Applications of UV–Visible, Fluorescence and Mid-Infrared Spectroscopic Methods Combined with Chemometrics for the Authentication of Apple Vinegar
Abstract
1. Introduction
2. Materials and Methods
2.1. Vinegar Samples and Adulteration
2.2. Measurement of Quality Parameters
2.3. Fluorescence Spectroscopy
2.4. UV–Visible Spectroscopy
2.5. Fourier Transform Infrared Spectroscopy
2.6. Statistical Analysis
3. Results and Discussion
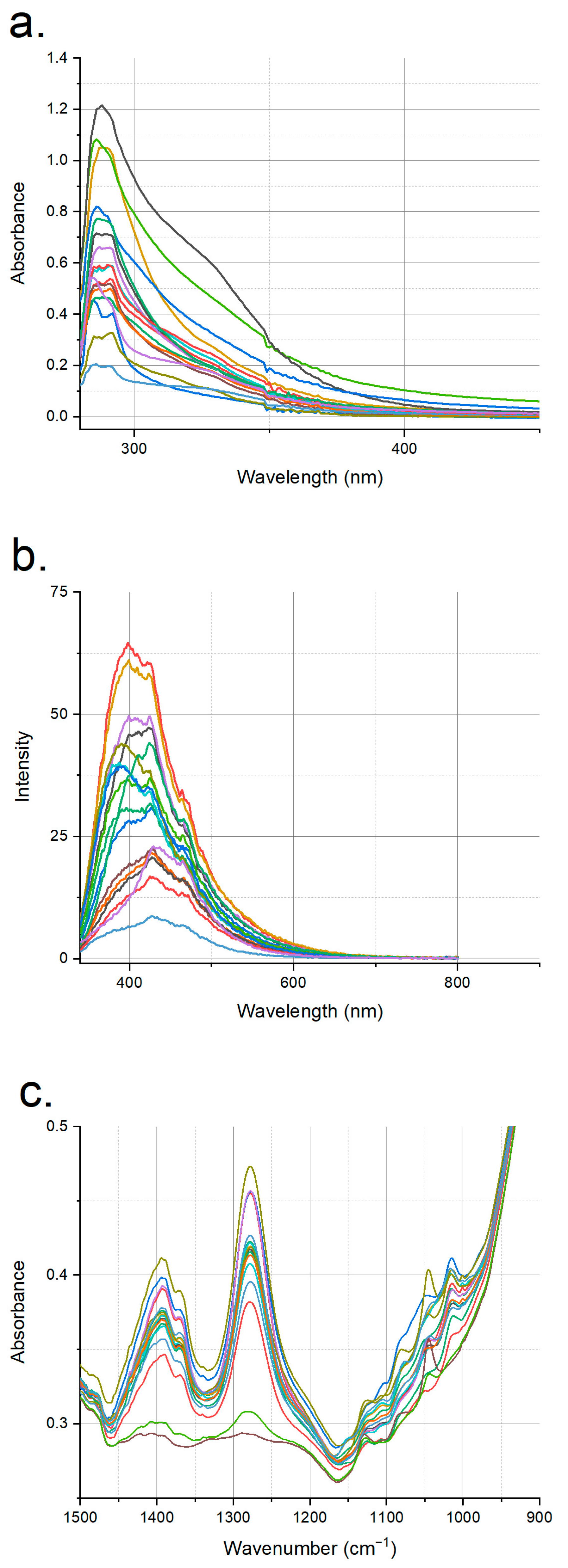
3.1. Spectroscopic Profiles
3.2. Chemometric Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Callejón, R.M.; Rios-Reina, R.; Morales, M.L.; Troncoso, A.M.; Thomas, F.; Camin, F. Vinegar. In Food Integrity Handbook. A Guide to Food Authenticity Issues and Analytical Solutions; Eurofins Analytics France: Nantes, France, 2018; pp. 273–294. ISBN 978-2-956630302. [Google Scholar]
- Expert Markets Search. Vinegar Market. Available online: https://www.expertmarketresearch.com/reports/vinegar-market (accessed on 14 January 2023).
