Analysis
2.5.9. Total Phenolic Content
2.5.10. Antioxidant Activity
2.5.11. Angiotensin-Converting Enzyme Inhibitory Activity (ACE ia (%))
2.5.12. Microbiological Analysis
2.5.13. Statistical Analysis
3. Results and Discussion
3.1. Changes in Proximal Composition Induced by Fungal Solid-State Fermentation of Lentils
3.2. Air Drying Kinetics of Fermented Lentils
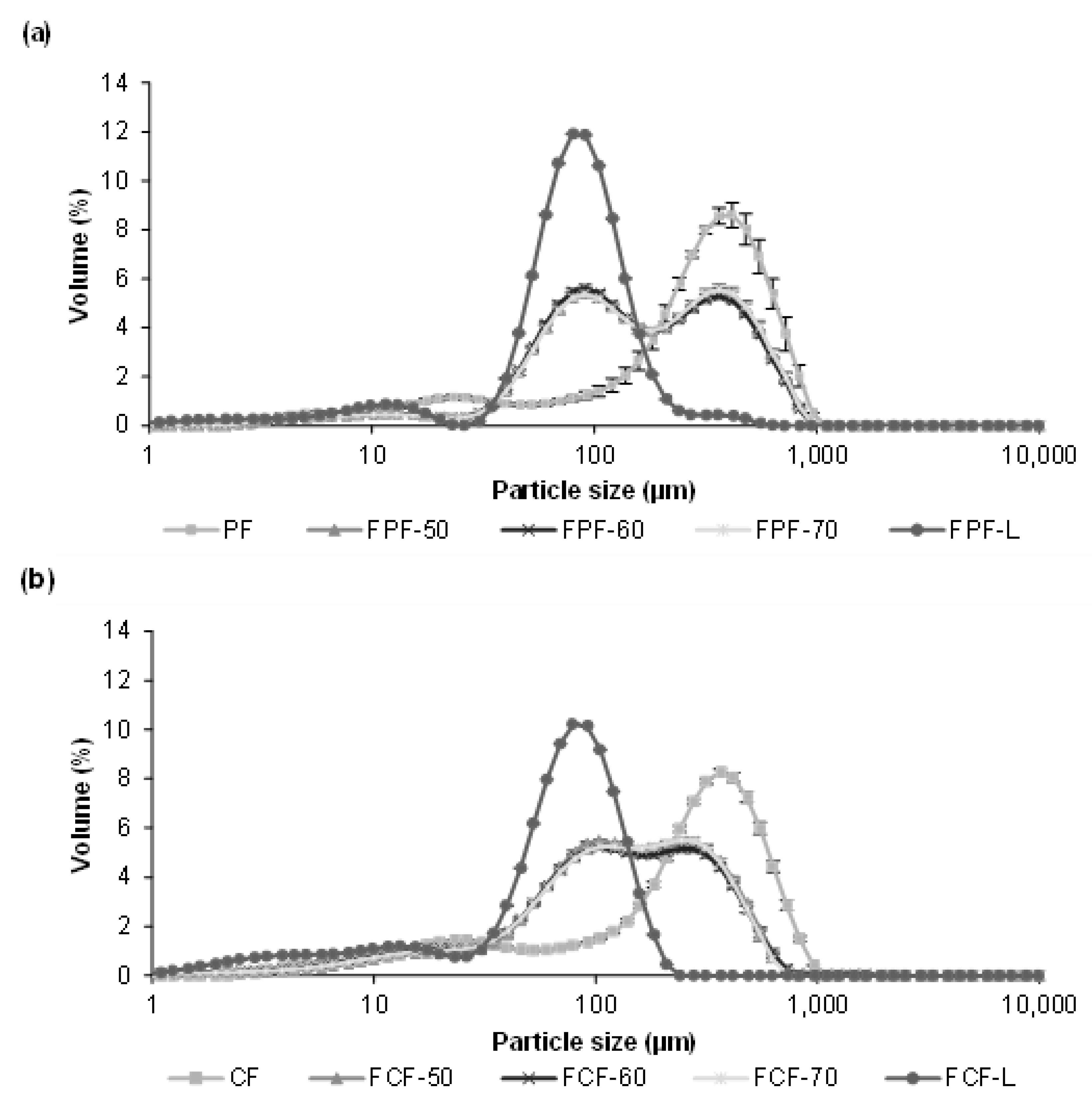
3.3. Impact of Processing on Particle Size, Colour, and Phytic Acid of Fermented Flours
3.4. Impact of Processing on Antioxidant and Anti- Hypertensive Properties of Fermented Flours
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maphosa, Y.; Jideani, V.A. The Role of Legumes in Human Nutrition. In Functional Food-Improve Health through Adequate Food; InTech: London, UK, 2017; Volume 1, pp. 103–122. [Google Scholar]
- Polak, R.; Phillips, E.M.; Campbell, A. Legumes: Health Benefits and Culinary Approaches to Increase Intake. Clin. Diabetes 2015, 33, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Calvo-Lerma, J.; Asensio-Grau, A.; García-Hernández, J.; Heredia, A.; Andrés, A. Exploring the Impact of Solid-State Fermentation on Macronutrient Profile and Digestibility in Chia (Salvia hispanica) and Sesame (Sesamum indicum) Seeds. Foods 2022, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Páez, E.; Alanis-Guzmán, M.G.; Hernández-Luna, C.E.; Báez-González, J.G.; Amaya-Guerra, C.A.; Andrés-Grau, A.M. Increasing Antioxidant Activity and Protein Digestibility in Phaseolus vulgaris and Avena sativa by Fermentation with the Pleurotus ostreatus Fungus. Molecules 2017, 22, 2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogeveen, G.W.M.; Hoogeveen, H.W. System for Solid State Fermentation and Use Thereof. EP2186876A1, 19 May 2010. [Google Scholar]
- Lyons, M.P.; Hoskins, B.J. Compositions and Methods for Conversion of Lignocellulosic Material to Fermentable Sugars and Products Produced Therefrom. CA2755449C, 25 February 2014. [Google Scholar]
- Sánchez-García, J.; Asensio-Grau, A.; García-Hernández, J.; Heredia, A.; Andrés, A. Nutritional and antioxidant changes in lentils and quinoa through fungal solid-state fermentation with Pleurotus ostreatus. Bioresour. Bioprocess. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. Evaluation of Selected Culinary-Medicinal Mushrooms for Antioxidant and ACE Inhibitory Activities. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tuck, M. Management of Hypertension in the Patient with Diabetes Mellitus: Focus on the Use of Angiotensin-Converting Enzyme Inhibitors. Am. J. Hypertens. 1988, 1, 384S–388S. [Google Scholar] [CrossRef]
- Marchini, M.; Carini, E.; Cataldi, N.; Boukid, F.; Blandino, M.; Ganino, T.; Vittadini, E.; Pellegrini, N. The use of red lentil flour in bakery products: How do particle size and substitution level affect rheological properties of wheat bread dough? LWT-Food Sci. Technol. 2021, 136, 110299. [Google Scholar] [CrossRef]
