Effect of a Change in the CaCl2/Pectin Mass Ratio on the Particle Size, Rheology and Physical Stability of Lemon Essential Oil/W Emulgels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Characterization
2.2.1. Particle Size Distribution
2.2.2. Multiple Light Scattering
2.2.3. Rheological Characterization
2.2.4. Optical Microscopy
2.2.5. Statistical Analysis
3. Results and Discussion
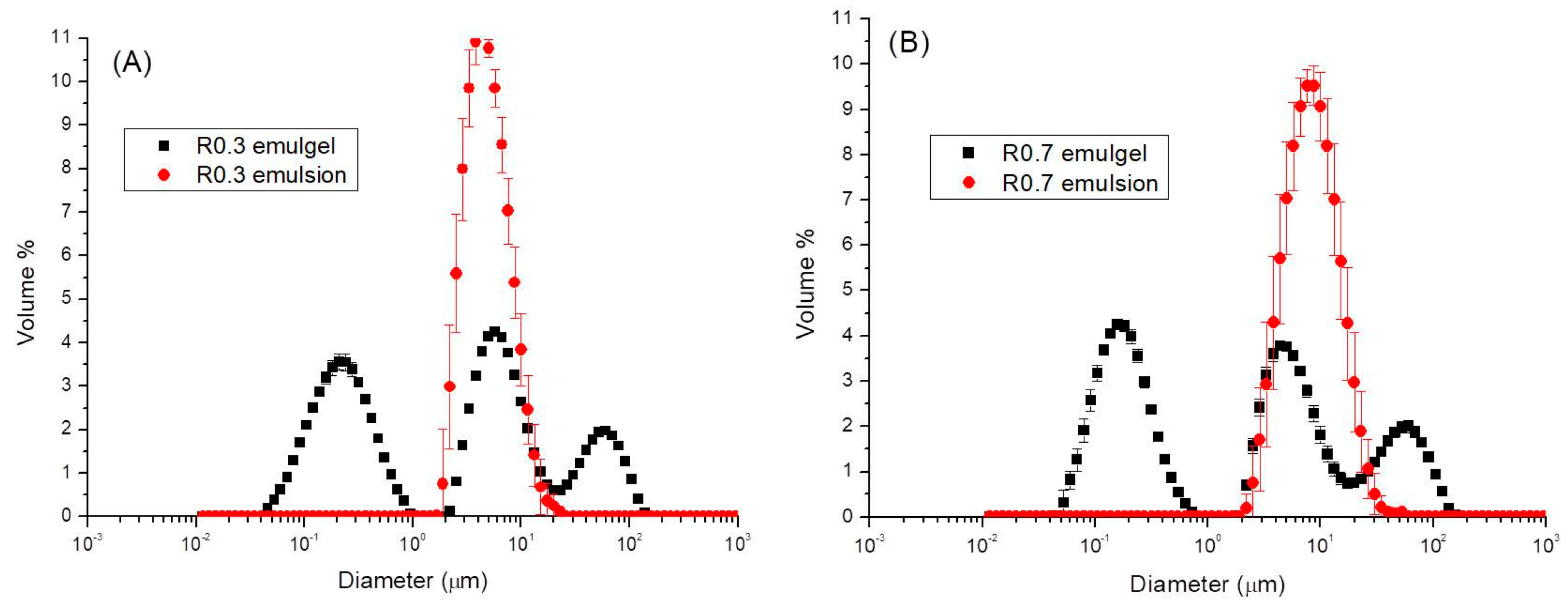
3.1. Particle Size Distribution
3.2. Physical Stability
3.3. Rheological Characterization
3.4. Optical Microscopy Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geremias-Andrade, I.M.; Souki, N.P.; Moraes, I.C.; Pinho, S.C. Rheology of emulsion-filled gels applied to the development of food materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Scholten, E.; Van Aken, G.A. Effect of fat hardness on large deformation rheology of emulsion-filled gels. Food Hydrocoll. 2015, 43, 299–310. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of emulsion gels for the delivery of functional food ingredients: From structure to functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Lorenzo, G.; Zaritzky, N.; Califano, A. Rheological analysis of emulsion-filled gels based on high acyl gellan gum. Food Hydrocoll. 2013, 30, 672–680. [Google Scholar] [CrossRef]
- Daudt, R.M.; Back, P.I.; Cardozo, N.S.M.; Marczak, L.D.F.; Külkamp-Guerreiro, I.C. Pinhão starch and coat extract as new natural cosmetic ingredients: Topical formulation stability and sensory analysis. Carbohydr. Polym. 2015, 134, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Fu, S.Y.; Hou, J.J.; Guo, J.; Wang, J.M.; Yang, X.Q. Zein based oil-in-glycerol emulgels enriched with β-carotene as margarine alternatives. Food Chem. 2016, 211, 836–844. [Google Scholar] [CrossRef]
- Bruno, E.; Lupi, F.R.; Mammolenti, D.; Mileti, O.; Baldino, N.; Gabriele, D. Emulgels Structured with Dietary Fiber for Food Uses: A Rheological Model. Foods 2022, 11, 3866. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Chodankar, R.; Shelke, O. Emulgels: A novel topical drug delivery system. Pharm. Biol. Eval. 2015, 2, 64–75. [Google Scholar]
- Ammad, F.; Moumen, O.; Gasem, A.; Othmane, S.; Hisashi, K.N.; Zebib, B.; Merah, O. The potency of lemon (Citrus limon L.) essential oil to control some fungal diseases of grapevine wood. Comptes Rendus Biol. 2018, 341, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. Int. Sch. Res. Not. 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou YB chang against Listeria monocytogenes. Food Control 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Tmušić, N.; Stanojević, L.; Stanojević, J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT 2022, 153, 112210. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutierrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E.S. Chemical constituents, in vitro antioxidant activity and in silico study on NADPH oxidase of Allium sativum L.(garlic) essential oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef]
- Pucci, M.; Raimondo, S.; Zichittella, C.; Tinnirello, V.; Corleone, V.; Aiello, G.; Moschetti, M.; Conigliaro, A.; Fontana, S.; Alessandro, R. Biological properties of a citral-enriched fraction of Citrus limon essential oil. Foods 2020, 9, 1290. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M. Introduction to the special issue: Application of essential oils in food systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef]
- Florence, A.T.; Whitehill, D. Stabilization of water/oil/water multiple emulsions by polymerization of the aqueous phases. J. Pharm. Pharmacol. 1982, 34, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Dominguez, M.; Pemartin, K.; Boutonnet, M. Preparation of inorganic nanoparticles in oil-in-water microemulsions: A soft and versatile approach. Curr. Opin. Colloid Interface Sci. 2012, 17, 297–305. [Google Scholar] [CrossRef]