- Guiné, R.P.; Barroca, M.J.; Coldea, T.E.; Bartkiene, E.; Anjos, O. Apple fermented products: An overview of technology, properties and health effects. Processes 2021, 9, 223. [Google Scholar] [CrossRef]
- Launholt, T.L.; Kristiansen, C.B.; Hjorth, P. Safety and side effects of apple vinegar intake and its effect on metabolic parameters and body weight: A systematic review. Eur. J. Nutr. 2020, 59, 2273–2289. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Codex Alimentarius—Discussion Paper on Food Integrity and Food Authenticity. In Proceedings of the Joint FAO/WHO Food Standards Programme. Codex Committee on Food Import and Export Inspection and Certification Systems. In Proceedings of the 24th Session, Brisbane, Australia, 22–26 October 2018; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-733-24%252FWorking%2BDocuments%252Ffc24_07e.pdf (accessed on 3 February 2022).
- Cavdaroglu, C.; Ozen, B. Authentication of vinegars with targeted and non-targeted methods. Food Rev. Int. 2021, 1–18. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Camiña, J.M.; Callejón, R.M.; Azcarate, S.M. Spectralprint techniques for wine and vinegar characterization, authentication and quality control: Advances and projections. TrAC Trends Anal. Chem. 2021, 134, 116121. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Graziosi, R.; Maietti, A.; Tedeschi, P.; Marchetti, A.; Plessi, M. Traditional balsamic vinegar and balsamic vinegar of Modena analyzed by nuclear magnetic resonance spectroscopy coupled with multivariate data analysis. LWT-Food Sci. Technol. 2015, 60, 1017–1024. [Google Scholar] [CrossRef]
- Camin, F.; Bontempo, L.; Perini, M.; Tonon, A.; Breas, O.; Guillou, C.; Morena-Rojas, J.M.; Gagliano, G. Control of wine vinegar authenticity through δ18O analysis. Food Control 2013, 29, 107–111. [Google Scholar] [CrossRef]
- Mohammadian, E.; Rahimpour, E.; Alizadeh-Sani, M.; Foroumadi, A.; Jouyban, A. An overview on terbium sensitized based-optical sensors/nanosensors for determination of pharmaceuticals. Appl. Spectrosc. Rev. 2022, 57, 39–76. [Google Scholar] [CrossRef]
- Hitabatuma, A.; Wang, P.; Su, X.; Ma, M. Metal-organic frameworks-based sensors for food safety. Foods 2022, 11, 382. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Dai, Z.; Cui, L.; Lin, H.; Li, Z.; Wu, K.; Liu, G. Quantitative detection of extra virgin olive oil adulteration, as opposed to peanut and soybean oil, employing LED-induced fluorescence spectroscopy. Sensors 2022, 22, 1227. [Google Scholar] [CrossRef]
- De la Haba, M.J.; Arias, M.; Ramírez, P.; López, M.I.; Sánchez, M.T. Characterizing and authenticating Montilla-Moriles PDO vinegars using near infrared reflectance spectroscopy (NIRS) technology. Sensors 2014, 14, 3528–3542. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Cagliani, L.R.; Benevelli, F.; Spraul, M.; Humpfer, E.; Stocchero, M. NMR and chemometric methods: A powerful combination for characterization of balsamic and traditional balsamic vinegar of Modena. Anal. Chim. Acta 2008, 611, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Rios-Reina, R.; Callejón, R.M.; Oliver-Pozo, C.; Amigo, J.M.; Garcia-Gonzalez, D.L. ATR-FTIR as a potential tool for controlling high quality vinegar categories. Food Control 2017, 78, 230–237. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Elcoroaristizabal, S.; Ocana-Gonzalez, J.A.; Garcia-Gonzalez, D.L.; Amigo, J.M.; Callejón, R.M. Characterization and authentication of Spanish PDO wine vinegars using multidimensional fluorescence and chemometrics. Food Chem. 2017, 230, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Rios-Reina, R.; Garcia-Gonzalez, D.L.; Callejón, R.M.; Amigo, J.M. NIR spectroscopy and chemometrics for the typification of Spanish wine vinegars with a protected designation of origin. Food Control 2018, 89, 108–116. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Azcarate, S.M.; Camina, J.; Callejón, R.M.; Amigo, J.M. Application of hierarchical classification models and reliability estimation by bootstrapping, for authentication and discrimination of wine vinegars by UV-Vis spectroscopy. Chemometr. Intel. Lab. 2019, 191, 42–53. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Callejón, R.M.; Savorani, F.; Arnigo, J.M.; Cocchi, M. Data fusion approaches in spectroscopic characterization and classification of PDO wine vinegars. Talanta 2019, 198, 560–572. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Azcarate, S.M.; Camiña, J.M.; Callejón, R.M. Sensory and spectroscopic characterization of Argentinean wine and balsamic vinegars: A comparative study with European vinegars. Food Chem. 2020, 323, 126791. [Google Scholar] [CrossRef]
- Lastra-Mejías, M.; González-Flores, E.; Izquierdo, M.; Cancilla, J.C.; Torrecilla, J.S. Cognitive chaos on spectrofluorometric data to quantitatively unmask adulterations of a PDO vinegar. Food Control 2020, 108, 106860. [Google Scholar] [CrossRef]
- Peng, T.Q.; Yin, X.L.; Sun, W.; Ding, B.; Ma, L.A.; Gu, H.W. Developing an excitation-emission matrix fluorescence spectroscopy method coupled with multi-way classification algorithms for the identification of the adulteration of Shanxi aged vinegars. Food Anal. Method. 2019, 12, 2306–2313. [Google Scholar] [CrossRef]
- Cavdaroglu, C.; Ozen, B. Detection of vinegar adulteration with spirit vinegar and acetic acid using UV–visible and Fourier transform infrared spectroscopy. Food Chem. 2022, 379, 132150. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine. OENO 52-2000 Wine Vinegars—Determination of Total Acidity Content. Compendium of Methods of Analysis of Wine Vinegars. 2000. Available online: https://www.oiv.int/public/medias/2697/oeno-52-2000.pdf (accessed on 14 January 2023).
- Ozturk, I.; Caliskan, O.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT-Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Callejón, R.M.; Amigo, J.M.; Pairo, E.; Garmón, S.; Ocaña, J.A.; Morales, M.L. Classification of Sherry vinegars by combining multidimensional fluorescence, parafac and different classification approaches. Talanta 2012, 88, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Särndal, C.E.; Swensson, B.; Wretman, J. Model Assisted Survey Sampling; Springer: Berlin, Germany, 2003; pp. 100–109. ISBN 0387406204. [Google Scholar]
- Bajoub, A.; Medina-Rodríguez, S.; Gómez-Romero, M.; Bagur-González, M.G.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Assessing the varietal origin of extra-virgin olive oil using liquid chromatography fingerprints of phenolic compound, data fusion and chemometrics. Food Chem. 2017, 215, 245–255. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Casolino, M.C.; Hysenaj, V.; Oliveri, P.; Zunin, P. A screening method based on UV–Visible spectroscopy and multivariate analysis to assess addition of filler juices and water to pomegranate juices. Food Chem. 2013, 140, 735–741. [Google Scholar] [CrossRef]
- Włodarska, K.; Piasecki, P.; Lobo-Prieto, A.; Pawlak-Lemańska, K.; Górecki, T.; Sikorska, E. Rapid screening of apple juice quality using ultraviolet, visible, and near infrared spectroscopy and chemometrics: A comparative study. Microchem. J. 2021, 164, 106051. [Google Scholar] [CrossRef]