- Romano, A.; Gallo, V.; Ferranti, P.; Masi, P. Lentil flour: Nutritional and technological properties, in vitro digestibility and perspectives for use in the food industry. Curr. Opin. Food Sci. 2021, 40, 157–167. [Google Scholar] [CrossRef]
- Patrón-Vázquez, J.; Baas-Dzul, L.; Medina-Torres, N.; Ayora-Talavera, T.; Sánchez-Contreras, A.; García-Cruz, U.; Pacheco, N. The Effect of Drying Temperature on the Phenolic Content and Functional Behavior of Flours Obtained from Lemon Wastes. Agronomy 2019, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- González, M.; Vernon-Carter, E.; Alvarez-Ramirez, J.; Carrera-Tarela, Y. Effects of dry heat treatment temperature on the structure of wheat flour and starch in vitro digestibility of bread. Int. J. Biol. Macromol. 2020, 166, 1439–1447. [Google Scholar] [CrossRef]
- Duan, J.-L.; Xu, J.-G. Effects of Drying Methods on Physico-Chemical Properties and Antioxidant Activity of Shiitake Mushrooms (Lentinus edodes). Agric. Food Sci. Res. 2015, 2, 51–55. [Google Scholar]
- Piskov, S.; Timchenko, L.; Grimm, W.-D.; Rzhepakovsky, I.; Avanesyan, S.; Sizonenko, M.; Kurchenko, V. Effects of Various Drying Methods on Some Physico-Chemical Properties and the Antioxidant Profile and ACE Inhibition Activity of Oyster Mushrooms (Pleurotus ostreatus). Foods 2020, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Chkir, I.; Balti, M.A.; Ayed, L.; Azzouz, S.; Kechaou, N.; Hamdi, M. Effects of air drying properties on drying kinetics and stability of cactus/brewer’s grains mixture fermented with lactic acid bacteria. Food Bioprod. Process. 2015, 94, 10–19. [Google Scholar] [CrossRef]
- Lewis, W.K. The Rate of Drying of Solid Materials. J. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Association of Official Analysis Chemists. AOAC Official Methods of Analysis of AOAC International; Association of Official Analysis Chemists International: Arlington, VA, USA, 2000; ISBN 0935584544. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sansano, M.; Juan-Borrás, M.; Escriche, I.; Andrés, A.M.; Heredia, A. Effect of Pretreatments and Air-Frying, a Novel Technology, on Acrylamide Generation in Fried Potatoes. J. Food Sci. 2015, 80, T1120–T1128. [Google Scholar] [CrossRef]
- Aidoo, K.E.; Hendry, R.; Wood, B.J.B. Estimation of fungal growth in a solid state fermentation system. Eur. J. Appl. Microbiol. Biotechnol. 1981, 12, 6–9. [Google Scholar] [CrossRef]
- Scotti, C.; Vergoignan, C.; Feron, G.; Durand, A. Glucosamine measurement as indirect method for biomass estimation of Cunninghamella elegans grown in solid state cultivation conditions. Biochem. Eng. J. 2001, 7, 1–5. [Google Scholar] [CrossRef]