- Velev, O.D.; Gurkov, T.D.; Chakarova, S.K.; Dimitrova, B.I.; Ivanov, I.B.; Borwankar, R.P. Experimental investigations on model emulsion systems stabilized with non-ionic surfactant blends. Colloids Surf. A Physicochem. Eng. Asp. 1994, 83, 43–55. [Google Scholar] [CrossRef]
- Huibers, P.D.; Shah, D.O. Evidence for synergism in nonionic surfactant mixtures: Enhancement of solubilization in water-in-oil microemulsions. Langmuir 1997, 13, 5762–5765. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Effect of surface character of filler particles on rheology of heat-set whey protein emulsion gels. Colloids Surf. B Biointerfaces 1999, 12, 373–381. [Google Scholar] [CrossRef]
- Kim, K.-H.; Renkema, J.M.S.; van Vliet, T. Rheological properties of soybean protein isolate gels containing emulsion droplets. Food Hydrocoll. 2001, 15, 295–302. [Google Scholar] [CrossRef]
- Chojnicka, A.; Sala, G.; Kruif, C.G.; Van de Velde, F. The interactions between oil droplets and gel matrix affect the lubrication properties of sheared emulsion-filled gels. Food Hydrocoll. 2009, 23, 1038–1046. [Google Scholar] [CrossRef]
- Sato, A.C.K.; Moraes, K.E.F.P.; Cunha, R.L. Development of gelled emulsions with improved oxidative and pH stability. Food Hydrocoll. 2014, 34, 184–192. [Google Scholar] [CrossRef]
- De Lavergne, M.D.; van Delft, M.; Van de Velde, F.; van Boekel, M.A.J.S.; Stieger, M. Dynamic texture perception and oral processing of semi-solid food gels: Part 1: A comparison between QDA, progressive profiling and TDS. Food Hydrocoll. 2015, 43, 207–217. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Giarnetti, M.; Summo, C.; Pasqualone, A.; Minervini, F.; Caponio, F. Production and characterization of emulsion-filled gels based on inulin and extra virgin olive oil. Food Hydrocoll. 2015, 45, 30–40. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Hou, J.J.; Guo, J.; Wang, J.M.; Yang, X.Q. Effect of interfacial composition and crumbliness on aroma release in soy protein/sugar beet pectin mixed emulsion gels. J. Agric. Food Chem. 2016, 96, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Jia, X.; Zhu, Q.; Liu, Y.; Li, J.; Yin, L. Investigation of the mechanical, rheological and microstructural properties of sugar beet pectin/soy protein isolate-based emulsion-filled gels. Food Hydrocoll. 2019, 89, 813–820. [Google Scholar] [CrossRef]
- Jiang, W.X.; Qi, J.R.; Liao, J.S.; Yang, X.Q. Acid/ethanol induced pectin gelling and its application in emulsion gel. Food Hydrocoll. 2021, 118, 106774. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, J.; Wang, Y.; Ye, X.; Chen, S.; Pan, H.; Chen, J. Fabrication of rhamnogalacturonan-I enriched pectin-based emulsion gels for protection and sustained release of curcumin. Food Hydrocoll. 2022, 128, 107592. [Google Scholar] [CrossRef]
- Ovodov, Y.S. Current views on pectin substances. Russ. J. Bioorganic Chem. 2009, 35, 269–284. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schütz, L.; Schuchmann, H.P. Interfacial and emulsifying properties of citrus pectin: Interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 2017, 62, 288–298. [Google Scholar] [CrossRef]
- Isusi, G.S.; Bindereif, B.; Karbstein, H.P.; Van der Schaaf, U.S. Polymer or microgel particle: Differences in emulsifying properties of pectin as microgel or as individual polymer chains. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124793. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Kastner, H.; Einhorn-Stoll, U.; Senge, B. Structure formation in sugar containing pectin gels–Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocoll. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- García, M.C.; Alfaro, M.C.; Calero, N.; Muñoz, J. Influence of gellan gum concentration on the dynamic viscoelasticity and transient flow of fluid gels. Biochem. Eng. J. 2011, 55, 73–81. [Google Scholar] [CrossRef]