- Rodriguez-Delgado, M.A.; Malovana, S.; Perez, J.P.; Borges, T.; Montelongo, F.G. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Mirzaee, E.; Rahmanpour, M.; Mostafaei, M. Detecting the adulteration in apple vinegar using olfactory machine coupled PCA and ANN methods. Agric. Eng. Int. 2022, 24, 164–173. [Google Scholar]



| Number of Samples | pH Range | Brix Range | Total Phenolic Content Range (mg Gallic Acid/L) |
|---|---|---|---|
| 17 | 2.74–2.99 | 0.6–5.3 | 163.15–547.40 |
| Statistical Measures * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LV | R2cal | R2val | RMSE | Sensitivity | Specificity | CCcal% | CCval% | ||
| MSC-transformed fluorescence data with PLS-DA | |||||||||
| First sample set | 3 | 0.96 | 0.11 | 0.303 | 1 | 0.92 | 90 | 92 | |
| Second sample set | 7 | 0.97 | 0.87 | 0.119 | 0.2 | 0.91 | 100 | 85 | |
| Third sample set | 4 | 0.96 | 0.43 | 0.243 | NaN | 0.9 | 94 | 90 | |
| SNV-transformed UV–visible data with OPLS-DA | First sample set | 1 + 7 | 0.98 | 0.47 | 0.241 | 0.67 | 0.94 | 93 | 92 |
| Second sample set | 1 + 7 | 0.99 | 0.46 | 0.243 | 0 | 0.9 | 93 | 88 | |
| Third sample set | 1 + 7 | 0.99 | 0.44 | 0.247 | NaN | 0.9 | 93 | 90 | |
| Statistical Measures * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LV | R2cal | R2val | RMSE | Sensitivity | Specificity | CCcal% | CCval% | ||
| PLS-DA | |||||||||
| Raw | First sample set | 8 | 0.99 | 0.77 | 0.159 | 1 | 0.92 | 99 | 92 |
| Second sample set | 10 | 0.99 | 0.85 | 0.128 | 1 | 0.94 | 100 | 94 | |
| Third sample set | 10 | 0.99 | 0.86 | 0.125 | 1 | 0.94 | 100 | 94 | |
| Square | First sample set | 10 | 0.99 | 0.85 | 0.13 | 1 | 0.94 | 100 | 94 |
| Second sample set | 10 | 0.99 | 0.84 | 0.135 | 1 | 0.94 | 99 | 94 | |
| Third sample set | 10 | 0.99 | 0.83 | 0.139 | 1 | 0.94 | 99 | 94 | |
| Savitzky–Golay | First sample set | 8 | 0.99 | 0.75 | 0.165 | 1 | 0.92 | 99 | 92 |
| Second sample set | 10 | 0.99 | 0.83 | 0.138 | 1 | 0.96 | 100 | 96 | |
| Third sample set | 10 | 0.99 | 0.84 | 0.134 | 1 | 0.94 | 100 | 94 | |
| OPLS-DA | |||||||||
| Raw | First sample set | 1 + 8 | 0.99 | 0.84 | 0.133 | 1 | 0.92 | 99 | 92 |
| Second sample set | 1 + 9 | 0.99 | 0.85 | 0.128 | 1 | 0.94 | 100 | 94 | |
| Third sample set | 1 + 9 | 0.99 | 0.86 | 0.125 | 1 | 0.96 | 100 | 96 | |
| Square | First sample set | 1 + 8 | 0.99 | 0.81 | 0.143 | 1 | 0.94 | 100 | 94 |
| Second sample set | 1 + 8 | 0.99 | 0.78 | 0.157 | 1 | 0.94 | 99 | 94 | |
| Third sample set | 1 + 7 | 0.00 | 0.83 | 0.139 | 1 | 0.96 | 99 | 96 | |
| Savitzky–Golay | First sample set | 1 + 8 | 0.99 | 0.82 | 0.14 | 1 | 0.92 | 99 | 92 |
| Second sample set | 1 + 9 | 0.99 | 0.83 | 0.138 | 1 | 0.96 | 100 | 96 | |
| Third sample set | 1 + 5 | 0.99 | 0.84 | 0.134 | 1 | 0.92 | 95 | 92 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavdaroglu, C.; Ozen, B. Applications of UV–Visible, Fluorescence and Mid-Infrared Spectroscopic Methods Combined with Chemometrics for the Authentication of Apple Vinegar. Foods 2023, 12, 1139. https://doi.org/10.3390/foods12061139
Cavdaroglu C, Ozen B. Applications of UV–Visible, Fluorescence and Mid-Infrared Spectroscopic Methods Combined with Chemometrics for the Authentication of Apple Vinegar. Foods. 2023; 12(6):1139. https://doi.org/10.3390/foods12061139
Chicago/Turabian StyleCavdaroglu, Cagri, and Banu Ozen. 2023. "Applications of UV–Visible, Fluorescence and Mid-Infrared Spectroscopic Methods Combined with Chemometrics for the Authentication of Apple Vinegar" Foods 12, no. 6: 1139. https://doi.org/10.3390/foods12061139
APA StyleCavdaroglu, C., & Ozen, B. (2023). Applications of UV–Visible, Fluorescence and Mid-Infrared Spectroscopic Methods Combined with Chemometrics for the Authentication of Apple Vinegar. Foods, 12(6), 1139. https://doi.org/10.3390/foods12061139