- Haug, W.; Lantzsch, H.-J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, T.; Tian, J.-C. Phytic Acid Contents of Wheat Flours from Different Mill Streams. Agric. Sci. China 2010, 9, 1684–1688. [Google Scholar] [CrossRef]
- Caprioli, G.; Nzekoue, A.F.K.; Giusti, F.; Vittori, S.; Sagratini, G. Optimization of an extraction method for the simultaneous quantification of sixteen polyphenols in thirty-one pulse samples by using HPLC-MS/MS dynamic-MRM triple quadrupole. Food Chem. 2018, 266, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef]
- Tanleque-Alberto, F.; Juan-Borrás, M.; Escriche, I. Antioxidant characteristics of honey from Mozambique based on specific flavonoids and phenolic acid compounds. J. Food Compos. Anal. 2019, 86, 103377. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Sharma, S.; Kataria, A.; Singh, B. Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa). LWT 2022, 160, 113256. [Google Scholar] [CrossRef]
- Akıllıoğlu, H.G.; Karakaya, S. Effects of heat treatment and in vitro digestion on the Angiotensin converting enzyme inhibitory activity of some legume species. Eur. Food Res. Technol. 2009, 229, 915–921. [Google Scholar] [CrossRef]
- Hernández-Olivas, E.; Muñoz-Pina, S.; García-Hernández, J.; Andrés, A.; Heredia, A. Impact of common gastrointestinal disorders in elderly on in vitro meat protein digestibility and related properties. Food Biosci. 2022, 46, 101560. [Google Scholar] [CrossRef]
- Aguilera, Y.; Dueñas, M.; Estrella, I.; Hernández, T.; Benitez, V.; Esteban, R.M.; Martín-Cabrejas, M.A. Evaluation of Phenolic Profile and Antioxidant Properties of Pardina Lentil as Affected by Industrial Dehydration. J. Agric. Food Chem. 2010, 58, 10101–10108. [Google Scholar] [CrossRef]
- Chawla, P.; Bhandari, L.; Sadh, P.K.; Kaushik, R. Impact of Solid-State Fermentation (Aspergillus oryzae) on Functional Properties and Mineral Bioavailability of Black-Eyed Pea (Vigna unguiculata) Seed Flour. Cereal Chem. 2017, 94, 437–442. [Google Scholar] [CrossRef]
- Mora-Uzeta, C.; Cuevas-Rodríguez, E.; López-Cervantes, J.; Milán-Carrillo, J.; Gutiérrez-Dorado, R.; Reyes-Moreno, C. Improvement Nutritional/Antioxidant Properties of Underutilized Legume Tepary Bean (Phaseolus acutifolius) by Solid State Fermentation. Agrociencia 2019, 53, 987–1003. [Google Scholar]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Plaza, J.; Morales-Corts, M.; Pérez-Sánchez, R.; Revilla, I.; Vivar-Quintana, A. Morphometric and Nutritional Characterization of the Main Spanish Lentil Cultivars. Agriculture 2021, 11, 741. [Google Scholar] [CrossRef]
- Guiné, R.P.F. The Drying of Foods and Its Effect on the Physical-Chemical, Sensorial and Nutritional Properties. ETP Int. J. Food Eng. 2018, 2, 93–100. [Google Scholar] [CrossRef]