- García, M.C.; Alfaro, M.C.; Muñoz, J. Yield stress and onset of nonlinear time-dependent rheological behaviour of gellan fluid gels. J. Food Eng. 2015, 159, 42–47. [Google Scholar] [CrossRef]
- García, M.C.; Trujillo, L.A.; Muñoz, J.; Alfaro, M.C. Gellan gum fluid gels: Influence of the nature and concentration of gel-promoting ions on rheological properties. Colloid Polym. Sci. 2018, 296, 1741–1748. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. Characterisation of instability of concentrated dispersions by a new optical analyser: The TURBISCAN MA 1000. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 111–123. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Martin-Piñero, M.J.; García, M.C.; Santos, J.; Alfaro-Rodriguez, M.C.; Muñoz, J. Characterization of novel nanoemulsions, with improved properties, based on rosemary essential oil and biopolymers. J. Sci. Food Agric. 2020, 100, 3886–3894. [Google Scholar] [CrossRef]
- Alfaro, M.C.; Guerrero, A.F.; Muñoz, J. Dynamic viscoelasticity and flow behavior of a polyoxyethylene glycol nonylphenyl ether/toluene/water system. Langmuir 2000, 16, 4711–4719. [Google Scholar] [CrossRef]
- Rincón, F.; Muñoz, J.; Ramírez, P.; Galán, H.; Alfaro, M.C. Physicochemical and rheological characterization of Prosopis juliflora seed gum aqueous dispersions. Food Hydrocoll. 2014, 35, 348–357. [Google Scholar] [CrossRef]
- Gabriele, D.; de Cindio, B.; D’Antona, P. A weak gel model for foods. Rheol. Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Farrés, I.F.; Moakes, R.J.A.; Norton, I.T. Designing biopolymer fluid gels: A microstructural approach. Food Hydrocoll. 2014, 42, 362–372. [Google Scholar] [CrossRef]







| wt% | R0.3 | R0.4 | R0.5 | R0.6 | R0.7 |
|---|---|---|---|---|---|
| Pectin | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| CaCl2 | 0.12 | 0.16 | 0.20 | 0.24 | 0.28 |
| Tween 80 | 7.95 | 7.95 | 7.95 | 7.95 | 7.95 |
| Span 20 | 7.05 | 7.05 | 7.05 | 7.05 | 7.05 |
| Lemon Oil | 15 | 15 | 15 | 15 | 15 |
| Water | 69.48 | 69.44 | 69.40 | 69.36 | 69.32 |
| Day | K (Pa·sn) | n | R2 | |
|---|---|---|---|---|
| R 0.3 | 1 | 10.65 ± 0.41 | 0.10 ± 0.01 | 0.991 |
| 15 | 9.96 ± 0.21 | 0.10 ± 0.00 | 0.992 | |
| R 0.7 | 1 | 3.31 ± 0.07 | 0.30 ± 0.01 | 0.985 |
| 15 | 3.17 ± 0.07 | 0.29 ± 0.00 | 0.986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, J.; Prieto-Vargas, P.; García, M.C.; Alfaro-Rodríguez, M.-C. Effect of a Change in the CaCl2/Pectin Mass Ratio on the Particle Size, Rheology and Physical Stability of Lemon Essential Oil/W Emulgels. Foods 2023, 12, 1137. https://doi.org/10.3390/foods12061137
Muñoz J, Prieto-Vargas P, García MC, Alfaro-Rodríguez M-C. Effect of a Change in the CaCl2/Pectin Mass Ratio on the Particle Size, Rheology and Physical Stability of Lemon Essential Oil/W Emulgels. Foods. 2023; 12(6):1137. https://doi.org/10.3390/foods12061137
Chicago/Turabian StyleMuñoz, José, Paula Prieto-Vargas, Mᵃ Carmen García, and María-Carmen Alfaro-Rodríguez. 2023. "Effect of a Change in the CaCl2/Pectin Mass Ratio on the Particle Size, Rheology and Physical Stability of Lemon Essential Oil/W Emulgels" Foods 12, no. 6: 1137. https://doi.org/10.3390/foods12061137
APA StyleMuñoz, J., Prieto-Vargas, P., García, M. C., & Alfaro-Rodríguez, M.-C. (2023). Effect of a Change in the CaCl2/Pectin Mass Ratio on the Particle Size, Rheology and Physical Stability of Lemon Essential Oil/W Emulgels. Foods, 12(6), 1137. https://doi.org/10.3390/foods12061137