- Choe, U.; Osorno, J.M.; Ohm, J.-B.; Chen, B.; Rao, J. Modification of physicochemical, functional properties, and digestibility of macronutrients in common bean (Phaseolus vulgaris L.) flours by different thermally treated whole seeds. Food Chem. 2022, 382, 132570. [Google Scholar] [CrossRef]
- Yi, J.-Y.; Lyu, J.; Bi, J.-F.; Zhou, L.-Y.; Zhou, M. Hot air drying and freeze drying pre-treatments coupled to explosion puffing drying in terms of quality attributes of mango, pitaya, and papaya fruit chips. J. Food Process. Preserv. 2017, 41, e13300. [Google Scholar] [CrossRef]
- Djekic, I.; Tomic, N.; Bourdoux, S.; Spilimbergo, S.; Smigic, N.; Udovicki, B.; Hofland, G.; Devlieghere, F.; Rajkovic, A. Comparison of three types of drying (supercritical CO2, air and freeze) on the quality of dried apple—Quality index approach. LWT 2018, 94, 64–72. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Effect of Different Cooking Conditions on Phenolic Compounds and Antioxidant Capacity of Some Selected Brazilian Bean (Phaseolus vulgaris L.) Cultivars. J. Agric. Food Chem. 2009, 57, 5734–5742. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Beans: A Source of Natural Antioxidants. In Phenolic Compounds in Foods and Natural Health Products; American Chemical Society: Washington, DC, USA, 2005; pp. 83–93. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Lui, W.-Y.; Chan, C.-L.; Corke, H. Hot Air Drying Induces Browning and Enhances Phenolic Content and Antioxidant Capacity in Mung Bean (Vigna radiata L.) Sprouts. J. Food Process. Preserv. 2016, 41, e12846. [Google Scholar] [CrossRef]
- Anton, A.A.; Ross, K.A.; Beta, T.; Fulcher, R.G.; Arntfield, S.D. Effect of pre-dehulling treatments on some nutritional and physical properties of navy and pinto beans (Phaseolus vulgaris L.). LWT 2008, 41, 771–778. [Google Scholar] [CrossRef]
- Zou, Y.; Gao, Y.; He, H.; Yang, T. Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. LWT 2018, 90, 246–253. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Bajpai, B.; Patil, S. A New Approach to Microbial Production of Gallic Acid. Braz. J. Microbiol. 2008, 39, 708–711. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Zárate, P.; Cruz-Hernández, M.; Montañez, J.; Belmares-Cerda, R.; Aguilar, C. Bacterial tannases: Production, properties and applications, Tanasas bacterianas: Producción, propiedades y aplicaciones. Rev. Mex. Ing. Química 2014, 13, 63–74. [Google Scholar]
- Thavarajah, P.; Thavarajah, D.; Vandenberg, A. Low Phytic Acid Lentils (Lens culinaris L.): A Potential Solution for Increased Micronutrient Bioavailability. J. Agric. Food Chem. 2009, 57, 9044–9049. [Google Scholar] [CrossRef]
- Farinde, E.O.; Olanipekun, O.T.; Olasupo, R.B. Nutritional Composition and Antinutrients Content of Raw and Processed Lima Bean (Phaseolus lunatus). Ann. Food Sci. Technol. 2018, 19, 250–264. [Google Scholar]
- Barahuie, F.; Dorniani, D.; Saifullah, B.; Gothai, S.; Hussein, M.Z.; Pandurangan, A.K.; Arulselvan, P. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017, 12, 2361–2372. [Google Scholar] [CrossRef] [Green Version]
- Shamsuddin, A.M. Anti-cancer function of phytic acid. Int. J. Food Sci. Technol. 2002, 37, 769–782. [Google Scholar] [CrossRef] [Green Version]
- Schröterová, L.; Hašková, P.; Rudolf, E.; Cervinka, M. Effect of phytic acid and inositol on the proliferation and apoptosis of cells derived from colorectal carcinoma. Oncol. Rep. 2010, 23, 787–793. [Google Scholar]
- Gu, M.; Roy, S.; Raina, K.; Agarwal, C.; Agarwal, R. Inositol Hexaphosphate Suppresses Growth and Induces Apoptosis in Prostate Carcinoma Cells in Culture and Nude Mouse Xenograft: PI3K-Akt Pathway as Potential Target. Cancer Res. 2009, 69, 9465–9472. [Google Scholar] [CrossRef] [Green Version]
- Vucenik, I.; Shamsuddin, A.M. Protection Against Cancer by Dietary IP6and Inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The Antihypertensive Effect of Peptides: A Novel Al-ternative to Drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef]
- Ansor, N.M.; Abdullah, N.; Aminudin, N. Anti-angiotensin converting enzyme (ACE) proteins from mycelia of Ganoderma lucidum (Curtis) P. Karst. BMC Complement. Altern. Med. 2013, 13, 256. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Lai, J.; He, P.; Pan, L.; Zhang, Y.; Zhang, M.; Wu, H. Screening, ACE-inhibitory mechanism and structure-activity relationship of a novel ACE-inhibitory peptide from Lepidium meyenii (Maca) protein hydrolysate. Food Biosci. 2023, 52, 102374. [Google Scholar] [CrossRef]


| Pardina (P) | Fermented Pardina (FP) | Castellana (C) | Fermented Castellana (FC) | |
|---|---|---|---|---|
| Moisture | 6.78 ± 0.06 a | 118.25 ± 0.17 b | 8.66 ± 0.05 a | 156.4 ± 0.3 b |
| Protein | 23.78 ± 0.4 a | 24.1 ± 0.6 a | 26.4 ± 0.2 a | 26.9 ± 0.4 a |
| Lipids | 1.06 ± 0.09 a | 1.31 ± 0.11 b | 1.38 ± 0.06 a | 1.68 ± 0.13 b |
| Ashes | 2.74 ± 0.02 b | 2.481 ± 0.014 a | 3.35 ± 0.02 b | 2.596 ± 0.009 a |
| Total Carbohydrates * | 72.32 ± 0.4 a | 72.1 ± 0.7 a | 68.9 ± 0.2 a | 68.9 ± 0.5 a |
| Reducing sugars | 0.22 ± 0.01 a | 0.92 ± 0.08 b | 0.22 ± 0.02 a | 2.94 ± 0.18 b |
| Total fibre | 16.0 ± 0.2 b | 14.3 ± 0.2 a | 17.3 ± 0.2 b | 13.6 ± 0.3 a |
| Soluble fibre | 1.39 ± 0.10 a | 2.25 ± 0.10 b | 1.94 ± 0.10 a | 1.78 ± 0.10 a |
| Insoluble fibre | 15.0 ± 0.3 b | 12.26 ± 0.10 a | 15.1 ± 0.2 b | 11.5 ± 0.2 a |
| Biomass ** | - | 14.1 ± 1.5 | - | 61.8 ± 1.4 |
| pH | 6.457 ± 0.006 a | 7.217 ± 0.006 b | 6.603 ± 0.006 b | 6.520 ± 0.010 a |
| aw | 0.367 ± 0.003 a | 0.9798 ± 0.0011 b | 0.5084 ± 0.0012 a | 0.9736 ± 0.0008 b |
| Temperature (°C) | Fermented Pardina | Fermented Castellana | ||
|---|---|---|---|---|
| k | R2 | k | R2 | |
| 50 | 0.2218 | 0.9788 | 0.2854 | 0.9746 |
| 60 | 0.3467 | 0.9771 | 0.3519 | 0.9768 |
| 70 | 0.4514 | 0.9698 | 0.4910 | 0.9732 |
| Aerobic Mesophilic Bacteria count (UFC/g) | Yeast and Mould Count (UFC/g) | E. coli Count (UFC/g) | Salmonella spp. Detection (Presence /Absence) | Listeria monocytogenes (Presence /Absence) | |
|---|---|---|---|---|---|
| PF | 2.5 × 103 | ˂102 | ˂102 | Absence | Absence |
| FPF-50 | 5 × 102 | 1 × 102 | ˂102 | Absence | Absence |
| FPF-60 | ˂102 | ˂102 | ˂102 | Absence | Absence |
| FPF-70 | ˂102 | ˂102 | ˂102 | Absence | Absence |
| FPF-L | 3 × 102 | ˂102 | ˂102 | Absence | Absence |
| CF | 6 × 102 | ˂102 | ˂102 | Absence | Absence |
| FCF-50 | 4 × 102 | ˂102 | ˂102 | Absence | Absence |
| FCF-60 | 1 × 102 | ˂102 | ˂102 | Absence | Absence |
| FCF-70 | ˂102 | ˂102 | ˂102 | Absence | Absence |
| FCF-L | 4 × 102 | ˂102 | ˂102 | Absence | Absence |
| Pardina Flours | Castellana Flours | ||||
|---|---|---|---|---|---|
 PF | L* | 80.2 ± 0.3 d |  CF | L* | 82.86 ± 0.09 d |
| a* | 1.70 ± 0.06 a | a* | 0.905 ± 0.007 a | ||
| b* | 15.5 ± 0.2 a | b* | 18.34 ± 0.10 e | ||
| C* | 15.5 ± 0.2 d | C* | 18.36 ± 0.10 e | ||
| h | 83.72 ± 0.15 d | h | 87.175 ± 0.008 c | ||
| ΔE | - | ΔE | - | ||
 FPF-50 | L* | 61.9 ± 0.2 b |  FCF-50 | L* | 58.7 ± 0.4 a |
| a* | 4.42 ± 0.03 c | a* | 7.18 ± 0.11 d | ||
| b* | 11.22 ± 0.09 ab | b* | 15.63 ± 0.09 b | ||
| C* | 12.06 ± 0.10 ab | C* | 17.20 ± 0.13 b | ||
| h | 68.49 ± 0.04 b | h | 65.3 ± 0.2 a | ||
| ΔE | 18.96 ± 0.41 b | ΔE | 25.1 ± 0.4 c | ||
 FPF-60 | L* | 61.73 ± 0.06 ab |  FCF-60 | L* | 60.4 ± 0.2 b |
| a* | 4.30 ± 0.02 b | a* | 6.972 ± 0.012 c | ||
| b* | 10.91 ± 0.05 a | b* | 16.18 ± 0.08 c | ||
| C* | 11.73 ± 0.06 a | C* | 17.61 ± 0.07 c | ||
| h | 68.48 ± 0.03 b | h | 66.68 ± 0.14 b | ||
| ΔE | 19.20 ± 0.23 b | ΔE | 23.4 ± 0.2 b | ||
 FPF-70 | L* | 61.3 ± 0.3 a |  FCF-70 | L* | 58.37 ± 0.14 a |
| a* | 4.43 ± 0.07 c | a* | 7.44 ± 0.02 e | ||
| b* | 11.44 ± 0.19 b | b* | 16.38 ± 0.09 d | ||
| C* | 12.3 ± 0.2 b | C* | 17.99 ± 0.07 d | ||
| h | 68.83 ± 0.02 c | h | 65.57 ± 0.17 a | ||
| ΔE | 19.51 ± 0.46 b | ΔE | 25.4 ± 0.2 c | ||
 FPF-L | L* | 62.5 ± 0.3 c |  FCF-L | L* | 63.01 ± 0.04 c |
| a* | 5.01 ± 0.09 d | a* | 6.51 ± 0.02 b | ||
| b* | 12.6 ± 0.3 c | b* | 14.32 ± 0.03 a | ||
| C* | 13.6 ± 0.3 c | C* | 15.73 ± 0.03 a | ||
| h | 68.30 ± 0.09 a | h | 65.55 ± 0.06 a | ||
| ΔE | 18.23 ± 0.17 a | ΔE | 21.02 ± 0.03 a | ||
| Antioxidant Activity | Total Phenol Content | Phytic Acid | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ABTS | ABTS Index | DPPH | DPPH Index | FRAP | FRAP Index | APCI * | |||
| PF | 9.5 ± 0.4 d | 100 | 2.07 ± 0.09 c | 100 | 7.62 ± 0.17 b | 100 | 100 | 3.8 ± 0.2 c | 9.7 ± 0.7 b |
| FP | 5.7 ± 0.5 c | 60.7 | 0.64 ± 0.04 b | 30.8 | 0.311 ± 0.019 a | 4.09 | 31.9 | 2.10 ± 0.08 b | 7.3 ± 0.6 a |
| FPF-50 | 3.81 ± 0.10 b | 40.3 | 0.49 ± 0.03 a | 23.6 | 0.30 ± 0.02 a | 3.9 | 22.6 | 2.21 ± 0.09 b | 7.4 ± 1.4 a |
| FPF-60 | 4.03 ± 0.19 b | 42.7 | 0.486 ± 0.016 a | 23.5 | 0.32 ± 0.02 a | 4.2 | 23.5 | 2.39 ± 0.19 b | 7.6 ± 1.2 a |
| FPF-70 | 3.91 ± 0.16 b | 41.4 | 0.516 ± 0.010 a | 25 | 0.351 ± 0.007 a | 4.6 | 23.7 | 2.37 ± 0.12 b | 7.7 ± 0.9 a |
| FPF-L | 3.20 ± 0.04 a | 33.9 | 0.502 ± 0.014 a | 24.3 | 0.310 ± 0.16 a | 4.07 | 20.7 | 1.59 ± 0.08 a | 8.0 ± 1.4 ab |
| CF | 8.4 ± 0.4 d | 100 | 1.634 ± 0.015 bc | 72 | 8.3 ± 0.2 e | 100 | 90.7 | 4.13 ± 0.10 c | 7.3 ± 0.3 d |
| FC | 2.50 ± 0.09 a | 29.9 | 2.27 ± 0.13 d | 100 | 1.10 ± 0.03 a | 13.4 | 47.7 | 2.75 ± 0.11 a | 0.9 ± 0.5 c |
| FCF-50 | 6.1 ± 0.3 c | 73.2 | 1.61 ± 0.02 b | 70.8 | 6.21 ± 0.19 c | 75.2 | 73.1 | 6.9 ± 0.3 d | 0.4 ± 0.4 bc |
| FCF-60 | 5.48 ± 0.07 b | 65.4 | 1.568 ± 0.016 b | 69.07 | 6.3 ± 0.2 c | 76.2 | 70.2 | 7.13 ± 0.12 e | 0.21 ± 0.07 b |
| FCF-70 | 6.2 ± 0.2 c | 73.9 | 1.71 ± 0.02 c | 75.1 | 7.0 ± 0.3 d | 85.3 | 78.1 | 7.71 ± 0.15 f | 0.02 ± 0.01 a |
| FCF-L | 2.32 ± 0.16 a | 27.7 | 1.093 ± 0.016 a | 48.2 | 2.14 ± 0.05 b | 25.9 | 33.9 | 3.42 ± 0.02 b | 0.26 ± 0.04 b |
| PF | FP | FPF-50 | FPF-60 | FPF-70 | FPF-L | |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| Gallic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Caffeic acid | 10.6 ± 0.4 BC | n.d. | n.d. | n.d. | n.d. | n.d. |
| p-Coumaric acid | 7.8 ± 0.2 bABC | 9.47 ± 0.04 dC | 8.5 ± 0.5 cC | 10.2 ± 0.5 dC | 9.5 ± 0.2 dC | 5.2 ± 0.1 aA |
| Sinapic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4-O-Caffeoylquinic | 264 ± 6 dG | 143.5 ± 0.06 cF | 121 ± 3 aG | 123 ± 2 aF | 127 ± 3 bF | 152 ± 5 cD |
| 4-Hydroxibezoic acid | 11.0 ± 0.2 aC | 11.78 ± 0.03 aD | 11.0 ± 0.3 aD | 11.2 ± 0.3 aC | 10.7 ± 0.3 aC | 10.1 ± 0.1 aB |
| Vanillic acid | 23.0 ± 0.3 aE | 34.7 ± 0.9 bE | 47 ± 3 cF | 47 ± 2 cE | 46 ± 4 cE | 34 ± 2 bC |
| Ferulic acid | 6.6 ± 0.2 cA | 5.3 ± 0.1 bA | 2.8 ± 0.01 aA | 2.92 ± 0.03 aA | 2.88 ± 0.06 aA | 2.90 ± 0.03 aA |
| trans-Cinnamic acid | traces | 6.6 ± 0.2 bB | 20.78 ± 0.04 cE | 29 ± 2 dD | 26.8 ± 0.6 dD | 4.98 ± 0.05 aA |
| Flavonoids | ||||||
| Rutin | 11.6 ± 0.8 C | n.d. | n.d. | n.d. | n.d. | n.d. |
| Epicatechin | 71 ± 2 F | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin 3-glucoside | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercitrin | 6.70 ± 0.02 cAB | traces | 3.38 ± 0.05 aAB | 3.30 ± 0.03 aA | 3.2 ± 0.2 aA | 3.76± 0.09 bA |
| Apigenin-7-glucoside | 16.9 ± 0.1 D | traces | traces | traces | traces | Traces |
| Quercetin | 5.7 ± 0.2 cA | 11.2 ± 0.1 dD | 5.89 ± 0.07 aB | 6.05 ± 0.03 aB | 5.95 ± 0.03 aB | 5.76 ± 0.05 bA |
| Naringenin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kaempferol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| CF | FC | FCF-50 | FCF-60 | FCF-70 | FCF-L | |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| Gallic acid | 45 ± 5 aE | 67 ± 2 bD | 143 ± 10 dE | 129 ± 15 dE | 181.6 ± 0.8 eE | 102 ± 7 cE |
| Caffeic acid | 13 ± 2 bC | 8.2 ± 0.5 aC | 8 ± 0.2 aC | 7.6 ± 0.4 aC | 8.1 ± 0.8 aBC | 8.3 ± 0.5 aC |
| p-Coumaric acid | 9 ± 0.1 C | traces | traces | traces | traces | Traces |
| Sinapic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4-O-Caffeoylquinic | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4-Hydroxibezoic acid | 4.5 ± 0.2 aB | 9.8 ± 0.4 bC | 12.1 ± 0.6 cD | 12.2 ± 0.3 cD | 10 ± 1 bC | 12.5 ± 0.5 cD |
| Vanillic acid | 2.7 ± 0.2 A | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ferulic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| trans-Cinnamic acid | n.d. | 5.9 ± 0.7 aB | 9.7 ± 0.2 bCD | 11.5 ± 0.4 cD | 14 ± 2 dD | 5.2 ± 0.3 aB |
| Flavonoids | ||||||
| Rutin | 43.3 ± 0.2 dF | 6.3 ± 0.7 cB | 5.5 ± 0.1 bB | 4.8 ± 0.2 abB | 4.7 ± 0.4 aB | 4.3 ± 0.3 aB |
| Epicatechin | 19 ± 4 D | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin 3-glucoside | 4.1 ± 0.1 bB | 4.2 ± 0.2 bA | 3.0 ± 0.2 aA | 2.8 ± 0.1 aA | 3.0 ± 0.5 aA | n.d. |
| Quercitrin | traces | traces | traces | traces | traces | 3.2 ± 0.8 A |
| Apigenin-7-glucoside | 3.4 ± 0.1 aA | traces | 4.7 ± 0.1 bB | 7.2 ± 0.3 cC | 7.5 ± 0.9 cB | 4.7 ± 0.3 bB |
| Quercetin | 3.9 ± 0.1 B | n.d. | n.d. | n.d. | n.d. | n.d. |
| Naringenin | n.d. | n.d. | 6 ± 1 bB | 6.5 ± 0.2 bC | 7.5 ± 0.9 bB | 3.0 ± 0.1 aA |
| Kaempferol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-García, J.; Muñoz-Pina, S.; García-Hernández, J.; Heredia, A.; Andrés, A. Impact of Air-Drying Temperature on Antioxidant Properties and ACE-Inhibiting Activity of Fungal Fermented Lentil Flour. Foods 2023, 12, 999. https://doi.org/10.3390/foods12050999
Sánchez-García J, Muñoz-Pina S, García-Hernández J, Heredia A, Andrés A. Impact of Air-Drying Temperature on Antioxidant Properties and ACE-Inhibiting Activity of Fungal Fermented Lentil Flour. Foods. 2023; 12(5):999. https://doi.org/10.3390/foods12050999
Chicago/Turabian StyleSánchez-García, Janaina, Sara Muñoz-Pina, Jorge García-Hernández, Ana Heredia, and Ana Andrés. 2023. "Impact of Air-Drying Temperature on Antioxidant Properties and ACE-Inhibiting Activity of Fungal Fermented Lentil Flour" Foods 12, no. 5: 999. https://doi.org/10.3390/foods12050999
APA StyleSánchez-García, J., Muñoz-Pina, S., García-Hernández, J., Heredia, A., & Andrés, A. (2023). Impact of Air-Drying Temperature on Antioxidant Properties and ACE-Inhibiting Activity of Fungal Fermented Lentil Flour. Foods, 12(5), 999. https://doi.org/10.3390/foods12050999







